Pediatric Septoplasty: Benefits, Challenges, and Clinical Recommendations—Comprehensive Review of Young ESPO
Abstract
1. Introduction
2. Background and Methods
3. Results
3.1. Population Size, Age, and Sex Distribution
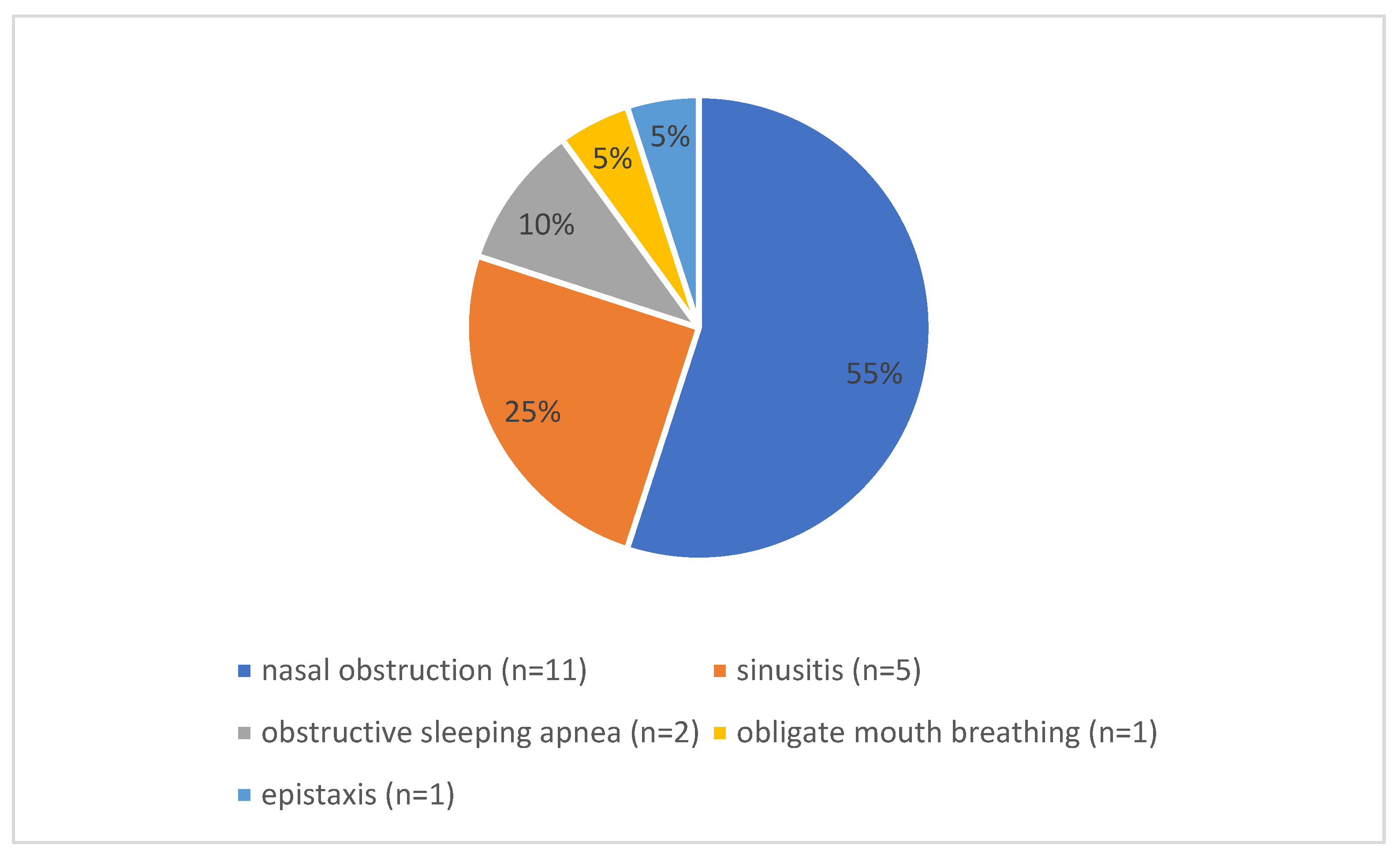
3.2. Preoperative Symptoms and Diagnostics
3.3. Septoplasty Techniques, Adjunct Procedures, and Postoperative Care
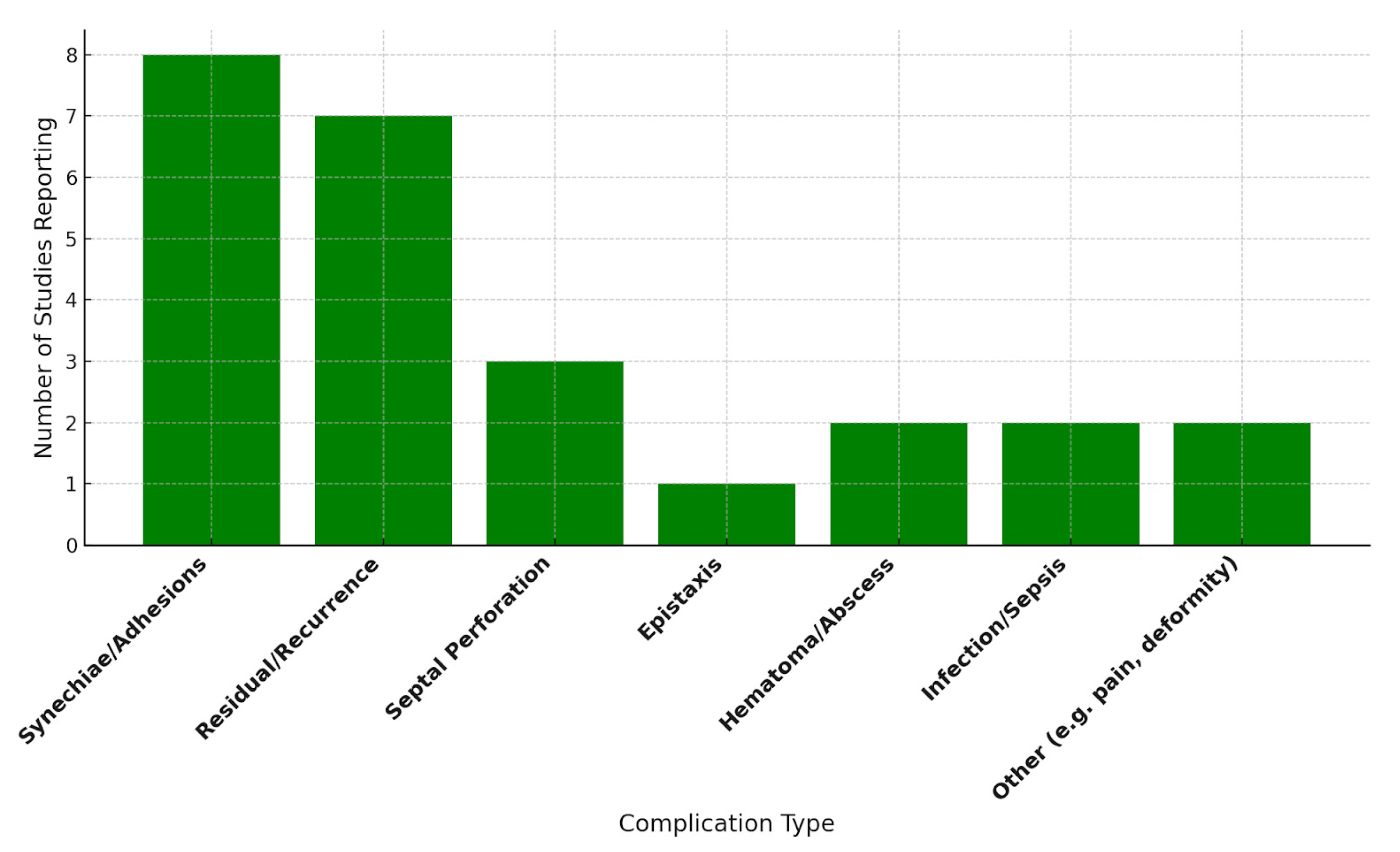
3.4. Complications
3.5. Craniofacial Growth and Development
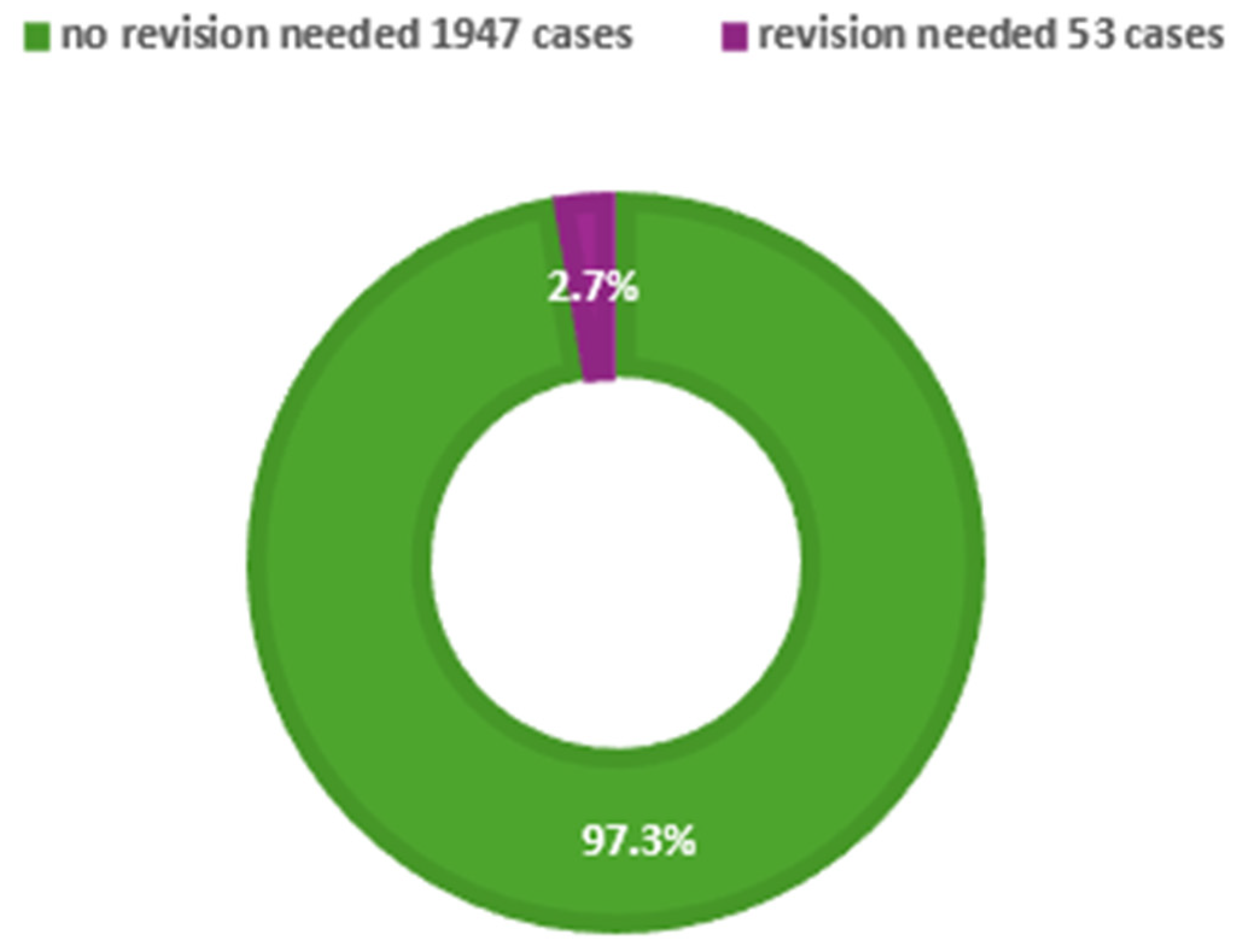
3.6. Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ESPO | European Society of Pediatric Otorhinolaryngology |
| NOSE | Nasal Obstruction Symptom Evaluation |
References
- Most, S.P.; Rudy, S.F. Septoplasty: Basic and advanced techniques. Facial Plast. Surg. Clin. N. Am. 2017, 25, 161–169. [Google Scholar] [CrossRef]
- Hinderer, K.H. Fundamentals of Anatomy and Surgery of the Nose; Aesculapius Pub. Co.: Birmingham, AL, USA, 1971. [Google Scholar]
- Adams, W. On the treatment of broken-nose by forcible straightening and mechanical retentive apparatus. Br. Med. J. 1875, 2, 421. [Google Scholar] [CrossRef]
- Bosworth, F.H. Deformities of the nasal septum. Med. Rec. 1887, 31, 115. [Google Scholar]
- Ingals, E.F. Deflection of the septum narium. Chic. Med. J. Exam. 1882, 45, 627–635. [Google Scholar] [PubMed] [PubMed Central]
- Jarvis, W.C. Cocaine in intra-nasal surgery. Reed. Rec. 1884, 3, 654–656. [Google Scholar]
