Effectiveness of Virtual Reality-Based Training Versus Conventional Exercise Programs on Fall-Related Functional Outcomes in Older Adults with Various Health Conditions: A Systematic Review
Abstract
1. Introduction
- In which subgroups of older adults (considering different medical conditions) is VR training more effective in reducing fall risk than conventional exercise programs?
- What is the impact of VR interventions and conventional programs on specific motor parameters such as static and dynamic balance, lower limb strength, functional mobility (e.g., Timed Up and Go), and gait characteristics?
- What are the adherence rates, safety indicators, and long-term effects (follow-up) of both types of interventions?
- What practical and clinical factors (e.g., technology acceptance, equipment requirements, therapist competence) influence the implementation of VR in fall prevention programs in geriatric care settings?
2. Methods
2.1. Search Strategy
- Scopus:TITLE-ABS-KEY ((“virtual reality” OR VR OR exergam*) AND (“circuit training” OR circuit-based OR multicomponent OR multimodal OR “combined exercise”) AND (“balance training” OR “isolated balance” OR “postural control” OR “postural stability”) AND (falls OR “fall prevention” OR “fall risk” OR “accidental falls”) AND (“older adults” OR elderly OR aged OR geriatric OR senior OR “community-dwelling”))
- PubMed:(“Virtual Reality” [Mesh] OR “virtual reality” [tiab] OR VR [tiab] OR exergam* [tiab]) AND (“Exercise Therapy” [Mesh] OR multicomponent [tiab] OR “combined exercise” [tiab] OR “circuit training” [tiab]) AND (“Accidental Falls” [Mesh] OR fall* [tiab] OR “fall risk” [tiab] OR “fall prevention” [tiab]) AND (“Aged” [Mesh] OR elderly [tiab] OR “older adults” [tiab]) AND (randomized controlled trial [pt] OR clinical trial [pt])
- EBSCO:((“virtual reality” OR VR OR exergam*) AND (“circuit training” OR circuit-based OR multicomponent OR multimodal OR “combined exercise”) AND (“balance training” OR “isolated balance” OR “postural control” OR “postural stability”) AND (falls OR “fall prevention” OR “fall risk” OR “accidental falls”) AND (“older adults” OR elderly OR aged OR geriatric OR senior OR “community-dwelling”))
- Web Of Science:TS = (“virtual reality” OR VR OR exergam*) AND TS = (multicomponent OR “combined exercise” OR “circuit training”) AND TS = (fall* OR “fall risk” OR “fall prevention”) AND TS = (elderly OR “older adults”)
2.2. PICOS Inclusion and Exclusion Criteria
2.3. Selection Process
2.4. Data Extraction
2.5. Data Items
- Publication characteristics: authors, year, title, and publication source.
- Study design: type (randomized, controlled/pre–post/cross-sectional), setting, number of participants, and inclusion criteria.
- Participants: age, sex, health status (e.g., idiopathic falls, Parkinson’s disease, mild cognitive impairment [MCI], osteoporosis, chronic dizziness, healthy older adults), and physical activity level.
- VR intervention: type, session duration (30–60 min), frequency (2–5 times/week), and intervention period (3–12 weeks).
- Control intervention: conventional balance exercises (traditional treadmill training, stretching/relaxation), home-based programs (educational materials, balance exercises), or no intervention.
- Balance assessments: Berg Balance Scale, tandem stance, one-leg stance test, Functional Reach Test, Fullerton Advanced Balance Scale.
- Mobility assessments: Timed Up and Go (TUG), 10-Meter Walk Test (10MWT), gait speed, and gait variability.
- Lower limb muscle strength: isokinetic strength of the quadriceps and hamstrings.
- Cognitive function: Montreal Cognitive Assessment (MoCA).
- Fall risk and fear of falling: Falls Efficacy Scale (FES), Physiological Profile Assessment (PPA).
- Dizziness: Vertigo Symptom Scale (VSS) and Dizziness Handicap Inventory (DHI).
- Parkinson’s disease symptoms: Movement Disorders Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), motor section.
- Number of falls: incidence of fall events before and after the intervention (falls per 6 months).
