Assessment of Ultrasound-Controlled Diagnostic Methods for Thyroid Lesions and Their Associated Costs in a Tertiary University Hospital in Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Clinical and Imaging Data
- I.
- Nondiagnostic;
- II.
- Benign (<3%);
- III.
- Atypia of Undetermined Significance or Follicular Lesion of Undetermined Significance (10–30%);
- IV.
- Follicular Neoplasm or Suspicious for Follicular Neoplasm (25–40%);
- V.
- Suspicious for Malignancy (50–75%);
- VI.
- Malignant (97–99%) [29].
2.3. Statistical Analysis
2.3.1. Univariate Analysis
2.3.2. Multivariate Logistic Regression
2.3.3. Receiver Operating Characteristics
2.3.4. Cost–Benefit Utility Functions
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| US | ultrasonography |
| FNAB | fine-needle aspiration biopsy |
| TI-RADS | Thyroid Imaging Reporting and Data System |
| SMI | superb microvascular imaging |
| SWE | shear wave elastography |
| TBSRTC | Bethesda System for Reporting Thyroid Cytopathology |
References
- Ali, S.Z.; Baloch, Z.W.; Cochand-Priollet, B.; Schmitt, F.C.; Vielh, P.; VanderLaan, P.A. The 2023 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2023, 33, 1039–1044. [Google Scholar] [CrossRef]
- Biswas, A.; Basu, K.; De, S.; Karmakar, S.; De, D.; Sengupta, M.; Ghosh, S. Correlation between Thyroid Imaging Reporting and Data System and Bethesda System of Reporting of Thyroid Cytopathology of Thyroid Nodule: A Single Center Experience. J. Cytol. 2020, 37, 193–199. [Google Scholar] [CrossRef]
- Cay, M.; Turan, I.; Mengen, E.; Çimen, A.M.; Celebi, Ş.T.; Yüksel, B. Optimal timing of repeat thyroid fine-needle aspiration biopsy. J. Pediatr. Endocrinol. Metab. 2025, 38, 494–500. [Google Scholar] [CrossRef]
- Codrich, M.; Biasotto, A.; D’Aurizio, F. Circulating Biomarkers of Thyroid Cancer: An Appraisal. J. Clin. Med. 2025, 14, 1582. [Google Scholar] [CrossRef]
- Drummond, M.F.; Sculpher, M.J.; Claxton, K.; Stoddart, G.L.; Torrance, G.W. Methods for the Economic Evaluation of Health Care Programmes, 4th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Elmadani, M.; Mokaya, P.O.; Omer, A.A.A.; Kiptulon, E.K.; Klara, S.; Orsolya, M. Cancer burden in Europe: A systematic analysis of the GLOBOCAN database (2022). BMC Cancer 2025, 25, 447. [Google Scholar] [CrossRef]
- Fei, M.; Ding, D.; Ouyang, X.; Shen, W.; Zhang, F.; Zhang, B.; Qin, L. The value of NGS-based multi-gene testing for differentiation of benign from malignant and risk stratification of thyroid nodules. Front. Oncol. 2024, 14, 1414492. [Google Scholar] [CrossRef]
- Gabriel, M.; Tomczak, J.; Snoch-Ziółkiewicz, M.; Dzieciuchowicz, Ł.; Strauss, E.; Oszkinis, G. Comparison of superb micro-vascular ultrasound imaging (SMI) and contrast-enhanced ultrasound (CEUS) for detection of endoleaks after endovascular aneurysm repair (EVAR) Am. J. Case Rep. 2016, 17, 43–46. [Google Scholar] [CrossRef]
- Gharib, H.; Papini, E.; Garber, J.R.; Duick, D.S.; Harrell, R.M.; Hegedüs, L.; Paschke, R.; Valcavi, R.; Vitti, P. AACE/ACE/AME Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules—2016 update. Endocr. Pract. 2016, 22, 622–639. [Google Scholar] [CrossRef]
- Goffi, A.; Al-Amoodi, A.; Buchanan, B. Principles of Doppler Ultrasonography and Basic Applications for the Clinician. Med. Clin. N. Am. 2025, 109, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.J.; Chung, S.R.; Na, D.G.; Ahn, H.S.; Chung, J.; Lee, J.Y.; Park, J.S.; Yoo, R.E.; Baek, J.H.; Baek, S.M.; et al. 2021 Korean thyroid imaging reporting and data system and imaging-based management of thyroid nodules: Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J. Radiol. 2021, 22, 2094–2123. [Google Scholar] [CrossRef]
- Hata, J.; Toshiba Medical System. Seeing the Unseen New Techniques in Vascular Imaging Superb Micro-Vascular Imaging. Available online: http://www.toshibamedicalsystems.com (accessed on 19 March 2025).