- Freer, O.T. The correction of deflections of the nasal septum with a minimum of traumatism. JAMA 1902, 38, 636–642. [Google Scholar] [CrossRef]
- Killian, G. Die submukose Fensterresektion der Nasenscheidewand. Arch. Laryngol. 1904, 16, 203. [Google Scholar]
- Metzenbaum, M. Replacement of the lower end of the dislocated septal cartilage versus submucous resection of the dislocated end of the septal cartilage. Arch. Otolaryngol. 1929, 9, 282–296. [Google Scholar] [CrossRef]
- Peer, L.A. An operation to repair lateral displacement of the lower border of the septal cartilage. Arch. Otolaryngol. 1937, 25, 475–477. [Google Scholar] [CrossRef]
- Cottle, M.H.; Loring, R.M. Surgery of the nasal septum—New operative procedures and indications. Ann. Otol. Rhinol. Laryngol. 1948, 57, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.P. Deviated nasal septum incidence and etiology. Ann. Otol. Rhinol. Laryngol. 1978, 87 (Suppl. S2), 3–20. [Google Scholar] [CrossRef]
- Moh, W.; Graham, J.M., Jr.; Wadhawan, I.; Sanchez-Lara, P.A. Extrinsic factors influencing fetal deformations and intrauterine growth restriction. J. Pregnancy 2012, 2012, 750485. [Google Scholar] [CrossRef]
- Pirsig, W. Growth of the deviated septum and its influence on midfacial development. Facial Plast. Surg. 1992, 8, 224–232. [Google Scholar] [CrossRef]
- Morris, L. Rhinoplasty in Kids: Why, How, and When. Curr. Otorhinolaryngol. Rep. 2022, 10, 155–161. [Google Scholar] [CrossRef]