2.6. Effect Measures
2.7. Synthesis Methods
2.8. Study Risk of Bias Assessment
3. Results
3.1. Article Identification
3.2. Study Characteristics
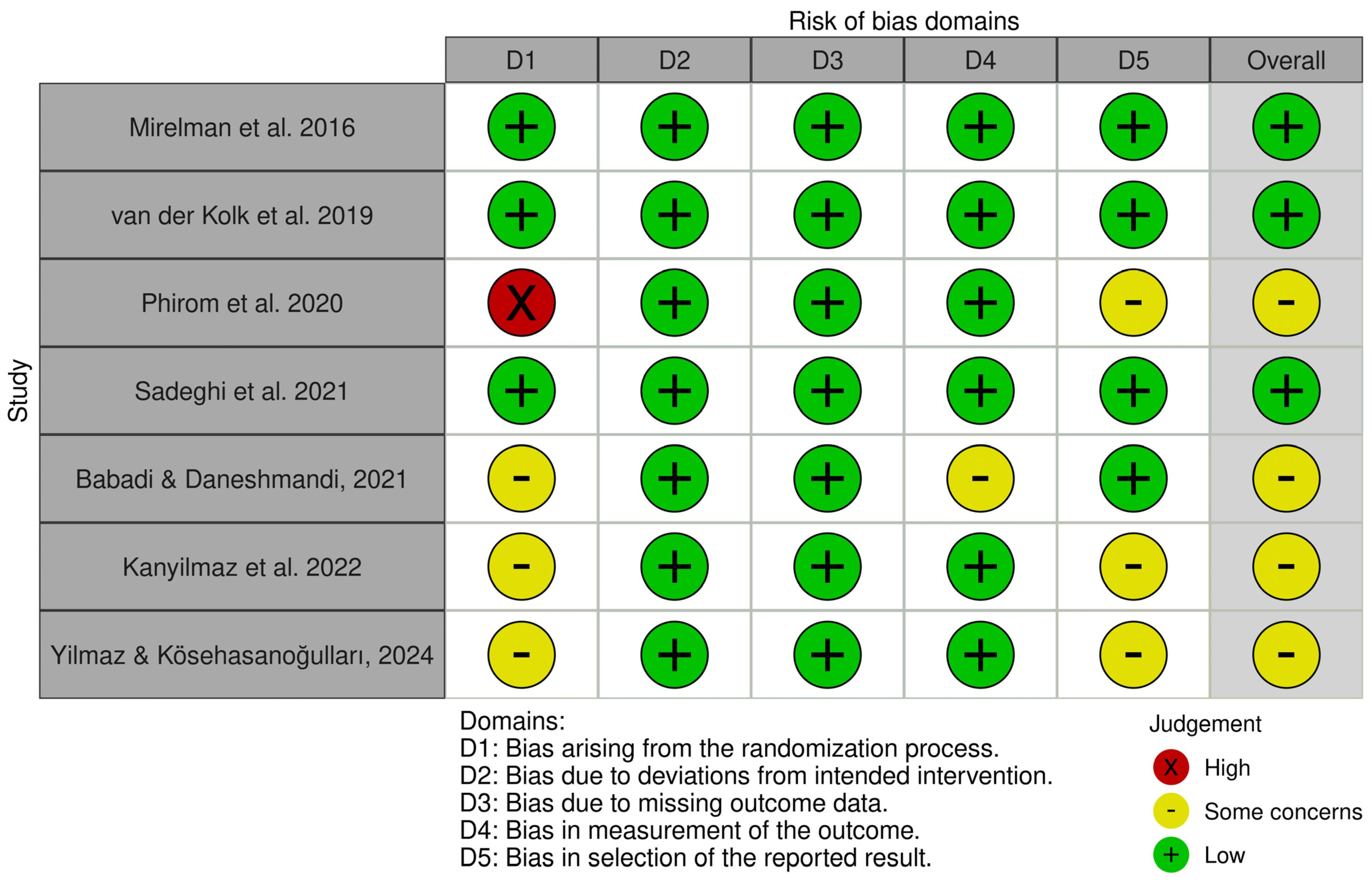
3.3. Risk of Bias in Included Studies
| Lp. | Study | 1. Randomisation | 2. Allocation Concealment | 3. Baseline Comparability | 4. Patient Blinding | 5. Therapist Blinding | 6. Assessor Blinding | 7. ≥85% Follow-Up | 8. Intention-to-Treat | 9. Between-Group Comparisons | 10. Point Measures & Variability | Total Score: |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mirelman et al., 2016 [39] | − | + | + | − | − | − | + | + | + | + | 7/10 |
| 2 | van der Kolk et al., 2019 [36] | + | + | + | + | − | + | + | + | + | + | 9/10 |
| 3 | Phirom et al., 2020 [37] | + | − | + | − | − | − | + | − | + | + | 5/10 |
| 4 | Sadeghi et al., 2021 [38] | + | − | + | − | − | + | + | − | + | + | 6/10 |
| 5 | Yousefi Babadi et al., 2021 [40] | + | − | + | − | − | − | + | − | + | + | 5/10 |
| 6 | Kanyılmaz et al., 2022 [41] | + | − | + | − | − | − | + | − | + | + | 5/10 |
| 7 | Yilmaz & Kösehasanoğulları 2024 [42] | + | − | + | − | − | − | + | − | + | + | 5/10 |
- Domain 1 (bias arising from the randomization process): Three out of seven studies [40,41,42] were rated as raising some concerns regarding the randomization process. One study [37] was rated as having a high risk of bias in this domain. The remaining two studies [36,39] demonstrated a low risk of bias.