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Socorro, C.R.; Saavedra, P.; López-Fernández, J.C.; Ruiz-Santana, S. Assessment of Muscle Wasting in Long-Stay ICU Patients Using a New Ultrasound Protocol. Nutrients 2018, 10, 1849. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Socorro, C.R.; Saavedra, P.; López-Fernández, J.C.; Lübbe-Vazquez, F.; Ruiz-Santana, S. Novel High-Quality Sonographic Methods to Diagnose Muscle Wasting in Long-Stay Critically Ill Patients: Shear Wave Elastography, Superb Microvascular Imaging and Contrast-Enhanced Ultrasound. Nutrients 2021, 13, 2224. [Google Scholar] [CrossRef] [PubMed]
- Horvath, E.; Majlis, S.; Rossi, R.; Franco, C.; Niedmann, J.P.; Castro, A.; Dominguez, M. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J. Clin. Endocrinol. Metab. 2009, 94, 1748–1751. [Google Scholar] [CrossRef]
- Januś, D.; Kujdowicz, M.; Kiszka-Wiłkojć, A.; Kaleta, K.; Taczanowska-Niemczuk, A.; Radliński, J.; Możdżeń, K.; Nowak, Z.; Górecki, W.; Starzyk, J.B. Ultrasound and histopathological assessment of benign, borderline, and malignant thyroid tumors in pediatric patients: An illustrative review and literature overview. Front. Endocrinol. 2025, 15, 1481804. [Google Scholar] [CrossRef]
- Jung, H.J.; Eun, N.L.; Son, E.J.; Kim, J.A.; Youk, J.H.; Lee, H.S.; Jeon, S. Ultrasound Findings Suggestive of Malignancy in Thyroid Nodules Classified as Follicular Lesion of Undetermined Significance or Follicular Neoplasm based on the 2017 Bethesda System for Reporting Thyroid Cytopathology. J. Korean Soc. Radiol. 2025, 86, 114–126. [Google Scholar] [CrossRef]
- Kang, Y.J.; Stybayeva, G.; Hwang, S.H. Surgical completeness and safety of minimally invasive thyroidectomy in patients with thyroid cancer: A network meta-analysis. Surgery 2023, 173, 1381–1390. [Google Scholar] [CrossRef]
- Kumari, S.; Suresh, T.N.; Sakalecha, A.K.; Azeem Mohiyuddin, S.M. Association between TI-RADS ultrasound categories and BETHESDA cytology categories of thyroid lesions. Indian J. Cancer 2024, 61, 775–780. [Google Scholar] [CrossRef]
- Kushchayev, S.V.; Kushchayeva, Y.S.; Tella, S.H.; Glushko, T.; Pacak, K.; Teytelboym, O.M. Medullary Thyroid Carcinoma: An Update on Imaging. J. Thyroid Res. 2019, 7, 1893047. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Xiong, Y.; Li, X.; Yao, J.; Ju, H.; Ren, F.; Zhang, L.; Wang, H. Diagnostic value of combined ultrasound contrast and elastography for differentiating benign and malignant thyroid nodules: A meta-analysis. Sci. Rep. 2024, 14, 12605. [Google Scholar] [CrossRef]
- Macvanin, M.T.; Gluvic, Z.M.; Zaric, B.L.; Essack, M.; Gao, X.; Isenovic, E.R. New biomarkers: Prospect for diagnosis and monitoring of thyroid disease. Front. Endocrinol. 2023, 14, 1218320. [Google Scholar] [CrossRef]
- Moraes, P.H.; Chammas, M.C.; Vanderlei, F.B.; Schelini, M.V.; Milani, C.M.; Chacon, D.A. 2D Shear Wave Elastography Increases Diagnostic Accuracy of the TI-RADS-ACR and ATA Classification System in Thyroid Nodule Selection: Cyto- and Histological Correlation. Ultraschall Med. 2025. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Lee, E.J.; Huang, M.G.; Park, Y.I.; Khullar, A.; Plodkowski, R.A. Diagnosis and treatment of patients with thyroid cancer. Am. Health Drug Benefits 2015, 8, 30–40. [Google Scholar] [PubMed] [PubMed Central]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 5 June 2025).