- Howard, T.; Williams, I.; Navaratnam, A.; Haloob, N.; Stoenchev, K.; Saleh, H. Should Pediatric Septal Surgery and Septorhino-plasty Be Performed for Nasal Obstruction?—A Systematic Review of the Literature. Facial Plast. Surg. 2024, 40, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Henríquez, C.; Neves, J.C.; Arancibia-Tagle, D.; Chiesa-Estomba, C.; Lechien, J.R.; Mayo-Yáñez, M.; Martinez-Capoccioni, G.; Martin-Martin, C. Does pediatric septoplasty compromise midfacial growth? A systematic review. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Rabie, A.N.; El Begermy, M.M.; El Shalma, A.A.A.; Fadel, M. Pediatric septoplasty impact on nasal breathing and when to consid-er a revision surgery-A meta-analysis study. Egypt. J. Otolaryngol. 2024, 40, 133. [Google Scholar] [CrossRef]
- Rose, A.S.; Makary, C.A.; Soler, Z.M.; Kimple, A.J.; Pearlman, A.N.; Ramaswamy, U.S.; Setzen, M.; Gudis, D.A. American Rhinologic Society Expert Practice Statement: Indications and Recommen-dations for Septoplasty in Children. Int. Forum Allergy Rhinol. 2024, 14, 1363–1374. [Google Scholar] [CrossRef]
- Zhao, Z.; Zheng, L.; Huang, X.; Li, C.; Liu, J.; Hu, Y. Effects of Mouth Breathing on Facial Skeletal Development in Children: A System-Atic Review and Meta-Analysis. BMC Oral. Health 2021, 21, 108. [Google Scholar] [CrossRef]
- Alshehri, A.A. Evaluating the Efficacy and Safety of Batten Grafts in Pediatric Caudal Septoplasty: A Prospective Observational Study. Aesthetic Plast. Surg. 2025. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Choudhary, A.; DasBiswas, K.A. Study on Septoplasty and Functional Septorhinoplasty in Children. Indian J. Otolaryngol. Head Neck Surg. 2024, 76, 5647–5651. [Google Scholar] [CrossRef]
