Pancreatic Cancer with Liver Oligometastases—Different Patterns of Disease Progression May Suggest Benefits of Surgical Resection
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDAC | pancreatic adenocarcinoma |
| LOM | liver oligometastases |
| MDT | multidisciplinary team |
| PLR | platelet to lymphocyte ration |
| NLR | neutrophil to lymphocyte ratio |
| OS | overall survival |
| BMI | body mass index |
| HTN | hypertension |
| CVA | cerebrovascular accident |
| CEA | carcinoembryonic antigen |
| WBC | white blood cell |
| SHR | subdistribution hazard ratios |
| HR | hazard ratio |
References
- Strobel, O.; Lorenz, P.; Hinz, U.M.; Gaida, M.; König, A.-K.; Hank, T.; Niesen, W.; Kaiser, J.; Al-Saeedi, M.; Bergmann, F.; et al. Actual Five-year Survival after Upfront Resection for Pancreatic Ductal Adenocarcinoma: Who Beats the Odds? Ann. Surg. 2022, 275, 962–971. [Google Scholar] [CrossRef]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer. A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef]
- Heestand, G.M.; Murphy, J.D.; Lowy, A.M. Approach to patients with pancreatic cancer without detectable metastases. J. Clin. Oncol. 2015, 33, 1770–1778. [Google Scholar] [CrossRef]
- Boggi, U.; Kauffmann, E.F.; Napoli, N.; Barreto, S.G.; Besselink, M.G.; Fusai, G.K.; Hackert, T.; Hilal, M.A.; Marchegiani, G.; Salvia, R.; et al. REDISCOVER guidelines for borderline-resectable and locally advanced pancreatic cancer: Management algorithm, unanswered questions, and future perspectives. Updates Surg. 2024, 76, 1573–1591. [Google Scholar] [CrossRef]
- Isaji, S.; Mizuno, S.; Windsor, J.A.; Bassi, C.; Castillo, C.F.-D.; Hackert, T.; Hayasaki, A.; Katz, M.H.; Kim, S.-W.; Kishiwada, M.; et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018, 18, 2–11. [Google Scholar] [CrossRef]
- Conroy, T.; Bachet, J.-B.; Ayav, A.; Huguet, F.; Lambert, A.; Caramella, C.; Maréchal, R.; Van Laethem, J.-L.; Ducreux, M. Current standards and new innovative approaches for treatment of pancreatic cancer. Eur. J. Cancer 2016, 57, 10–22. [Google Scholar] [CrossRef]
- Hamad, A.; Underhill, J.; Ansari, A.; Thayaparan, V.; Cloyd, J.M.; Li, Y.; Pawlik, T.M.; Tsung, A.; Abushahin, L.; Ejaz, A. Surgical treatment of hepatic oligometastatic pancreatic ductal adenocarcinoma: An analysis of the National Cancer Database. Surgery 2022, 171, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzoli, S. Currently Debated Topics on Surgical Treatment of Pancreatic Ductal Adenocarcinoma: A Narrative Review on Surgical Treatment of Borderline Resectable, Locally Advanced, and Synchronous or Metachronous Oligometastatic Tumor. J. Clin. Med. 2023, 12, 6461. [Google Scholar] [CrossRef] [PubMed]
- Kaslow, S.R.; Sacks, G.D.; Berman, R.S.; Lee, A.Y.; Correa-Gallego, C. Natural History of Stage IV Pancreatic Cancer. Identifying Survival Benchmarks for Curative-intent Resection in Patients with Synchronous Liver-only Metastases. Ann. Surg. 2023, 278, e798–e804. [Google Scholar] [CrossRef] [PubMed]
- Clements, N.; Gaskins, J.; Martin, R.C.G. Surgical Outcomes in Stage IV Pancreatic Cancer with Liver Metastasis Current Evidence and Future Directions: A Systematic Review and Meta-Analysis of Surgical Resection. Cancers 2025, 17, 688. [Google Scholar] [CrossRef]