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TI-RADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Steward, D.L.; Carty, S.E.; Sippel, R.S.; Yang, S.P.; Sosa, J.A.; Sipos, J.A.; Figge, J.J.; Mandel, S.; Haugen, B.R.; Burman, K.D.; et al. Performance of a multigene genomic classifier in thyroid nodules with indeterminate cytology: A Prospective Blinded Multicenter Study. JAMA Oncol. 2019, 5, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection via the Lasso. J. R. Stat. Soc. Ser. B Stat. Methodol. 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.; Zhang, S.; Cao, K.; Yang, J. Research progress on artificial intelligence technology-assisted diagnosis of thyroid diseases. Front. Oncol. 2025, 5, 1536039. [Google Scholar] [CrossRef]
- Yoo, M.H.; Kim, H.J.; Choi, I.H.; Park, S.; Kim, S.J.; Park, H.K.; Byun, D.W.; Suh, K. Shear wave elasticity by tracing total nodule showed high reproducibility and concordance with fibrosis in thyroid cancer. BMC Cancer 2020, 20, 118. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, X.; Liu, L.; Wang, Y.; Jiang, J.; Li, H.; Fei, W.; Zhong, T.; Jiang, Z. Clinical value of multi-gene testing in distinguishing benign and malignant thyroid nodules. Medicine 2024, 103, e35960. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Zhang, Y.; Wang, H.; Tang, L.; Xie, X.; Fu, Q.; Wu, P.Y.; Song, B. Thyroid imaging reporting and data system with MRI morphological features for thyroid nodules: Diagnostic performance and unnecessary biopsy rate. Cancer Imaging 2024, 24, 74. [Google Scholar] [CrossRef] [PubMed]