- Benyo, S.; Moroco, A.E.; Saadi, R.A.; Patel, V.A.; King, T.S.; Wilson, M.N. Postoperative Outcomes in Pediatric Septoplasty. Ann. Otol. Rhinol. Laryngol. 2023, 132, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, T.M.; Badie, S.; Ibrahim, A.A.; Eldib, D.B.; Abdelsamie, A. Endoscopic septoplasty below the age of 17 years. Egypt. J. Otolaryngol. 2023, 39, 163. [Google Scholar] [CrossRef]
- Yaseen, N.K. Outcomes of septoplasty in children. Pol. Merkur. Lek. 2023, 51, 140–143. [Google Scholar] [CrossRef]
- Shah, J.P.; Youn, G.M.; Wei, E.X.; Kandathil, C.; Most, S.P. Septoplasty Revision Rates in Pediatric vs Adult Populations. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 1044–1050. [Google Scholar] [CrossRef]
- Raghavan, M.; Carr, M. Age and indication for pediatric septoplasty in the NSQIP-P database. Int. J. Pediatr. Otorhinolaryngol. 2022, 154, 111046. [Google Scholar] [CrossRef]
- Ori, M.; Ricci, G.; Capalbo, M.; Maranzano, M.; Sarno, A.; di Stadio, A.; D’Ascanio, L. Quick septoplasty in children: Long-term effects on nasal breathing and dentofacial morphology. A prospective cephalometric study. Auris Nasus Larynx 2021, 48, 914–921. [Google Scholar] [CrossRef]
- Bishop, R.; Sethia, R.; Allen, D.; Elmaraghy, C.A. Pediatric nasal septoplasty outcomes. Transl. Pediatr. 2021, 10, 2883–2887. [Google Scholar] [CrossRef] [PubMed]