3.4. Balance Outcomes
3.5. Mobility
3.6. Cognitive Functions
3.7. Risk of Falls
3.8. Analysis of Functional Parameters and Evaluation of Intervention Effectiveness
3.9. Comparison of the Effectiveness of VR and Conventional Methods
3.10. Adherence, Safety, and Long-Term Maintenance of Effects
| Author | Population | Number of Participants (INT/Control) | Mean Age (±SD) | Intervention | Comparator (Traditional Training) | Duration | Assessed Outcomes | Measurement Tools | Main Findings | Adherence (%) | Follow-Up, Long-Term Effects |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mirelman et al., 2016 [39] | Older adults aged 60–90 years with a high risk of falls (≥2 falls in 6 months): idiopathic (n = 109), mild cognitive impairment (MCI) (n = 43), Parkinson’s disease (n = 130). | Randomization: 302 (INT: 154; Control: 148) ITT Analysis: 282 (INT: 146; Control: 136) | INT: 74.2 (±6.9) Control: 73.3 (±6.4) | Treadmill training + VR (3×/week, 6 weeks): obstacle, pathway, and distractor simulation. | Treadmill training without VR (same intensity and duration). | Intervention: 6 weeks. Follow-up: 6 months. | Primary: Fall incidence rate Secondary: Gait speed, gait variability, endurance (2-min walk), SPPB, SF-36, cognitive function | VR (Microsoft Kinect), Zeno platform, inertial sensors (Opal), NeuroTrax, SPPB, SF-36. | INT vs. Control: IRR = 0.58 (95% CI: 0.36–0.96; p = 0.033) Improved gait variability (p = 0.018), greater foot clearance (p = 0.002). | INT: 92% (16.62/18 sessions) Control: 93% (16.82/18 sessions) | 6-month follow-up: sustained improvement in the INT group, particularly among individuals with Parkinson’s disease (IRR = 0.45; p = 0.015). |
| van der Kolk et al., 2019 [36] | Patients with early-stage Parkinson’s disease (Hoehn and Yahr ≤ 2), on stable dopaminergic medication. | Randomization: 130 (INT: 65; Control: 65) ITT Analysis: 125 (INT: 61; Control: 64) | INT: 59.3 (±8.3) Control: 59.4 (±9.3) | Aerobic cycling training with VR (3×/week, 6 months): target heart rate 50–80% HRR, supported by a motivational app and remote supervision. | Stretching and relaxation exercises (3×/week, 6 months): similar support via a motivational app and remote supervision. | Intervention: 6 months. Follow-up: 6 months. | Primary: MDS-UPDRS-III (in the “off” medication state) Secondary: VO2 max, quality of life (PDQ-39), cognitive function, number of falls | MDS-UPDRS, VO2 max test, motivational app, VR system. | INT vs. Control: difference in MDS-UPDRS-III = −4.2 (95% CI: −6.9 to −1.6; p = 0.002) VO2 max improvement in INT: Δ = 2.4 mL/kg/min; p < 0.0001. | INT: 75% (54/72 sessions) Control: 83% (60/72 sessions) | 6-month follow-up: sustained improvement in the INT group (MDS-UPDRS-III), no significant differences in quality of life. |
| Phirom et al., 2020 [37] | Community-dwelling older adults aged ≥65 years, ambulating independently without assistive devices. | Randomization: 40 (INT: 20; Control: 20) Analysis: 39 (INT: 19; Control: 20) | INT: 70.21 ± 4.18 years Control: 69.40 ± 3.38 years | Cognitive–motor training using Xbox Kinect (3 sessions/week; games requiring stepping, balance, and cognitive tasks). | Educational materials + weekly phone calls to monitor health status (no active training). | 12 weeks (36 sessions). | - Fall risk (PPA, TUG) - Cognitive function (MoCA) | PPA (Physiological Profile Assessment), TUG (Timed Up and Go), MoCA (Montreal Cognitive Assessment). | After 12 weeks: INT: Significant improvement in PPA (p = 0.002), dual-task TUG (p = 0.045), MoCA (p = 0.001) vs. control. Control: Worsening of postural sway (p = 0.025). INT: Improvement in reaction time, postural sway, executive function and attention. | 98.8% (INT: 35.6/36 sessions) | Lack of long-term follow-up; majority of participants were women (82.5%); no data on SRD/MCID for outcomes. |
