Impact of Continuous Veno-Venous Hemodiafiltration on Thyroid Homeostasis in Critically Ill Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Protocol
- β31—clearance exponent for T3 (8 × 10−6 s−1);
- KM1—dissociation constant of type-1-deiodinase (5 × 10−7 mol/L);
- α31—dilution factor for T3 (0.026 L−1);
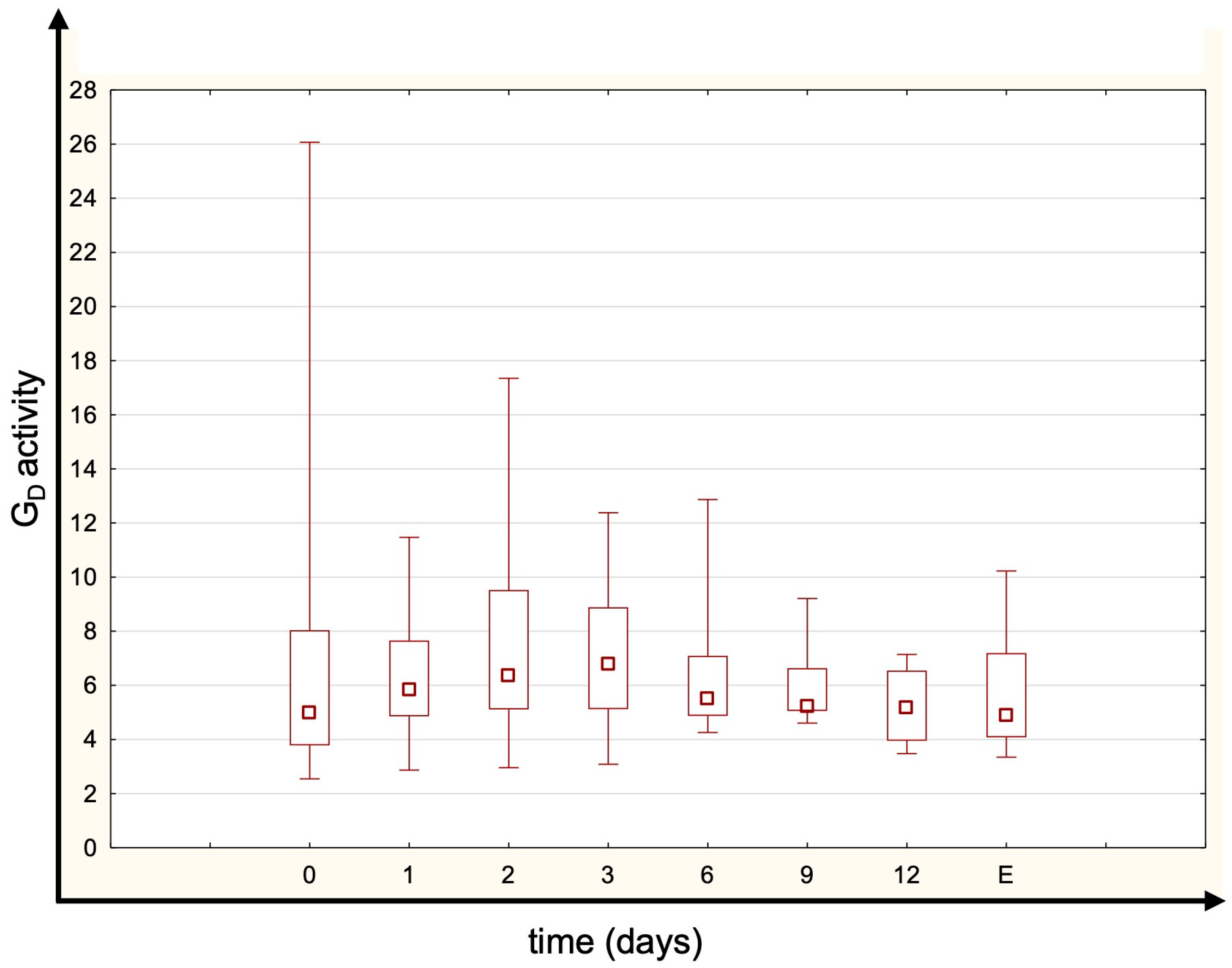
- GD—normal reference range is 20–60 nmoL s−1.