- Koirala, K.P. Septoplasty in Pediatric Population: A Ten Years’ Experience. Birat J. Health Sci. 2020, 5, 1040–1044. [Google Scholar] [CrossRef]
- Manteghi, A.; Din, H.; Bundogji, N.; Leuin, S.C. Pediatric septoplasty and functional septorhinoplasty: A quality of life outcome study. Int. J. Pediatr. Otorhinolaryngol. 2018, 111, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Fuller, J.C.; Levesque, P.A.; Lindsay, R.W. Functional septorhinoplasty in the pediatric and adolescent patient. Int. J. Pediatr. Otorhinolaryngol. 2018, 111, 97–102. [Google Scholar] [CrossRef]
- Sabry, O.; Dewidar, H.; Aziz Mabdel Elemam, A.; Nassar, A. Efficiency of modified Goldman’s technique in open pediatric septoplasty. Egypt. J. Otolaryngol. 2021, 37, 16. [Google Scholar] [CrossRef]
- Lee, V.S.; Gold, R.M.; Parikh, S.R. Short-term quality of life outcomes following pediatric septoplasty. Acta Oto-Laryngol. 2017, 137, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Ritchie, K.; Chorney, J.M.; Bezuhly, M.; Hong, P. The impact of septoplasty on health-related quality of life in paediatric patients. Clin. Otolaryngol. 2016, 41, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Matin, M.A.; Islam, N.; Ali, R.; Azad, A.K. Septoplasty in children: Results of 250 cases. Bangladesh J. Otorhinolaryngol. 2015, 21, 110–114. [Google Scholar] [CrossRef]
- Maniglia, C.P.; Maniglia, J.V. Rinosseptoplastia em crianças. Braz. J. Otorhinolaryngol. 2017, 83, 416–419. [Google Scholar] [CrossRef]