| Sadeghi et al., 2021 [38] | Older men aged ≥60 years, community-dwelling, able to walk independently. | Randomization: 64 (BT: 16, VR: 16, MIX: 16, CON: 16) ITT Analysis: 58 (BT: 14, VR: 15, MIX: 14, CON: 15) | - BT: 70.4 (±4.3) - VR: 74.1 (±7.0) - MIX: 70.5 (±5.1) - CON: 72.2 (±7.2) | MIX: Combination of balance training (BT) and VR (3×/week, 8 weeks). VR: Virtual reality games (e.g., Xbox Kinect). BT: Static/dynamic exercises. | CON: Control group without intervention. | 8 weeks (3 sessions/week, 40 min/session). | Primary: Muscle strength (quadriceps and hamstrings). Secondary: Balance (one-leg stance, tandem), mobility (TUG, 10mWT) | Biodex isokinetic dynamometer, balance tests (one-leg stance, tandem), TUG, 10mWT. | MIX > VR > BT > CON in improving strength, balance, and mobility (e.g., TUG: MIX −4.2 s; η2 = 0.85; p < 0.001). VR and BT improved outcomes compared to CON (p < 0.05). | - BT: 87.9% - VR: 90.4% - MIX: 92.1% | 8 weeks: maintenance of improvements in intervention groups. No long-term follow-up. |
| Yousefi Babadi & Daneshmandi, 2021 [40] | Older adults (60–75 years) residing in nursing homes (both men and women). | Randomization: 36 (VRT: 12, CBT: 12, Control: 12) ITT Analysis: 36 (no participant loss) | VRT: 66.5 ± 3.8 years CBT: 67.5 ± 3.1 years Control: 66.7 ± 3.2 years | VRT: Training using Xbox Kinect (sports games, e.g., boxing, table tennis). CBT: Conventional balance training (static/dynamic exercises). | Control: No intervention, maintenance of daily activities. | 9 weeks (3 sessions/week, 60 min/session). | Primary: Balance (SLS with eyes open/closed, FRT, TUG, FAB) | Tests: SLS, FRT, TUG, FAB. | VRT and CBT: Significant improvement in all balance parameters (p < 0.05). No difference between VRT and CBT (p > 0.05). Control: No improvement. | 100% adherence in VRT and CBT groups (no missed sessions) | 9 weeks: maintenance of improvements in intervention groups. No long-term follow-up. |
| Kanyilmaz et al., 2022 [41] | Older adults (65+ years) with dizziness. | Randomization: 32 (INT: 16, Control: 16) Analysis: 26 (INT: 13, Control: 13) | 69.7 ± 6.3 years (all ≥ 65) | VR: Vestibular rehabilitation supported by VR (360° videos using VR goggles + smartphone, e.g., motion simulation in a supermarket). | Conventional vestibular rehabilitation (without VR). | 3 weeks (5 sessions/week, 15 sessions). | Dizziness symptoms (VSS) Disability (DHI) Balance (BBT, PST) Mobility (TUG) Anxiety/depression (HAS, GDS) Fear of falling (FES-I) | VSS, DHI, BBT, TUG, FES-I, PST, GDS, HAS. Measurements: before, after 3 weeks, and at 6 months. | After treatment: INT: Significant improvement in emotional DHI and TUG vs. control (p < 0.05). At 6 months: INT: Significant improvement in VSS, all DHI subscales, BBT, HAS vs. control (p < 0.05). No differences in PST, FES-I, GDS. | 87% (2 missed sessions out of 15) | 6 months: Maintenance of improvement in VSS, DHI, BBT, HAS in the INT group. No differences in other parameters. |
| Yilmaz & Kösehasanoğulları, 2024 [42] | Women with osteoporosis (≥45 years). | Randomization: 60 (INT: 30, Control: 30) Analysis: 60 (no participant loss) | WEG: 67 ± 10.64 years HEG: 68 ± 9.06 years | VR: Balance exercises using Nintendo Wii (3 sessions/week, supervised by a physiotherapist). | Conventional home exercises (3 sessions/week). | 12 weeks (36 sessions). | Balance (BBS, TUG) Fear of falling (FES) | BBS (Berg Balance Scale), TUG (Timed Up and Go), FES (Falls Efficacy Scale). | After treatment: WEG: Significant improvement in BBS vs. HEG (52.9 ± 3.63 vs. 47.1 ± 2.89; p < 0.05). Both groups: Improvement in TUG and FES (p < 0.05), but no between-group differences. Difference in BBS (WEG: +10.2 vs. HEG: +5.17; p < 0.05). | 100% (no missed sessions) | No long-term follow-up. Results reported only after 12 weeks. |
4. Discussion
4.1. Balance
4.2. Mobility
4.3. Cognitive Function
4.4. Fall Risk
4.5. Technology Acceptance, Equipment Requirements, and Therapist Competence
4.6. Adherence, Safety, and Long-Term Effects
4.7. Comparison of Results in the Context of Other Meta-Analyses
4.8. Limitations and Practical Implications
5. Conclusions