- Jacobs, C.S.; Vitello, D.J.; Chawla, A. Surgical Palliation for Advanced Pancreas Cancer. Surg. Clin. N. Am. 2024, 104, 1121–1135. [Google Scholar] [CrossRef]
- Hashimoto, D.; Satoi, S.; Fujii, T.; Sho, M.; He, J.; Hackert, T.; Del Chiaro, M.; Jang, J.-Y.; Gulla, A.; Yoon, Y.-S.; et al. Is surgical resection justified for pancreatic ductal adenocarcinoma with distant abdominal organ metastasis? A position paper by experts in pancreatic surgery at the Joint Meeting of the International Association of Pancreatology (IAP) & the Japan Pancreas Society (JPS) 2022 in Kyoto. Pancreatology 2023, 23, 682–688. [Google Scholar] [CrossRef]
- Kong, Q.; Teng, F.; Li, H.; Chen, Z. Radical resection benefits patients suffering pancreatic ductal adenocarcinoma with liver oligometastases. Ann. Surg. Treat. Res. 2024, 106, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Hackert, T.; Niesen, W.; Hinz, U.; Tjaden, C.; Strobel, O.; Ulrich, A.; Michalski, C.; Büchler, M. Radical surgery of oligometastatic pancreatic cancer. Eur. J. Surg. Oncol. 2017, 43, 358–363. [Google Scholar] [CrossRef]
- Leonhardt, C.S.; Stamm, T.; Hank, T.; Prager, G.; Strobel, O. Defining oligometastatic pancreatic cancer: A systematic review and critical synthesis of consensus. ESMO Open 2023, 8, 102067. [Google Scholar] [CrossRef] [PubMed]
- Damanakis, A.I.; Ostertag, L.; Waldschmidt, D.; Kütting, F.; Quaas, A.; Plum, P.; Bruns, C.J.; Gebauer, F.; Popp, F. Proposal for a definition of “Oligometastatic disease in pancreatic cancer”. BMC Cancer 2019, 19, 1261. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Ban, D.; Mizui, T.; Takamoto, T.; Nara, S.; Esaki, M.; Shimada, K. Oligo-like liver metastasis: A novel prognostic indicator to improve survival in pancreatic cancer. Ann. Gastroenterol. Surg. 2024, 8, 481–489. [Google Scholar] [CrossRef]
- Koti, S.; Demyan, L.; Deutsch, G.; Weiss, M. Surgery for Oligometastatic Pancreatic Cancer: Defining Biologic Resectability. Ann. Surg. Oncol. 2024, 31, 4031–4041. [Google Scholar] [CrossRef]
- Cardillo, N.; Seible, D.M.; Fero, K.E.; Bruggeman, A.R.; Sarkar, R.R.; Azuara, A.; Simpson, D.R.; Murphy, J.D. Clinical Impact of Local Progression in Pancreatic Cancer. J. Natl. Compr. Cancer Netw. 2018, 16, 711–717. [Google Scholar] [CrossRef]
- Pencovich, N.; Nachmany, I. Should we resect distant metastases?—Reconsidering radical resection of pancreatic cancer with liver metastases. HepatoBiliary Surg. Nutr. 2024, 13, 905–907. [Google Scholar] [CrossRef]
- Fine, J.P.; Gray, R.J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT 14.3 User’s Guide: High-Performance Procedures; SAS Institute Inc: Cary, NC, USA, 2017. [Google Scholar]
- De Dosso, S.; Siebenhüner, A.R.; Winder, T.; Meisel, A.; Fritsch, R.; Astaras, C.; Szturz, P.; Borner, M. Treatment landscape of metastatic pancreatic cancer. Cancer Treat. Rev. 2021, 96, 102180. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Sasaki, T.; Okamoto, T.; Kasuga, A.; Matsuyama, M.; Ozaka, M.; Inoue, Y.; Takahashi, Y.; Saiura, A.; Sasahira, N. Outcomes of pancreatic cancer with liver oligometastasis. J. Hepato-Biliary-Pancreat. Sci. 2023, 30, 229–239. [Google Scholar] [CrossRef]