- Yilmaz, M.S.; Guven, M.; Akidil, O.; Kayabasoglu, G.; Demir, D.; Mermer, H. Does septoplasty improve the quality of life in children? Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1274–1276. [Google Scholar] [CrossRef]
- Dąbrowska-Bień, J.; Skarżyński, P.H.; Gwizdalska, I.; Łazęcka, K.; Skarżyński, H. Complications in septoplasty based on a large group of 5639 patients. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 1789–1794. [Google Scholar] [CrossRef]
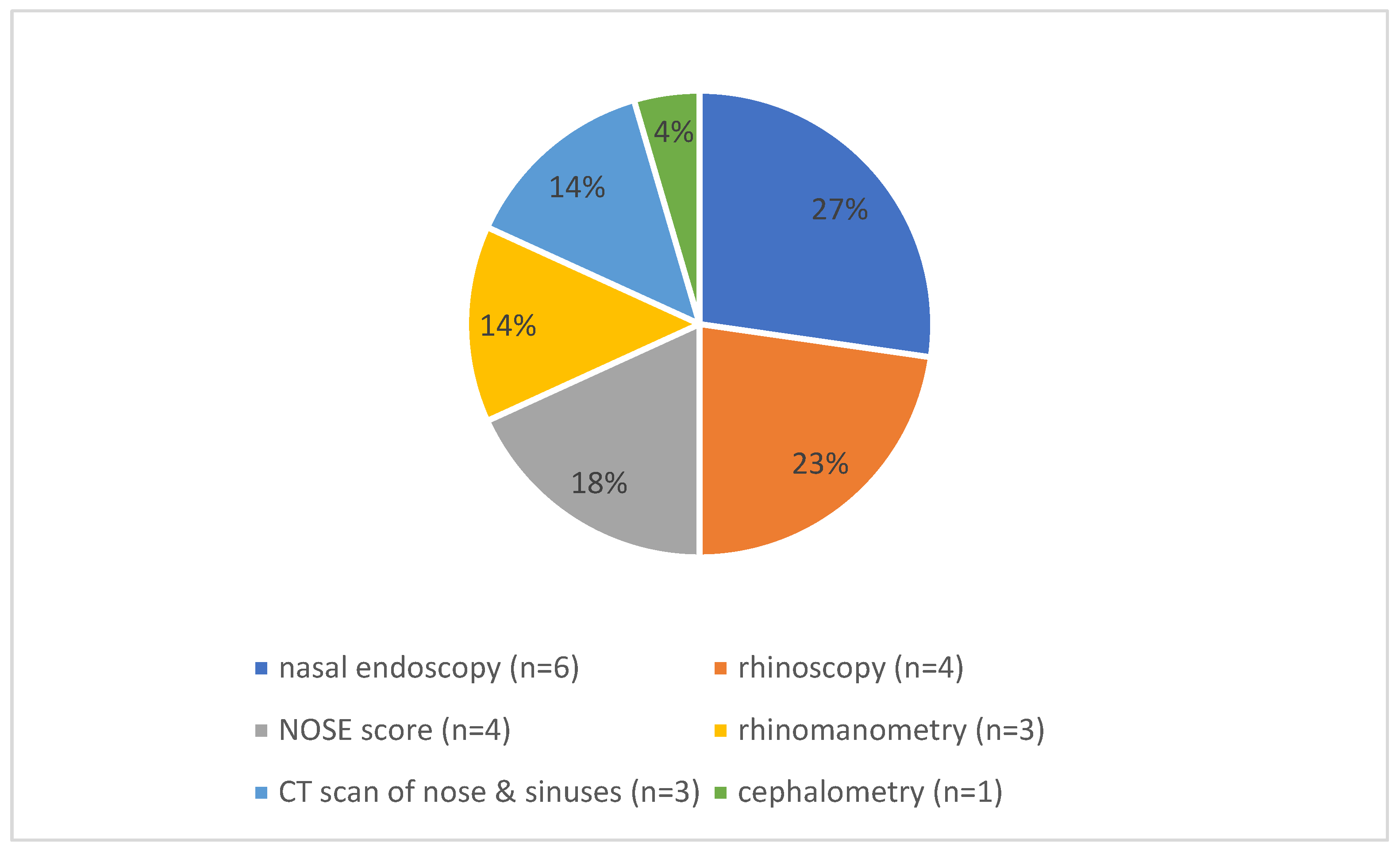
| Authors | Population (n) | Male (n) | Female (n) | Range of Age (Years) | Mean Age |
|---|---|---|---|---|---|
| Alshehri et al. [21] | 29 | 13 | 16 | - | 9.45 |
| Ghosh et al. [22] | 25 | 14 | 11 | 8–14 | 11.5 |
| Benyo et al. [23] | 729 | 503 | 226 | 6–16 | - |
| Abdelaal et al. [24] | 39 | 30 | 9 | 12–15.8 | 14.2 |
| Yaseen et al. [25] | 100 | 60 | 40 | 3–18 | - |
| Shah et al. [26] | 24,322 | 14,618 | 9704 | ≤18 | - |
| Raghavan et al. [27] | 2290 | - | - | - | 14.2 |
| Ori et al. [28] | 111 | - | - | 6–13 | 9.4 |
| Bishop et al. [29] | 194 | - | - | - | 14.6 |
| Koirala [30] | 50 | 37 | 13 | - | 12.78 |
| Manteghi et al. [31] | 136 | 94 | 42 | - | 15.7 |
| Fuller et al. [32] | 39 | - | - | 7–18 | 15.9 |
| Sabry et al. [33] | 30 | - | - | 5–17 | 12.7 |
| Lee et al. [34] | 28 | - | - | - | - |
| Anderson et al. [35] | 29 | 16 | 13 | 5–16 | 11.2 |
| Matin et al. [36] | 250 | 163 | 87 | 7–14 | - |
| Maniglia et al. [37] | 202 | 124 | 78 | 4–16 | - |