Future Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Q.; Ou, X.; Li, J. The Risk of Falls among the Aging Population: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 902599. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Darvishi, N.; Ahmadipanah, M.; Shohaimi, S.; Mohammadi, M. Global Prevalence of Falls in the Older Adults: A Comprehensive Systematic Review and Meta-Analysis. J. Orthop. Surg. Res. 2022, 17, 334. [Google Scholar] [CrossRef] [PubMed]
- Haagsma, J.A.; Olij, B.F.; Majdan, M.; Van Beeck, E.F.; Vos, T.; Castle, C.D.; Dingels, Z.V.; Fox, J.T.; Hamilton, E.B.; Liu, Z.; et al. Falls in Older Aged Adults in 22 European Countries: Incidence, Mortality and Burden of Disease from 1990 to 2017. Inj. Prev. 2020, 26, i67–i74. [Google Scholar] [CrossRef] [PubMed]
- Bergen, G.; Stevens, M.R.; Burns, E.R. Falls and Fall Injuries among Adults Aged ≥65 Years—United States, 2014. Morb. Mortal. Wkly. Rep. 2016, 65, 993–998. [Google Scholar] [CrossRef]
- Florence, C.S.; Bergen, G.; Atherly, A.; Burns, E.; Stevens, J.; Drake, C. Medical Costs of Fatal and Nonfatal Falls in Older Adults. J. Am. Geriatr. Soc. 2018, 66, 693–698. [Google Scholar] [CrossRef]
- Trevisan, K.; Cristina-Pereira, R.; Silva-Amaral, D.; Aversi-Ferreira, T.A. Theories of Aging and the Prevalence of Alzheimer’s Disease. BioMed Res. Int. 2019, 2019, 71424. [Google Scholar] [CrossRef]
- Darweesh, S.K.L.; Raphael, K.G.; Brundin, P.; Matthews, H.; Wyse, R.K.; Chen, H.; Bloem, B.R. Parkinson Matters. J. Park. Dis. 2018, 8, 495–498. [Google Scholar] [CrossRef]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D.L. The Prevalence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Xanthopoulos, A.; Butler, J. Cardiovascular Aging and Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 804–813. [Google Scholar] [CrossRef]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef]
- Chadda, K.R.; Ajijola, O.A.; Vaseghi, M.; Shivkumar, K.; Huang, C.L.H.; Jeevaratnam, K. Ageing, the Autonomic Nervous System and Arrhythmia: From Brain to Heart. Ageing Res. Rev. 2018, 48, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Sadighi Akha, A.A. Aging and the Immune System: An Overview. J. Immunol. Methods 2018, 463, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Grote, C.; Reinhardt, D.; Zhang, M.; Wang, J. Regulatory Mechanisms and Clinical Manifestations of Musculoskeletal Aging. J. Orthop. Res.® 2019, 37, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Jehu, D.A.; Davis, J.C.; Falck, R.S.; Bennett, K.J.; Tai, D.; Souza, M.F.; Cavalcante, B.R.; Zhao, M.; Liu-Ambrose, T. Risk Factors for Recurrent Falls in Older Adults: A Systematic Review with Meta-Analysis. Maturitas 2021, 144, 23–28. [Google Scholar] [CrossRef]
- Fawzan, S.; Kozou, H.; Baki, F.; Asal, S. Fall Risk Assessment and Effect of Vestibular Rehabilitation in the Elderly Population. Egypt. J. Otolaryngol. 2022, 38, 88. [Google Scholar] [CrossRef]
- Politi, L.; Salerni, L.; Bubbico, L.; Ferretti, F.; Carucci, M.; Rubegni, G.; Mandalà, M. Risk of Falls, Vestibular Multimodal Processing, and Multisensory Integration Decline in the Elderly–Predictive Role of the Functional Head Impulse Test. Front. Neurol. 2022, 13, 964017. [Google Scholar] [CrossRef]
- Rubega, M.; Di Marco, R.; Zampini, M.; Brain, E.F.-A. Muscular and Cortical Activation during Dynamic and Static Balance in the Elderly: A Scoping Review. Aging Brain 2021, 1, 100013. [Google Scholar] [CrossRef]
- Esposito, G.; Altavilla, G.; Domenico, F.D.; Aliberti, S.; D’isanto, T.; D’elia, F. Proprioceptive Training to Improve Static and Dynamic Balance in Elderly. Int. J. Stat. Med. Res. 2021, 10, 194–199. [Google Scholar] [CrossRef]
- Bullo, V.; Roma, E.; Gobbo, S.; Duregon, F.; Bergamo, M.; Bianchini, G.; Doria, E.; Cugusi, L.; di Blasio, A.; Bocalini, D.S.; et al. Lower Limb Strength Profile in Elderly with Different Pathologies: Comparisons with Healthy Subjects. Geriatrics 2020, 5, 83. [Google Scholar] [CrossRef]