2.3. Statistical Analyses
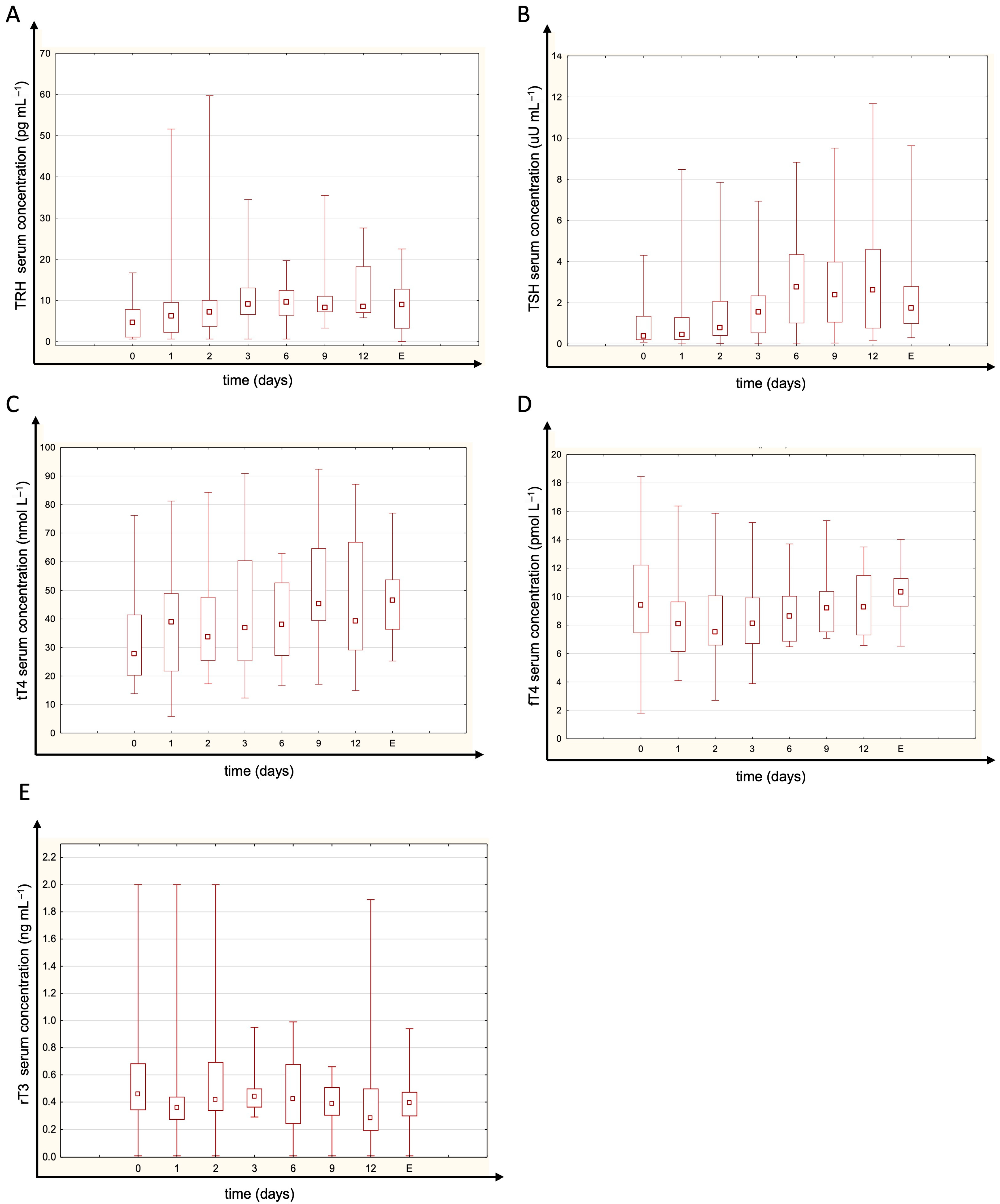
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Téblick, A.; Langouche, L.; Berghe, G.V.D. Anterior Pituitary Function in Critical Illness. Endocr. Connect. 2019, 8, R131–R143. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berghe, G. Non-Thyroidal Illness in the ICU: A Syndrome with Different Faces. Thyroid 2014, 24, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Fliers, E.; Bianco, A.C.; Langouche, L.; Boelen, A. Thyroid Function in Critically Ill Patients. Lancet Diabetes Endocrinol. 2015, 3, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Michalaki, M.; Vagenakis, A.G.; Makri, M.; Kalfarentzos, F.; Kyriazopoulou, V. Dissociation of the Early Decline in Serum T3 Concentration and Serum IL-6 Rise and TNFα in Nonthyroidal Illness Syndrome Induced by Abdominal Surgery. J. Clin. Endocrinol. Metab. 2001, 86, 4198–4205. [Google Scholar] [CrossRef]
- Arem, R.; Thornby, J.I.; Deppe, S.A. Comparison of Thyroid Hormone and Cortisol Measurements with APACHE II and TISS Scoring Systems as Predictors of Mortality in the Medical Intensive Care Unit. J. Intensive Care Med. 1997, 12, 12–17. [Google Scholar] [CrossRef]
- Gerdes, A.M.; Iervasi, G. Thyroid Replacement Therapy and Heart Failure. Circulation 2010, 122, 385–393. [Google Scholar] [CrossRef]
- Foks, M.; Dudek, A.; Polok, K.; Nowak-Kózka, I.; Fronczek, J.; Szczeklik, W. Thyroid Hormones as Potential Prognostic Factors in Sepsis. In Anaesthesiology Intensive Therapy; Termedia: Poznań, Poland, 2019; Volume 51, pp. 205–209. [Google Scholar]
- Boelen, A.; Kwakkel, J.; Fliers, E. Beyond Low Plasma T 3: Local Thyroid Hormone Metabolism during Inflammation and Infection. Endocr. Rev. 2011, 32, 670–693. [Google Scholar] [CrossRef]
- Pang, X.P.; Hershman, J.M.; Mirell, C.J.; Pekary, A.E. Impairment of Hypothalamic-Pituitary-Thyroid Function in Rats Treated with Human Recombinant Tumor Necrosis Factor-α (Cachectin). Endocrinology 1989, 125, 76–84. [Google Scholar] [CrossRef]
- Mebis, L.; Debaveye, Y.; Visser, T.J.; Van den Berghe, G. Changes Within the Thyroid Axis During the Course of Critical Illness. Endocrinol. Metab. Clin. N. Am. 2006, 35, 807–821. [Google Scholar] [CrossRef]
- Boelen, A.; Wiersinga, W.M.; Fliers, E. Fasting-Induced Changes in the Hypothalamus-Pituitary-Thyroid Axis. Thyroid 2008, 18, 123–129. [Google Scholar] [CrossRef]