- Żyłka, A.; Dobruch-Sobczak, K.; Piotrzkowska-Wróblewska, H.; Jędrzejczyk, M.; Bakuła-Zalewska, E.; Góralski, P.; Gałczyński, J.; Dedecjus, M. The Utility of Contrast-Enhanced Ultrasound (CEUS) in Assessing the Risk of Malignancy in Thyroid Nodules. Cancers 2024, 16, 1911. [Google Scholar] [CrossRef] [PubMed]
| Thyroid Cancer | ||||
|---|---|---|---|---|
| Overall N = 296 | No N = 271 | Yes N = 25 | p-Value | |
| Age (years) | 58.4 ± 14.2 | 58.1 ± 14.3 | 61.7 ± 13.7 | 0.23 |
| Sex male | 74 (25.0) | 63 (23.2) | 11 (44.0) | 0.022 |
| Thyroiditis | 47 (15.9) | 44 (16.2) | 3 (12.0) | 0.777 |
| Goiter | 292 (98.7) | 269 (99.3) | 23 (92.0) | 0.037 |
| Carcinoma/lymphoma | 24 (8.1) | 7 (2.6) | 17 (68.0) | <0.001 |
| Papillary cancer | 11 (3.7) | 3 (1.1) | 8 (32.0) | <0.001 |
| Follicular cancer | 8 (2.7) | 3 (1.1) | 5 (20.0) | <0.001 |
| TI-RADS | 4 (3; 5) | 4 (3; 4.5) | 7 (4; 8) | <0.001 |
| TI-RADS > 4 | 86 (29.1) | 68 (25.1) | 18 (72.0) | <0.001 |
| Thyroidectomy | 24 (8.1) | 14 (5.2) | 10 (40.0) | <0.001 |
| Elastography | <0.001 | |||
| Benign | 272 (91.9) | 264 (97.4) | 8 (32.0) | |
| Malignant | 24 (8.1) | 7 (2.6) | 17 (68.0) | |
| Doppler | <0.001 | |||
| Peripheral | 240 (81.1) | 226 (83.4) | 14 (56.0) | |
| Intranodular | 7 (2.4) | 2 (0.7) | 5 (20.0) | |
| Both | 49 (16.6) | 43 (15.9) | 6 (24.0) | |
| SMI | <0.001 | |||
| Peripheral | 232 (78.4) | 221 (81.5) | 11 (44.0) | |
| Intranodular | 21 (7.1) | 8 (3.0) | 13 (52.0) | |
| Both | 43 (14.5) | 42 (15.5) | 1 (4.0) | |
| NT composition | 0.011 | |||