- Xing, L.; Bao, Y.; Wang, B.; Shi, M.; Wei, Y.; Huang, X.; Dai, Y.; Shi, H.; Gai, X.; Luo, Q.; et al. Falls Caused by Balance Disorders in the Elderly with Multiple Systems Involved: Pathogenic Mechanisms and Treatment Strategies. Front. Neurol. 2023, 14, 1128092. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Soh, K.G.; Omar Dev, R.D.; Talib, O.; Xiao, W.; Soh, K.L.; Ong, S.L.; Zhao, C.; Galeru, O.; Casaru, C. Aerobic Exercise Combination Intervention to Improve Physical Performance Among the Elderly: A Systematic Review. Front. Physiol. 2022, 12, 798068. [Google Scholar] [CrossRef] [PubMed]
- Racey, M.; Markle-Reid, M.; Fitzpatrick-Lewis, D.; Ali, M.U.; Gagne, H.; Hunter, S.; Ploeg, J.; Sztramko, R.; Harrison, L.; Lewis, R.; et al. Fall Prevention in Community-Dwelling Adults with Mild to Moderate Cognitive Impairment: A Systematic Review and Meta-Analysis. BMC Geriatr. 2021, 21, 689. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Almagro, D.; Achalandabaso-Ochoa, A.; Ibáñez-Vera, A.J.; Góngora-Rodríguez, J.; Rodríguez-Huguet, M. Effectiveness of Virtual Reality Therapy on Balance and Gait in the Elderly: A Systematic Review. Healthcare 2024, 12, 158. [Google Scholar] [CrossRef]
- Neri, S.G.; Cardoso, J.R.; Cruz, L.; Lima, R.M.; de Oliveira, R.J.; Iversen, M.D.; Carregaro, R.L. Do virtual reality games improve mobility skills and balance measurements in community-dwelling older adults? Systematic review and meta-analysis. Clin. Rehabil. 2017, 31, 1292–1304. [Google Scholar] [CrossRef]
- Ren, Y.; Lin, C.; Zhou, Q.; Yingyuan, Z.; Wang, G.; Lu, A. Effectiveness of virtual reality games in improving physical function, balance and reducing falls in balance-impaired older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2023, 108, 104924. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, K.; Chen, Y.; Zhou, L.; Bao, D.; Zhou, J. Is Virtual Reality Training More Effective than Traditional Physical Training on Balance and Functional Mobility in Healthy Older Adults? A Systematic Review and Meta-Analysis. Front. Hum. Neurosci. 2022, 16, 843481. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Q.; He, C.Q.; Bian, R. Effect of Virtual Reality on Balance in Individuals with Parkinson Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phys. Ther. 2020, 100, 933–945. [Google Scholar] [CrossRef]
- Zhong, Y.-J.; Meng, Q.; Su, C.-H. Mechanism-Driven Strategies for Reducing Fall Risk in the Elderly: A Multidisciplinary Review of Exercise Interventions. Healthcare 2024, 12, 2394. [Google Scholar] [CrossRef]
- Ghous, M.; Masood, Q.; Nawaz Malik, A.; Afridi, A.; Mehmood, Q. Comparison of Nonimmersive Virtual Reality and Task-Oriented Circuit Training on Gait, Balance, and Cognition Among Elderly Population: A Single-Blind Randomized Control Trial. Games Health J. 2024, 13, 164–171. [Google Scholar] [CrossRef]
- Cavalcante, M.M.; Fraga, I.; Dalbosco, B.; De Marchi, P.; Iraci, L.; da Silva, M.B.; Dani, C.; Bosco, A.D.; Elsner, V. Exergame Training-Induced Neuroplasticity and Cognitive Improvement in Institutionalized Older Adults: A Preliminary Investigation. Physiol. Behav. 2021, 241, 113589. [Google Scholar] [CrossRef]
- Henrique, P.P.B.; Perez, F.M.P.; Dorneles, G.; Peres, A.; Korb, A.; Elsner, V.; De Marchi, A.C.B. Exergame and/or conventional training-induced neuroplasticity and cognitive improvement by engaging epigenetic and inflammatory modulation in elderly women: A randomized clinical trial. Physiol. Behav. 2023, 258, 113996. [Google Scholar] [CrossRef]
- Saragih, I.D.; Chen, Y.M.; Suarilah, I.; Susanto, H.; Lee, B.O. Virtual Reality Intervention for Fall Prevention in Older Adults: A Meta-Analysis. J. Nurs. Scholarsh. 2025; Advance online publication. [Google Scholar] [CrossRef]