- Pappa, T.A.; Vagenakis, A.G.; Alevizaki, M. The Nonthyroidal Illness Syndrome in the Non-Critically Ill Patient. Eur. J. Clin. Investig. 2011, 41, 212–220. [Google Scholar] [CrossRef]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P. Acute Renal Failure-Definition, Outcome Measures, Animal Models, Fluid Therapy and Information Technology Needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef] [PubMed]
- Singbartl, K.; Kellum, J.A. AKI in the ICU: Definition, Epidemiology, Risk Stratification, and Outcomes. Kidney Int. 2012, 81, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.G. Do Volatile Anaesthetics Depress Urine Output? Anaesthesiol. Intensive Ther. 2024, 56, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Pasieka, P.M.; Kurek, M.; Skupnik, W.; Skwara, E.; Bezshapkin, V.; Fronczek, J.; Kluzik, A.; Kudliński, B.; Białka, S.; Studzińska, D.; et al. Predictors of Outcomes of Patients ≥ 80 Years Old Admitted to Intensive Care Units in Poland—A Post-Hoc Analysis of the VIP2 Prospective Observational Study. Anaesthesiol. Intensive Ther. 2024, 56, 61–69. [Google Scholar] [CrossRef]
- Danel, J.K.; Taborek, M.; Nowotarska, A.; Winiarska, K.; Dylczyk-Sommer, A.; Szczeklik, W.; Białka, S.; Czarnik, T.; Sołek-Pastuszka, J.K.; Krzych, Ł.J. Nutritional Management in Critically Ill Patients with COVID-19: A Retrospective Multicentre Study. Anaesthesiol. Intensive Ther. 2024, 56, 70–76. [Google Scholar] [CrossRef]
- Bellomo, R.; Ronco, C. Continuous Haemofiltration in the Intensive Care Unit. Crit. Care 2000, 4, 339–345. [Google Scholar] [CrossRef]
- Iglesias, P.; Díez, J.J. Thyroid Dysfunction and Kidney Disease. Eur. J. Endocrinol. 2009, 160, 503–515. [Google Scholar] [CrossRef]
- Dietrich, J.W.; Landgrafe-Mende, G.; Wiora, E.; Chatzitomaris, A.; Klein, H.H.; Midgley, J.E.M.; Hoermann, R. Calculated Parameters of Thyroid Homeostasis: Emerging Tools for Differential Diagnosis and Clinical Research. Front. Endocrinol. 2016, 7, 57. [Google Scholar] [CrossRef]
- Bergstrand, M.; Karlsson, M.O. Handling Data below the Limit of Quantification in Mixed Effect Models. AAPS J. 2009, 11, 371–380. [Google Scholar] [CrossRef]
- Gao, W.; Guo, W.; Guo, Y.; Shi, M.; Dong, G.; Wang, G.; Ge, Q.; Zhu, J.; Zhou, X. Thyroid Hormone Concentrations in Severely or Critically Ill Patients with COVID-19. J. Endocrinol. Investig. 2021, 44, 1031–1040. [Google Scholar] [CrossRef]
- Rothberger, G.D.; Valestra, P.K.; Knight, K.; Desai, A.K.; Calixte, R.; Shapiro, L.E. Low Free T3 Is Associated With Worse Outcomes in Patients in the ICU Requiring Invasive Mechanical Ventilation. J. Intensive Care Med. 2021, 36, 313–318. [Google Scholar] [CrossRef]