| Yilmaz et al. [38] | 35 | 24 | 11 | 8–16 | 13.4 |
| Total population | 28,638 | ||||
| Sex distribution | 25,946 | 15,696 | 10,250 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zieliński, J.; Costa, S.; Cherkes, M.; Glibbery, N.; Kovács, P.; Mitrea-Sirețeanu, L.; Ciller, M.; Yılmaz Topçuoğlu, M.-S. Pediatric Septoplasty: Benefits, Challenges, and Clinical Recommendations—Comprehensive Review of Young ESPO. J. Clin. Med. 2025, 14, 5537. https://doi.org/10.3390/jcm14155537
Zieliński J, Costa S, Cherkes M, Glibbery N, Kovács P, Mitrea-Sirețeanu L, Ciller M, Yılmaz Topçuoğlu M-S. Pediatric Septoplasty: Benefits, Challenges, and Clinical Recommendations—Comprehensive Review of Young ESPO. Journal of Clinical Medicine. 2025; 14(15):5537. https://doi.org/10.3390/jcm14155537
Chicago/Turabian StyleZieliński, Jakub, Sara Costa, Maryana Cherkes, Natalia Glibbery, Petra Kovács, Luiza Mitrea-Sirețeanu, Marek Ciller, and Miray-Su Yılmaz Topçuoğlu. 2025. "Pediatric Septoplasty: Benefits, Challenges, and Clinical Recommendations—Comprehensive Review of Young ESPO" Journal of Clinical Medicine 14, no. 15: 5537. https://doi.org/10.3390/jcm14155537
APA StyleZieliński, J., Costa, S., Cherkes, M., Glibbery, N., Kovács, P., Mitrea-Sirețeanu, L., Ciller, M., & Yılmaz Topçuoğlu, M.-S. (2025). Pediatric Septoplasty: Benefits, Challenges, and Clinical Recommendations—Comprehensive Review of Young ESPO. Journal of Clinical Medicine, 14(15), 5537. https://doi.org/10.3390/jcm14155537