- Pugalenthi, A.; Protic, M.; Gonen, M.; Kingham, T.P.; Angelica, M.I.; Dematteo, R.P.; Fong, Y.; Jarnagin, W.R.; Allen, P.J. Postoperative complications and overall survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J. Surg. Oncol. 2016, 113, 188–193. [Google Scholar] [CrossRef]
- Ausania, F.; Vallance, A.E.; Manas, D.M.; Prentis, J.M.; Snowden, C.P.; White, S.A.; Charnley, R.M.; French, J.J.; Jaques, B.C. Double bypass for inoperable pancreatic malignancy at laparotomy: Postoperative complications and long-term outcome. Ann. R. Coll. Surg. Engl. 2012, 94, 563–568. [Google Scholar] [CrossRef]
- Azari, F.S.; Vollmer, C.M.; Roses, R.E.; Keele, L.; DeMatteo, R.P.; Drebin, J.A.; Lee, M.K. A contemporary analysis of palliative procedures in aborted pancreatoduodenectomy: Morbidity, mortality, and impact on future therapy. Surgery 2020, 168, 1026–1031. [Google Scholar] [CrossRef]
- Bosetti, C.; Lucenteforte, E.; Silverman, D.T.; Petersen, G.; Bracci, P.M.; Ji, B.T.; Negri, E.; Li, D.; Risch, H.A.; Olson, S.H.; et al. Cigarette smoking and pancreatic cancer: An analysis from the International Pancreatic Cancer Case-Control Consortium (PANC4). Ann. Oncol. 2012, 23, 1880–1888. [Google Scholar] [CrossRef]
- Edderkaoui, M.; Xu, S.; Chheda, C.; Morvaridi, S.; Hu, R.W.; Grippo, P.J.; Mascariñas, E.; Principe, D.R.; Knudsen, B.; Xue, J.; et al. HDAC3 mediates smoking-induced pancreatic cancer. Oncotarget 2016, 7, 7747–7760. [Google Scholar] [CrossRef]
- Momi, N.; Ponnusamy, M.P.; Kaur, S.; Rachagani, S.; Kunigal, S.S.; Chellappan, S.; Ouellette, M.M.; Batra, S.K. Nicotine/cigarette smoke promotes metastasis of pancreatic cancer through α7nAChR-mediated MUC4 upregulation. Oncogene 2013, 32, 1384–1395. [Google Scholar] [CrossRef]
- Yang, J.; Chheda, C.; Lim, A.B.; Hauptschein, D.B.; Zayou, L.; Tang, J.B.; Pandol, S.J.; Edderkaoui, M. HDAC4 Mediates Smoking-Induced Pancreatic Cancer Metastasis. Pancreas 2022, 51, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Kruger, D.; Lahoud, N.; Yako, Y.Y.; Devar, J.; Smith, M.; Kestler, H.A. Pancreatic ductal adenocarcinoma: Prognostic indicators of advanced disease. PLoS ONE 2022, 17, e0262439. [Google Scholar] [CrossRef] [PubMed]
- Rémond, M.; Smolenschi, C.; Tarabay, A.; Gelli, M.; Fernandez-De-Sevilla, E.; Mouawia, A.; Cosconea, S.; Tselikas, L.; Barbe, R.; Fuerea, A.; et al. Clinical and molecular features of early onset pancreatic adenocarcinoma. Int. J. Cancer 2024, 155, 1969–1981. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, H.; Yamaue, H.; Unno, M.; Mizuma, M.; Hamada, S.; Igarashi, H.; Kuroki, T.; Satoi, S.M.; Shimizu, Y.; Tani, M.; et al. Clinicopathological Characteristics of Young Patients with Pancreatic Cancer. Pancreas 2016, 45, 1411–1417. [Google Scholar] [CrossRef]
- Worapongpaiboon, R.; Siranart, N.; Pajareya, P.; Phutinart, S. Inflammatory markers in predicting survival in pancreatic cancer: A Systematic review and Meta-Analysis. Pancreatology 2025, 25, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Prognostic Factors After Pancreatectomy for Pancreatic Cancer Initially Metastatic to the Liver|Annals of Surgical Oncology. Available online: https://link.springer.com/article/10.1245/s10434-022-12385-4 (accessed on 19 July 2025).