- Peeters, R.P.; Wouters, P.J.; Kaptein, E.; Van Toor, H.; Visser, T.J.; Van Den Berghe, G. Reduced Activation and Increased Inactivation of Thyroid Hormone in Tissues of Critically Ill Patients. J. Clin. Endocrinol. Metab. 2003, 88, 3202–3211. [Google Scholar] [CrossRef]
- Burger, A.; Suter, P.; Nicod, P.; Vallotton, M.B.; Vagenakis, A.; Braverman, L. Reduced active thyroid hormone levels in acute illness. Lancet 1976, 307, 653–655. [Google Scholar] [CrossRef]
- Kwakkel, G.J. Understanding the Non-Thyroidal Illness Syndrome from In Vivo and In Vitro Studies. Ph.D. Thesis, University of Amsterdam, Amsterdam, The Netherlands, 2010. [Google Scholar]
- Maia, A.L.; Kim, B.W.; Huang, S.A.; Harney, J.W.; Larsen, P.R. Type 2 Iodothyronine Deiodinase Is the Major Source of Plasma T3 in Euthyroid Humans. J. Clin. Investig. 2005, 115, 2524–2533. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Pan, W.; Wang, H.; Wang, S.; Pan, S.; Ge, J. Relationship between Thyroid Function and ICU Mortality: A Prospective Observation Study. Crit. Care 2012, 16, R11. [Google Scholar] [CrossRef] [PubMed]
- Gutch, M.; Kumar, S.; Gupta, K. Prognostic Value of Thyroid Profile in Critical Care Condition. Indian J. Endocrinol. Metab. 2018, 22, 387. [Google Scholar] [CrossRef] [PubMed]
- Plikat, K.; Langgartner, J.; Buettner, R.; Bollheimer, L.C.; Woenckhaus, U.; Schölmerich, J.; Wrede, C.E. Frequency and Outcome of Patients with Nonthyroidal Illness Syndrome in a Medical Intensive Care Unit. Metabolism 2007, 56, 239–244. [Google Scholar] [CrossRef]
- Brent, G.A.; Hershman, J.M. Thyroxine Therapy in Patients with Severe Nonthyroidal Illnesses and Low Serum Thyroxine Concentration. J. Clin. Endocrinol. Metab. 1986, 63, 1–8. [Google Scholar] [CrossRef]
- Mullis-Jansson, S.L.; Argenziano, M.; Corwin, S.; Homma, S.; Weinberg, A.D.; Williams, M.; Rose, E.A.; Smith, C.R.; Wechsler, A.S.; Schaff, C.R. A Randomized Double-Blind Study of the Effect of Triiodothyronine on Cardiac Function and Morbidity after Coronary Bypass Surgery. J. Thorac. Cardiovasc. Surg. 1999, 117, 1128–1135. [Google Scholar] [CrossRef]
- Holndonner–Kirst, E.; Nagy, A.; Czobor, N.R.; Fazekas, L.; Dohan, O.; Kertai, M.D.; Lex, D.J.; Sax, B.; Hartyanszky, I.; Merkely, B.; et al. The Impact of L-Thyroxine Treatment of Donors and Recipients on Postoperative Outcomes After Heart Transplantation. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1629–1635. [Google Scholar] [CrossRef]