- Zhu, S.; Sui, Y.; Shen, Y.; Zhu, Y.; Ali, N.; Guo, C.; Wang, T. Effects of Virtual Reality Intervention on Cognition and Motor Function in Older Adults with Mild Cognitive Impairment or Dementia: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2021, 13, 586999. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- van der Kolk, N.M.; de Vries, N.M.; Kessels, R.P.C.; Joosten, H.; Zwinderman, A.H.; Post, B.; Bloem, B.R. Effectiveness of Home-Based and Remotely Supervised Aerobic Exercise in Parkinson’s Disease: A Double-Blind, Randomised Controlled Trial. Lancet Neurol. 2019, 18, 998–1008. [Google Scholar] [CrossRef]
- Phirom, K.; Kamnardsiri, T.; Sungkarat, S. Beneficial Effects of Interactive Physical-Cognitive Game-Based Training on Fall Risk and Cognitive Performance of Older Adults. Int. J. Environ. Res. Public Health 2020, 17, 6079. [Google Scholar] [CrossRef]
- Sadeghi, H.; Jehu, D.A.; Daneshjoo, A.; Shakoor, E.; Razeghi, M.; Amani, A.; Hakim, M.N.; Yusof, A. Effects of 8 Weeks of Balance Training, Virtual Reality Training, and Combined Exercise on Lower Limb Muscle Strength, Balance, and Functional Mobility Among Older Men: A Randomized Controlled Trial. Sports Health 2021, 13, 606–612. [Google Scholar] [CrossRef]
- Mirelman, A.; Rochester, L.; Maidan, I.; Del Din, S.; Alcock, L.; Nieuwhof, F.; Rikkert, M.O.; Bloem, B.R.; Pelosin, E.; Avanzino, L.; et al. Addition of a Non-Immersive Virtual Reality Component to Treadmill Training to Reduce Fall Risk in Older Adults (V-TIME): A Randomised Controlled Trial. Lancet 2016, 388, 1170–1182. [Google Scholar] [CrossRef]
- Yousefi Babadi, S.; Daneshmandi, H. Effects of Virtual Reality versus Conventional Balance Training on Balance of the Elderly. Exp. Gerontol. 2021, 153, 111498. [Google Scholar] [CrossRef]
- Kanyılmaz, T.; Topuz, O.; Ardıç, F.N.; Alkan, H.; Öztekin, S.N.S.; Topuz, B.; Ardıç, F. Effectiveness of Conventional versus Virtual Reality-Based Vestibular Rehabilitation Exercises in Elderly Patients with Dizziness: A Randomized Controlled Study with 6-Month Follow-Up. Braz. J. Otorhinolaryngol. 2022, 88, S41–S49. [Google Scholar] [CrossRef]
- Yilmaz, N.; Kösehasanoğulları, M. The Effectiveness of Virtual Reality Exercise Games on Balance Functions and Fear of Falling in Women with Osteoporosis. Rheumatol. Int. 2024, 44, 1071–1076. [Google Scholar] [CrossRef]
- Lubetzky, A.V.; Gospodarek, M.; Arie, L.; Kelly, J.; Roginska, A.; Cosetti, M.; Affiliations, A. Auditory Input and Postural Control in Adults: A Narrative Review. Arch. Otolaryngol. Neck Surg. 2020, 146, 480–487. [Google Scholar] [CrossRef]
- Mohebbi, A.; Amiri, P.; Kearney, R.E. Identification of Human Balance Control Responses to Visual Inputs Using Virtual Reality. J. Neurophysiol. 2022, 127, 1159–1170. [Google Scholar] [CrossRef]
- Li, H.; Liao, Y. Application and Effectiveness of Adaptive AI in Elderly Healthcare. Psychogeriatrics 2024, 25, e13214. [Google Scholar] [CrossRef]
- Pitsik, E.; Grubov, V.; Zakharov, A. Age-Related Differences in Sensorimotor Responce during VR-Based Task Performance: Time-Frequency Analysis and Functional Connectivity. In Proceedings of the 2023 7th Scientific School Dynamics of Complex Networks and Their Applications (DCNA), Kaliningrad, Russia, 18–20 September 2023. [Google Scholar]
- Sapmaz, M.; Mujdeci, B. The Effect of Fear of Falling on Balance and Dual Task Performance in the Elderly. Exp. Gerontol. 2021, 147, 111250. [Google Scholar] [CrossRef]
- Nobari, H.; Rezaei, S.; Sheikh, M.; Fuentes-García, J.P.; Pérez-Gómez, J. Effect of Virtual Reality Exercises on the Cognitive Status and Dual Motor Task Performance of the Aging Population. Int. J. Environ. Res. Public Health 2021, 18, 8005. [Google Scholar] [CrossRef] [PubMed]
- Stanmore, E.; Stubbs, B.; Vancampfort, D.; de Bruin, E.D.; Firth, J. The Effect of Active Video Games on Cognitive Functioning in Clinical and Non-Clinical Populations: A Meta-Analysis of Randomized Controlled Trials. Neurosci. Biobehav. Rev. 2017, 78, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.G.; Stanmore, E.; Caamano, J.; Vences, K.; Gell, N.M. A Feasibility Study of Multi-Component Fall Prevention for Homebound Older Adults Facilitated by Lay Coaches and Using a Tablet-Based, Gamified Exercise Application. J. Appl. Gerontol. 2021, 40, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, C.; Michaleff, Z.A.; Fairhall, N.; Paul, S.S.; Tiedemann, A.; Whitney, J.; Cumming, R.G.; Herbert, R.D.; Close, J.C.T.; Lord, S.R. Exercise to Prevent Falls in Older Adults: An Updated Systematic Review and Meta-Analysis. Br. J. Sports Med. 2017, 51, 1750–1758. [Google Scholar] [CrossRef]