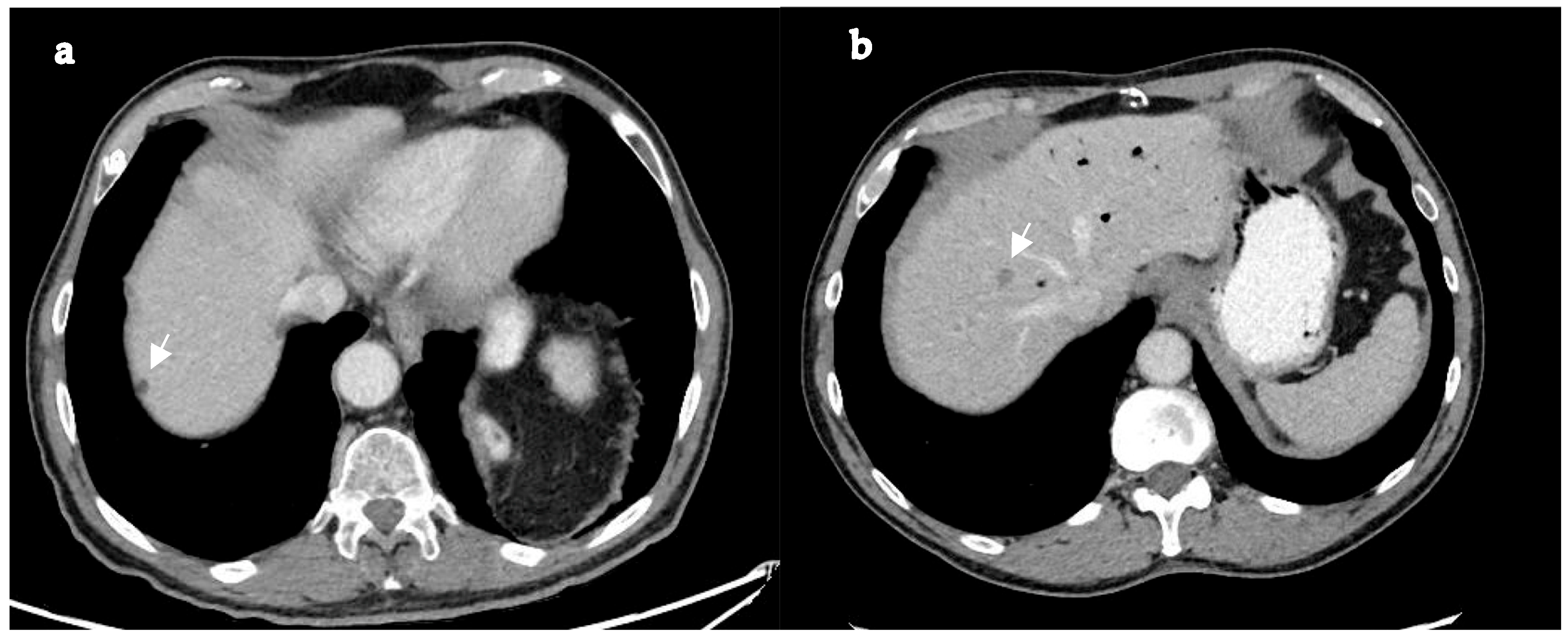

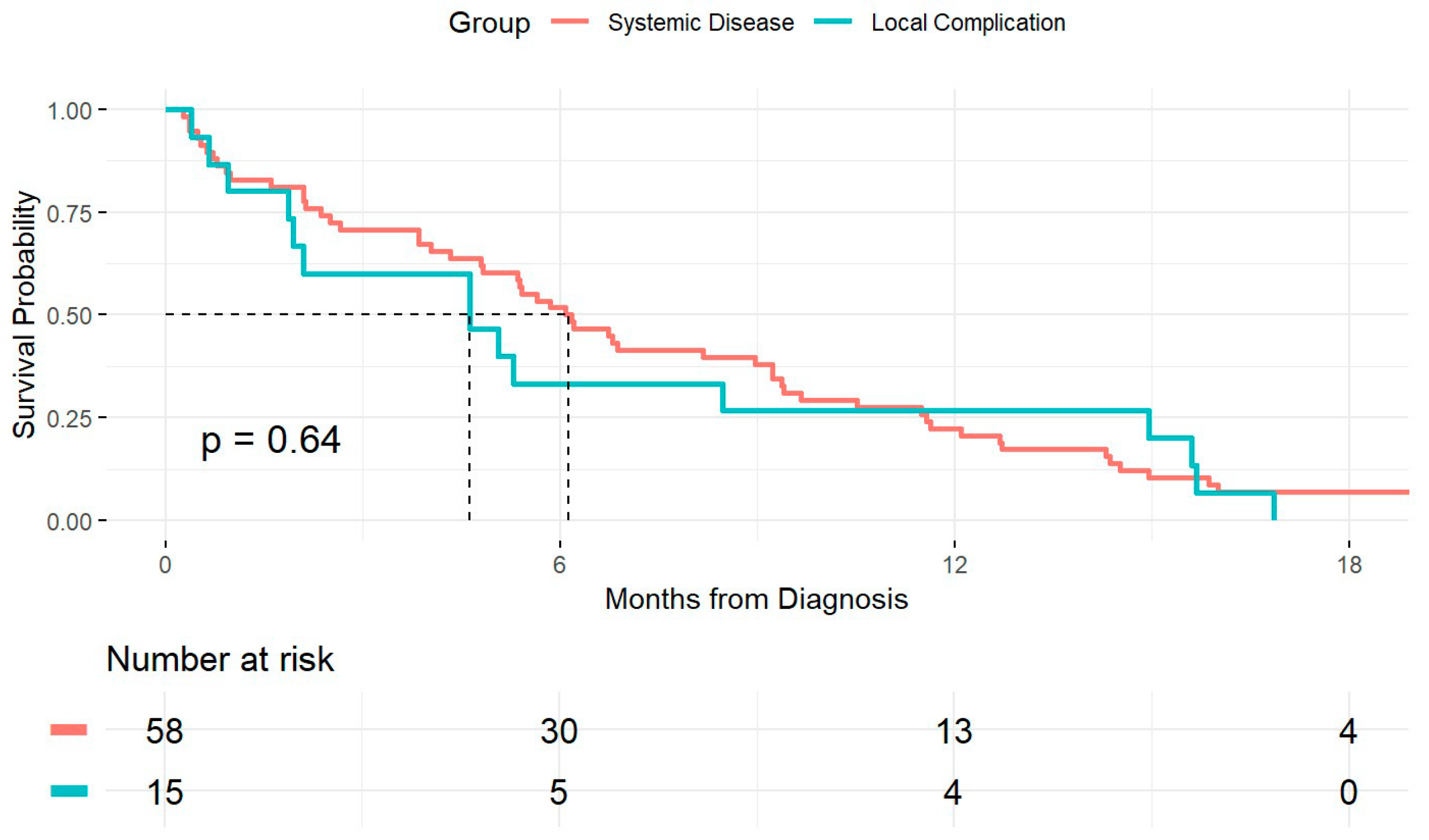
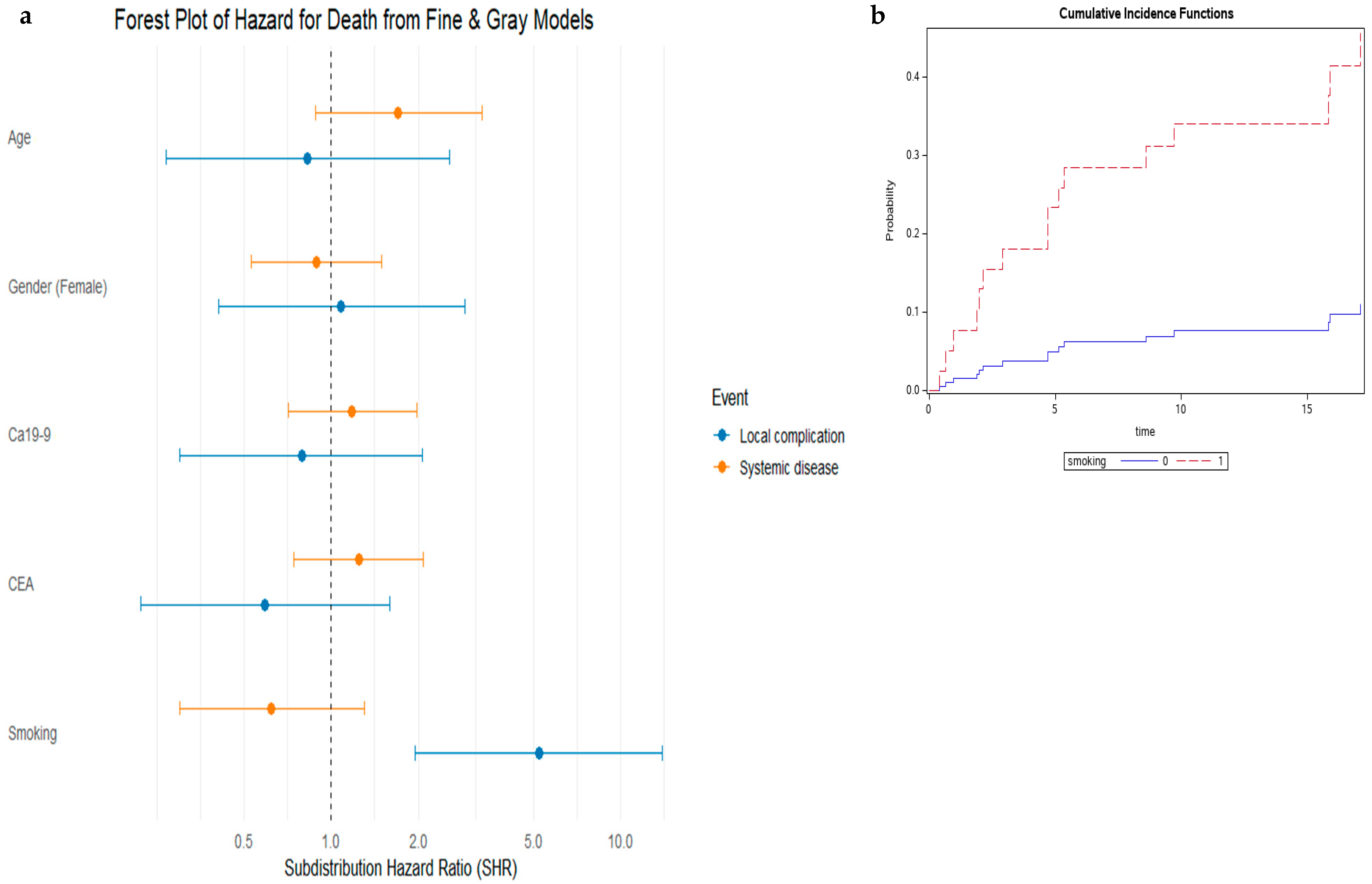
| Characteristic 1 | Total PDAC-LOM Patients with Known Cause of Death n = 74 | Death from Local Complications n = 16 | Death from Systemic Disease n = 58 | p-Value 2 |
|---|---|---|---|---|
| Age at diagnosis | 73 (10.7) | 77.5 (23) | 72 (8) | 0.02 |
| Female gender | 30 (39%) | 7 (44%) | 23 (40%) | 0.99 |
| BMI (kg/m2) | 24 (4) | 24 (3.5) | 24 | 0.69 |
| Smoking history | 14 (18%) | 7 (43%) | 7 (12%) | 0.01 |
| Diabetes | 23 (29%) | 5 (31%) | 18 (31%) | >0.9 |
| HTN | 36 (46%) | 9 (56%) | 27 (46%) | 0.68 |
| CVA | 4 (5%) | 1 (6%) | 3 (5%) | >0.9 |
| CA19-9 | 788 (4528) | 392 (2204.75) | 825 (6777) | 0.79 |
| CEA | 5 (14.1) | 3.35 (4.5) | 6.5 (17.8) | 0.16 |
| Bilirubin (mg/dL) | 0.6 (2.5) | 1 (5.8) | 0.6 (1.98) | 0.34 |
| Albumin (g/dL) | 4 (0.3) | 3.9 (1) | 4 (0.3) | 0.25 |