- Novitzky, D.; Fontanet, H.; Snyder, M.; Coblio, N.; Smith, D.; Parsonnet, V. Impact of Triiodothyronine on the Survival of High-Risk Patients Undergoing Open Heart Surgery. Cardiology 1996, 87, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Tandukar, S.; Palevsky, P.M. Continuous Renal Replacement Therapy: Who, When, Why, and How. Chest 2019, 155, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Nešković, N.; Drenjančević, D.; Kvolik, S.; Škiljić, S.; Budrovac, D.; Drenjančević, I.H. Predictive Role of Selected Biomarkers in Differentiating Gram-Positive from Gram-Negative Sepsis in Surgical Patients: A Retrospective Study. Anaesthesiol. Intensive Ther. 2023, 55, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Wang, K.; Lui, P.L.; Ramanathan, K.; Yang, S.P. Crash Landing of Thyroid Storm: A Case Report and Review of the Role of Extra-Corporeal Systems. Front. Endocrinol. 2021, 12, 1043. [Google Scholar] [CrossRef]
- Forest, J.C.; Dube, J.; Talbot, J. Thyroid Hormones in Patients with Chronic Renal Failure Undergoing Maintenance Hemodialysis. Am. J. Clin. Pathol. 1982, 77, 580–586. [Google Scholar] [CrossRef]
- Kerr, D.J.; Singh, V.K.; Tsakiris, D.; McConnell, K.N.; Junor, B.J.; Alexander, W.D. Serum and Peritoneal Dialysate Thyroid Hormone Levels in Patients on Continuous Ambulatory Peritoneal Dialysis. Nephron 1986, 43, 164–168. [Google Scholar] [CrossRef]
- Bashan, Z.; Guangming, L. The Clinical Significance of Dynamic Observations on the Changes of Serum Cortisol (COR), TNF-α and IL-6 Levels in Critical Patients on Continuous Renal Replacement Therapy (CRRT). J. Neuroimmunol. 2004, 17, 178–179. [Google Scholar]
- Boer, W.; Fivez, T.; Vander Laenen, M.; Bruckers, L.; Grön, H.J.; Schetz, M.; Oudemans-van Straaten, H. Citrate Dose for Continuous Hemofiltration: Effect on Calcium and Magnesium Balance, Parathormone and Vitamin D Status, a Randomized Controlled Trial. BMC Nephrol. 2021, 22, 409. [Google Scholar] [CrossRef]
- Bartalena, L.; Robbins, J. Variations in Thyroid Hormone Transport Proteins and Their Clinical Implications. Thyroid. Off. J. Am. Thyroid Assoc. 1992, 2, 237–245. [Google Scholar] [CrossRef]
- Böhler, J.; Donauer, J.; Keller, F. Pharmacokinetic Principles during Continuous Renal Replacement Therapy: Drugs and Dosage. Kidney Int. Suppl. 1999, 56, S24–S28. [Google Scholar] [CrossRef]
- Zhang, J.N.; Zhao, X. Le The Changes of Thyroid Function and Related Factors in Critical Patients without Thyroid Illness in ICU: A Retrospective Cross-Sectional Study. Ther. Clin. Risk Manag. 2022, 18, 571–578. [Google Scholar] [CrossRef]