| Cystic/spongiform | 63 (21.4) | 62 (22.9) | 1 (4.2) | |
| Mixed | 58 (19.7) | 56 (20.7) | 2 (8.3) | |
| Solid | 174 (59.0) | 153 (56.5) | 21 (87.5) | |
| Bethesda System category | <0.001 | |||
| II, III | 277 (94.5) | 271 (100.0) | 6 (27.3) | |
| IV, V, VI | 16 (5.5) | 0 | 16 (72.7) | |
| TN echogenicity | <0.001 | |||
| Anechoic | 17 (5.8) | 17 (6.3) | 0 | |
| Isoechoic/hyperechoic | 157 (53.2) | 152 (56.1) | 5 (20.8) | |
| Hypoechoic | 109 (36.9) | 93 (34.3) | 16 (66.7) | |
| Very hypoechoic | 12 (4.1) | 9 (3.3) | 3 (12.5) | |
| TN. Shape | <0.001 | |||
| Smooth/well defined | 258 (87.5) | 243 (89.7) | 15 (62.5) | |
| Loculated/irregular | 17 (5.8) | 10 (3.7) | 7 (29.2) | |
| Extra-thyroid extension | 20 (6.8) | 18 (6.6) | 2 (8.3) | |
| TN. Echogenic foci | 0.041 | |||
| None/artifacts | 187 (63.4) | 177 (65.3) | 10 (41.7) | |
| Macrocalcifications | 37 (12.5) | 34 (12.5) | 3 (12.5) | |
| Peripheral calcifications | 37 (12.5) | 32 (11.8) | 5 (20.8) | |
| Punctate foci | 34 (11.5) | 28 (10.3) | 6 (25.0) | |
| Tru-cut biopsy | <0.001 | |||
| Yes | 9 (3.0) | 4 (1.5) | 5 (20.0) | |
| No | 287 (97.0) | 267 (98.5) | 20 (80.0) | |
| Mechanism | Selected Variables | Coefficient (95% CI) * | p-Value * |
|---|---|---|---|
| Doppler only | Doppler. Peripheral | −2.399 (−4.153; −0.227) | <0.001 |
| Doppler. Intranodular | 0.826 (0.000; 1.697) | 0.001 | |
| SMI only | SMI. Intranodular | 3.614 (0.562; 4.764) | <0.001 |
| SMI. Both | −3.491 (−5.042; −0.657) | <0.001 | |
| SMI + elastography (if all 4 variables are included) | Malignant elastography | 3.611 (1.832; 5.364) | <0.001 |