- Mascret, N.; Delbes, L.; Voron, A.; Temprado, J.J.; Montagne, G. Acceptance of a Virtual Reality Headset Designed for Fall Prevention in Older Adults: Questionnaire Study. J. Med. Internet Res. 2020, 22, e20691. [Google Scholar] [CrossRef]
- Wong, J.; Wong, K.L.Y.; Kan, W.; Wu, C.; Upreti, M.; Van, M.; Temirova, A.; Alfares, H.; Wen, K.; Sharma, V.; et al. The staff perspectives of facilitators and barriers to implementing virtual reality for people living with dementia in long-term care. Front. Dement. 2024, 3, 1462946. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.; Schlomann, A. The Use of Virtual and Augmented Reality by Older Adults: Potentials and Challenges. Front. Virtual Real. 2021, 2, 639718. [Google Scholar] [CrossRef]
- Xu, G.; Chen, J.; Yan, S.; Yin, H.; Lin, D.; Mei, Z.; Ding, Z.; Wang, M.; Bai, Y. Virtual reality technology improves the gait and balance function of the elderly: A meta-analysis of randomized controlled trials. Arch. Med. Sci. 2024, 20, 1918–1929. [Google Scholar] [CrossRef]

| PICOS | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | mean age of ≥60 years, community-dwelling, ≥1 fall within the past 12 months or a high fall risk score | Participants in severe clinical conditions |
| Intervention | VR-based training (immersive, non-immersive, exergaming, VR treadmill, telerehabilitation) | Conventional exercises without the use of VR |
| Comparison | Any exercise program: isolated balance training, strength or aerobic training, multicomponent programs, or no exercise (“usual care”/no exercise) | No control group |
| Outcomes | At least one of the following: fall incidents; TUG; gait speed; 6MWT; Berg or Y Balance; gait variability; adherence rate (%) | No quantitative measurements of function or falls |
| Study | Randomized controlled trials; intervention duration ≥ 6 weeks; ≥10 participants | Pilot studies, observational studies, case reports, qualitative studies, reviews |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasicki, K.; Klimek Piskorz, E.; Rydzik, Ł.; Ambroży, T.; Ceranowicz, P.; Belcarz Ciuraj, M.; Król, P.; Błach, W. Effectiveness of Virtual Reality-Based Training Versus Conventional Exercise Programs on Fall-Related Functional Outcomes in Older Adults with Various Health Conditions: A Systematic Review. J. Clin. Med. 2025, 14, 5550. https://doi.org/10.3390/jcm14155550
Kasicki K, Klimek Piskorz E, Rydzik Ł, Ambroży T, Ceranowicz P, Belcarz Ciuraj M, Król P, Błach W. Effectiveness of Virtual Reality-Based Training Versus Conventional Exercise Programs on Fall-Related Functional Outcomes in Older Adults with Various Health Conditions: A Systematic Review. Journal of Clinical Medicine. 2025; 14(15):5550. https://doi.org/10.3390/jcm14155550
Chicago/Turabian StyleKasicki, Krzysztof, Ewa Klimek Piskorz, Łukasz Rydzik, Tadeusz Ambroży, Piotr Ceranowicz, Maria Belcarz Ciuraj, Paweł Król, and Wiesław Błach. 2025. "Effectiveness of Virtual Reality-Based Training Versus Conventional Exercise Programs on Fall-Related Functional Outcomes in Older Adults with Various Health Conditions: A Systematic Review" Journal of Clinical Medicine 14, no. 15: 5550. https://doi.org/10.3390/jcm14155550
APA StyleKasicki, K., Klimek Piskorz, E., Rydzik, Ł., Ambroży, T., Ceranowicz, P., Belcarz Ciuraj, M., Król, P., & Błach, W. (2025). Effectiveness of Virtual Reality-Based Training Versus Conventional Exercise Programs on Fall-Related Functional Outcomes in Older Adults with Various Health Conditions: A Systematic Review. Journal of Clinical Medicine, 14(15), 5550. https://doi.org/10.3390/jcm14155550