- Czarnik, A.; Gawda, R.; Piwoda, M.; Marszalski, M.; Molsa, M.; Pietka, M.; Bolanowski, M.; Czarnik, T. Parathyroid Hormone Serum Concentration Kinetic Profile in Critically Ill Patients Undergoing Continuous Renal Replacement Therapies: A Prospective Observational Study. Endokrynol. Pol. 2021, 72, 329–335. [Google Scholar] [CrossRef]
| Study Group (N = 30) | |
|---|---|
| Female sex | N = 8 [27%] |
| Age, year | 67.1 (11.7) |
| SAPS II score | 53 (16.9) |
| APACHE II score | 27.1 (7.6) |
| PDR | 0.59 (0.2) |
| Days from ICU admission to CVVHDF implementation | 1 [1–3] |
| Length of CVVHDF duration | 8.5 [4–13.5] |
| Reason for ICU admission | Septic shock N = 11 [37%] |
| Acute respiratory failure N = 4 [13%] | |
| Trauma N = 3 [10%] | |
| Post-resuscitation care N = 3 [10%] | |
| Acute cardiac failure N = 2 [7%] | |
| Hematological malignancy N = 2 [7%] | |
| Hemorrhagic shock N = 1 [3%] | |
| Acute pancreatitis N = 1 [3%] | |
| Toxic epidermal necrolysis N = 1 [3%] | |
| Multiorgan failure N = 1 [3%] | |
| Pulmonary thromboembolism N = 1 [3%] | |
| Acute Kidney Injury cause | Septic shock N = 21 [70%] |
| Exacerbation of chronic kidney disease N = 3 [10%] | |
| Multiorgan failure N = 2 [7%] | |
| Rhabdomyolysis N = 2 [7%] | |
| Hemorrhagic shock N = 1 [3%] | |
| Unknown N = 1 [3%] | |
| Reason of CVVHDF discontinuation | Recovery of renal function N = 16 [53%] |
| Death N = 12 [40%] | |
| Withdrawal of care due to futility N = 2 [7%] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipczyk, A.; Wujtewicz, M.A.; Okrągły, M.; Steckiewicz, K.P. Impact of Continuous Veno-Venous Hemodiafiltration on Thyroid Homeostasis in Critically Ill Patients. J. Clin. Med. 2025, 14, 5542. https://doi.org/10.3390/jcm14155542
Filipczyk A, Wujtewicz MA, Okrągły M, Steckiewicz KP. Impact of Continuous Veno-Venous Hemodiafiltration on Thyroid Homeostasis in Critically Ill Patients. Journal of Clinical Medicine. 2025; 14(15):5542. https://doi.org/10.3390/jcm14155542
Chicago/Turabian StyleFilipczyk, Alicja, Magdalena A. Wujtewicz, Michał Okrągły, and Karol P. Steckiewicz. 2025. "Impact of Continuous Veno-Venous Hemodiafiltration on Thyroid Homeostasis in Critically Ill Patients" Journal of Clinical Medicine 14, no. 15: 5542. https://doi.org/10.3390/jcm14155542
APA StyleFilipczyk, A., Wujtewicz, M. A., Okrągły, M., & Steckiewicz, K. P. (2025). Impact of Continuous Veno-Venous Hemodiafiltration on Thyroid Homeostasis in Critically Ill Patients. Journal of Clinical Medicine, 14(15), 5542. https://doi.org/10.3390/jcm14155542