| SMI. Intranodular | 0.735 (0.000; 2.769) | 0.03 | |
| SMI. Both | −1.395 (−3.349; 0.000) | 0.009 | |
| SMI + TI-RADS (if only elastography is excluded) | TI-RADS | 0.402 (0.167; 0.708) | <0.001 |
| SMI. Intranodular | 3.020 (0.728; 4.585) | 0.001 | |
| SMI. Both | −3.170 (−5.390; −0.462) | <0.001 |
| Mechanism | Score | Cutoff * |
|---|---|---|
| TI-RADS | - | 5.5 |
| Doppler only | −1.986 | |
| SMI only | 1.845 | |
| SMI + elastography | 0.367 | |
| SMI + TI-RADS | 2.61 |
| TI-RADS | Doppler Only | SMI only | Elastography | SMI + Elastography | SMI + TI-RADS | |
|---|---|---|---|---|---|---|
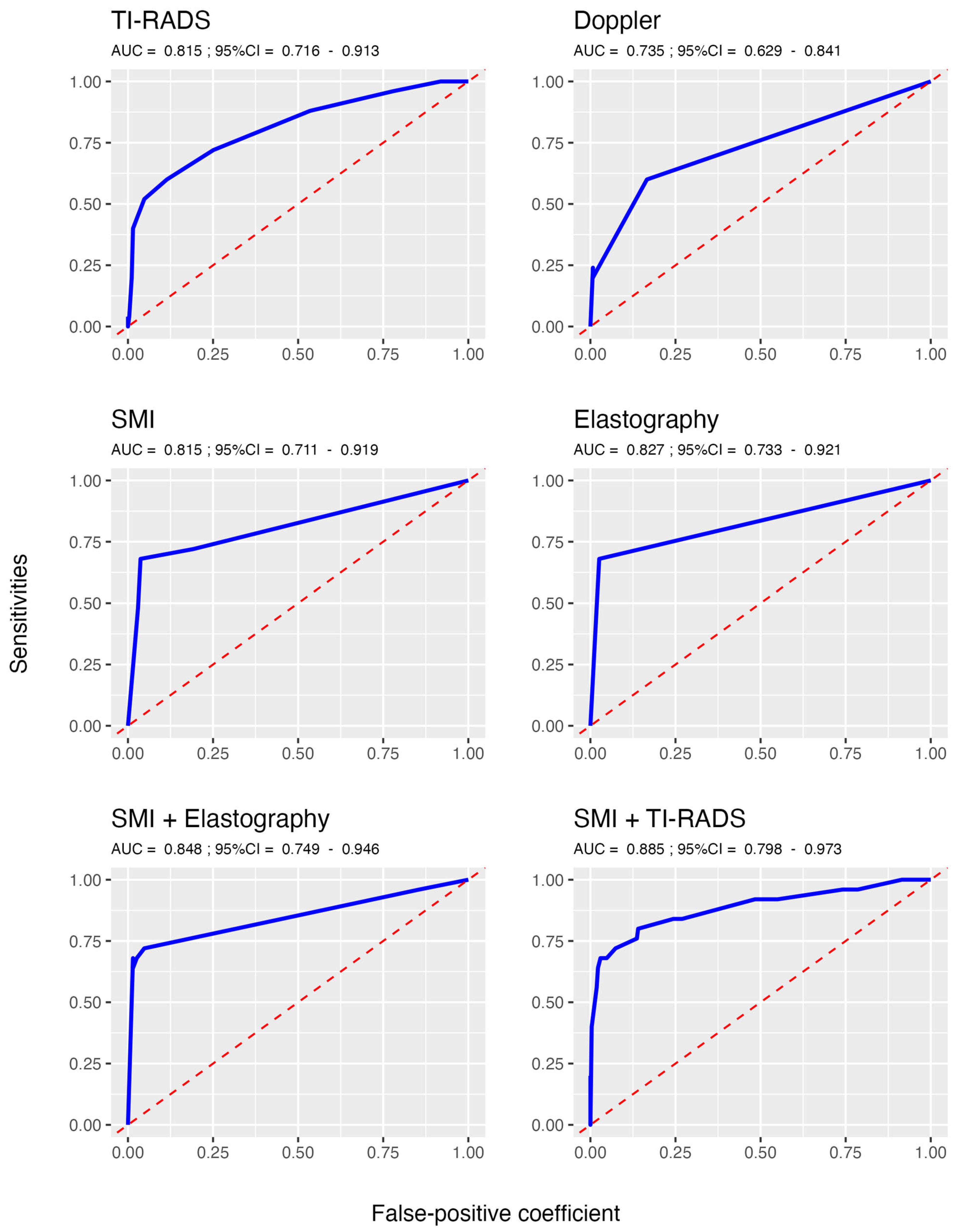
| Youden index * | 0.52 (0.34; 0.70) | 0.43 (0.24; 0.62) | 0.64 (0.44; 0.83) | 0.65 (0.48; 0.83) | 0.69 (0.50; 0.85) | 0.69 (0.52; 0.84) |
| Accuracy | 86.8 (69.9; 94.3) | 81.4 (77.0; 86.5) | 93.9 (90.5; 96.6) | 94.9 (91.6; 97.0) | 94.9 (91.2; 97.6) | 90.9 (77.7; 96.6) |
| Sensitivity | 64.0 (44.0; 88.0) | 60.0 (40.0; 80.0) | 68.0 (48.0; 88.0) | 68.0 (46.4; 84.3) | 72.0 (52.0; 88.0) | 76.0 (60.0; 92.0) |
| Specificity | 88.9 (69.4; 97.80) | 83.4 (78.6; 88.2) | 96.3 (93.7; 98.5) | 97.4 (94.5; 98.9) | 97.0 (93.4; 99.6) | 92.3 (76.8; 98.9) |
| PPV | 34.9 (17.3; 70.8) | 25.0 (17.7; 35.3) | 63.3 (45.4; 80.8) | 70.8 (48.8; 86.6) | 69.1 (48.5; 94.7) | 47.5 (25.6; 84.20) |
| NPV | 96.5 (94.7; 98.6) | 95.8 (93.8; 97.8) | 97.0 (95.3; 98.8) | 97.1 (94.1; 98.6) | 97.4 (95.7; 98.9) | 97.8 (96.2; 99.3) |
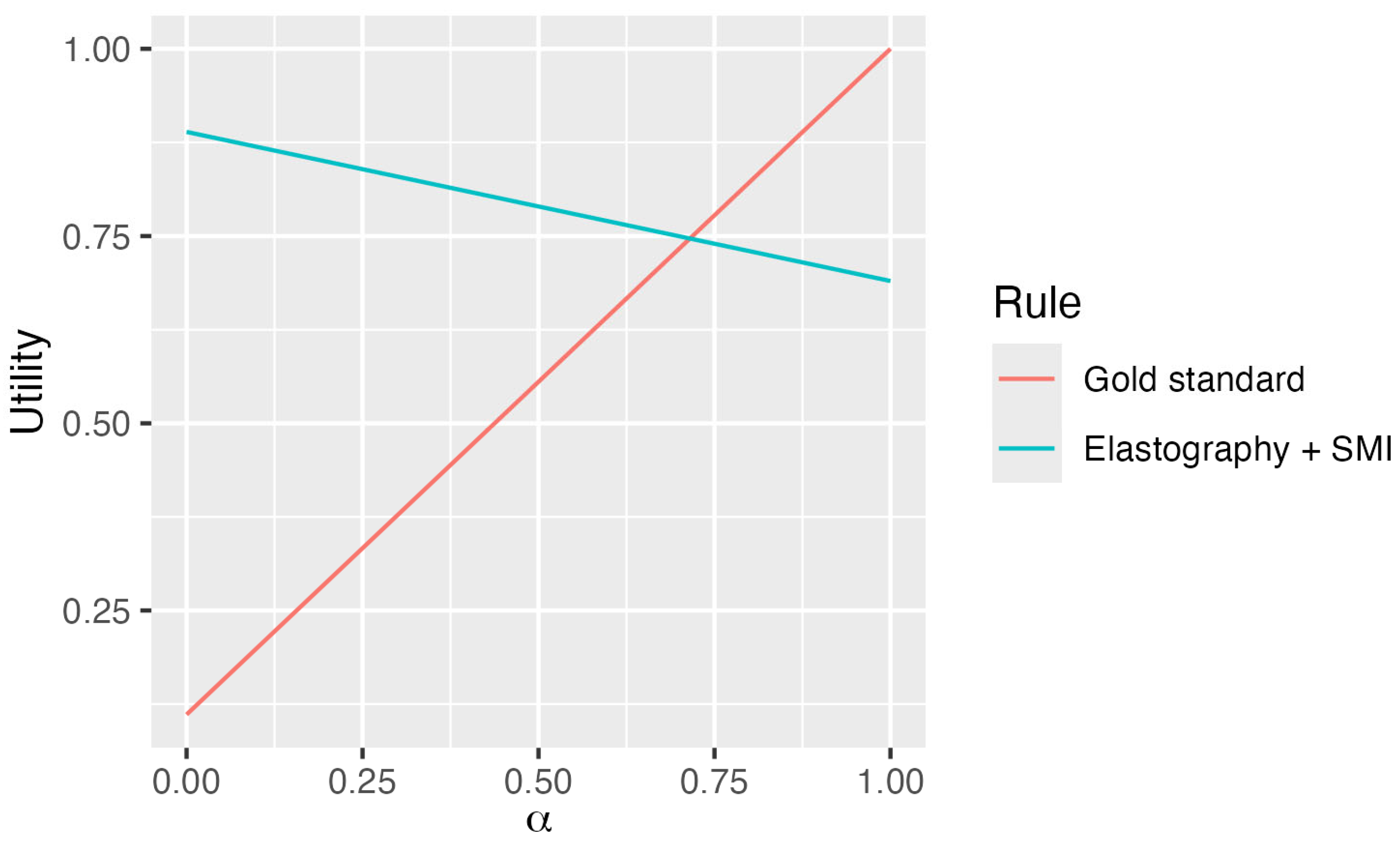
| Costs | Youden | ||
|---|---|---|---|