| Creatinine (mg/dL) | 0.7 (0.3) | 0.75 (0.34) | 0.7 (0.2) | 0.28 |
| WBC (K/microL) | 7 (3) | 5.5 (3.25) | 7 (3) | <0.01 |
| PLR (K/microL) | 130.56 (108.5) | 115 (76) | 141.4 (102) | 0.37 |
| NLR (K/microL) | 2.75 (1.8) | 3.12 (1.58) | 2.64 (1.84) | 0.9 |
| Characteristic | Total 1 |
|---|---|
| No progression of metastases | 6 (7%) |
| Progression of liver disease and new disseminated metastases 2 | 41 (47%) |
| • Progression of known LOM + new non-liver metastases | 13 (32%) |
| • New liver lesions + new non-liver metastases | 28 (68%) |
| New non-liver lesions only | 5 (6%) |
| Progression of liver disease only | 35 (40%) |
| • Growing of the original LOM + new lesions | 22 (63%) |
| • Growing of the original LOM only | 7 (20%) |
| • New liver lesions only | 6 (17%) |
| Variable | Multivariable Analysis— Local Complication | Multivariable Analysis— Systemic Disease | ||
|---|---|---|---|---|
| Hazard Ratio 95% CI | p-Value | Hazard Ratio 95% CI | p-Value | |
| Age | 0.89 (0.31–2.56) | 0.83 | 1.68 (0.84–3.39) | 0.14 |
| Female gender | 1.24 (0.46–3.30) | 0.67 | 0.81 (0.46–1.44) | 0.47 |
| Smoking history | 5.08 (1.93–13.39) | 0.001 | 0.62 (0.30–1.29) | 0.21 |
| CA19-9 | 1.06 (0.31–3.66) | 0.92 | 1.05 (0.56–1.97) | 0.88 |
| CEA | 0.62 (0.18–2.14) | 0.45 | 1.09 (0.58–2.04) | 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahamid, N.; Jacover, A.; Zabeda, A.; Beller, T.; Murad, H.; Elizur, Y.; Pery, R.; Eshkenazy, R.; Golan, T.; Nachmany, I.; et al. Pancreatic Cancer with Liver Oligometastases—Different Patterns of Disease Progression May Suggest Benefits of Surgical Resection. J. Clin. Med. 2025, 14, 5538. https://doi.org/10.3390/jcm14155538
Mahamid N, Jacover A, Zabeda A, Beller T, Murad H, Elizur Y, Pery R, Eshkenazy R, Golan T, Nachmany I, et al. Pancreatic Cancer with Liver Oligometastases—Different Patterns of Disease Progression May Suggest Benefits of Surgical Resection. Journal of Clinical Medicine. 2025; 14(15):5538. https://doi.org/10.3390/jcm14155538
Chicago/Turabian StyleMahamid, Nedaa, Arielle Jacover, Angam Zabeda, Tamar Beller, Havi Murad, Yoav Elizur, Ron Pery, Rony Eshkenazy, Talia Golan, Ido Nachmany, and et al. 2025. "Pancreatic Cancer with Liver Oligometastases—Different Patterns of Disease Progression May Suggest Benefits of Surgical Resection" Journal of Clinical Medicine 14, no. 15: 5538. https://doi.org/10.3390/jcm14155538
APA StyleMahamid, N., Jacover, A., Zabeda, A., Beller, T., Murad, H., Elizur, Y., Pery, R., Eshkenazy, R., Golan, T., Nachmany, I., & Pencovich, N. (2025). Pancreatic Cancer with Liver Oligometastases—Different Patterns of Disease Progression May Suggest Benefits of Surgical Resection. Journal of Clinical Medicine, 14(15), 5538. https://doi.org/10.3390/jcm14155538