| Rule diagnosis | Real (EUR) | Normalized | Estimated |
| Gold Standard: FNA-Tru-cut Biopsies | 302.44 | 0.889 | 1 |
| = Elastography + SMI | 37.74 | 0.111 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Hernández, L.; Hernández-Socorro, C.R.; Saavedra, P.; de la Vega-Pérez, M.; Ruiz-Santana, S. Assessment of Ultrasound-Controlled Diagnostic Methods for Thyroid Lesions and Their Associated Costs in a Tertiary University Hospital in Spain. J. Clin. Med. 2025, 14, 5551. https://doi.org/10.3390/jcm14155551
Ruiz-Hernández L, Hernández-Socorro CR, Saavedra P, de la Vega-Pérez M, Ruiz-Santana S. Assessment of Ultrasound-Controlled Diagnostic Methods for Thyroid Lesions and Their Associated Costs in a Tertiary University Hospital in Spain. Journal of Clinical Medicine. 2025; 14(15):5551. https://doi.org/10.3390/jcm14155551
Chicago/Turabian StyleRuiz-Hernández, Lelia, Carmen Rosa Hernández-Socorro, Pedro Saavedra, María de la Vega-Pérez, and Sergio Ruiz-Santana. 2025. "Assessment of Ultrasound-Controlled Diagnostic Methods for Thyroid Lesions and Their Associated Costs in a Tertiary University Hospital in Spain" Journal of Clinical Medicine 14, no. 15: 5551. https://doi.org/10.3390/jcm14155551
APA StyleRuiz-Hernández, L., Hernández-Socorro, C. R., Saavedra, P., de la Vega-Pérez, M., & Ruiz-Santana, S. (2025). Assessment of Ultrasound-Controlled Diagnostic Methods for Thyroid Lesions and Their Associated Costs in a Tertiary University Hospital in Spain. Journal of Clinical Medicine, 14(15), 5551. https://doi.org/10.3390/jcm14155551







