Bispecific Antibodies—A New Hope for Patients with Diffuse Large B-Cell Lymphoma
Abstract
1. Introduction
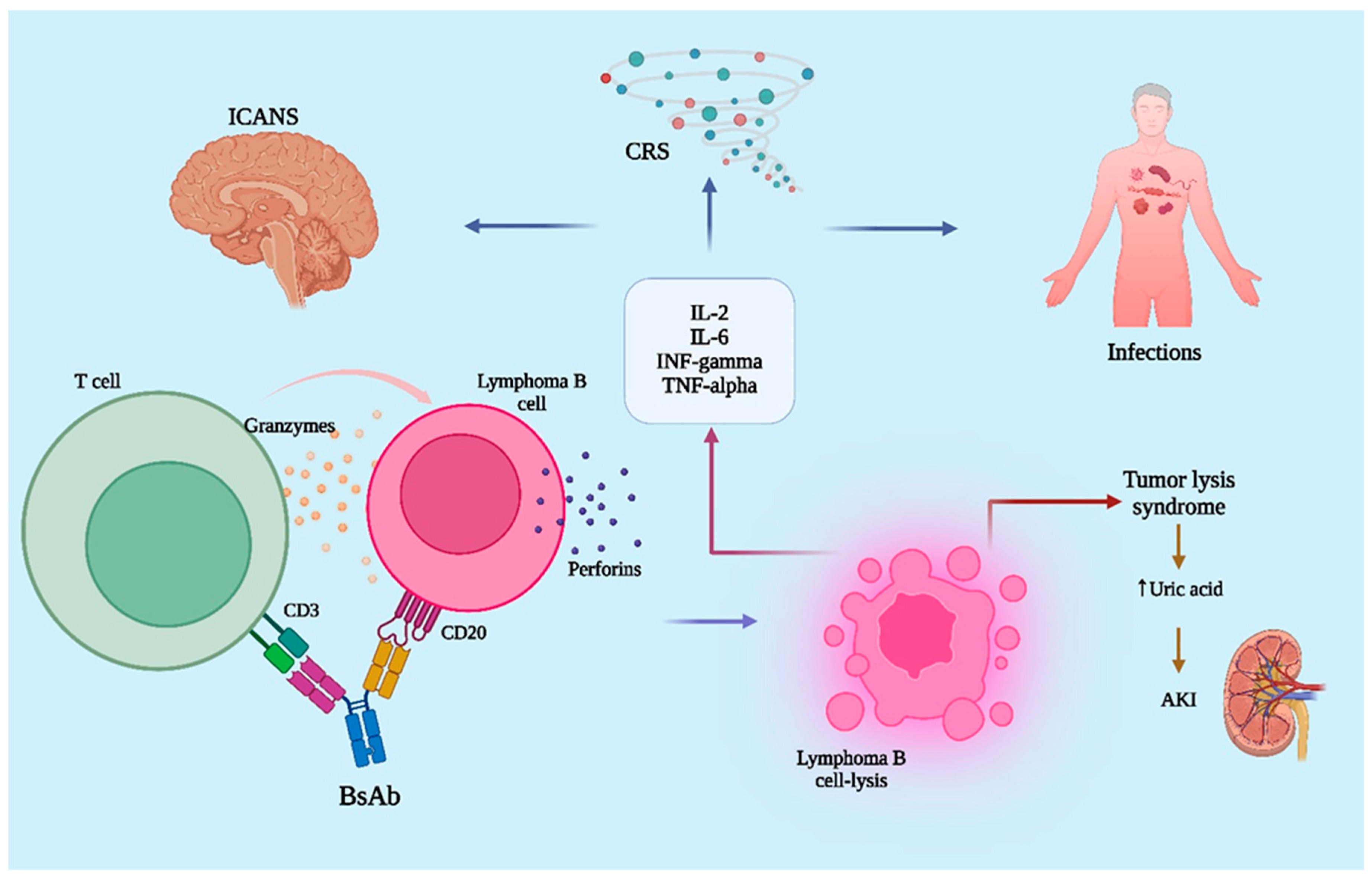
2. Pharmacodynamics of Bispecific Antibodies
3. Bispecific Antibodies Products
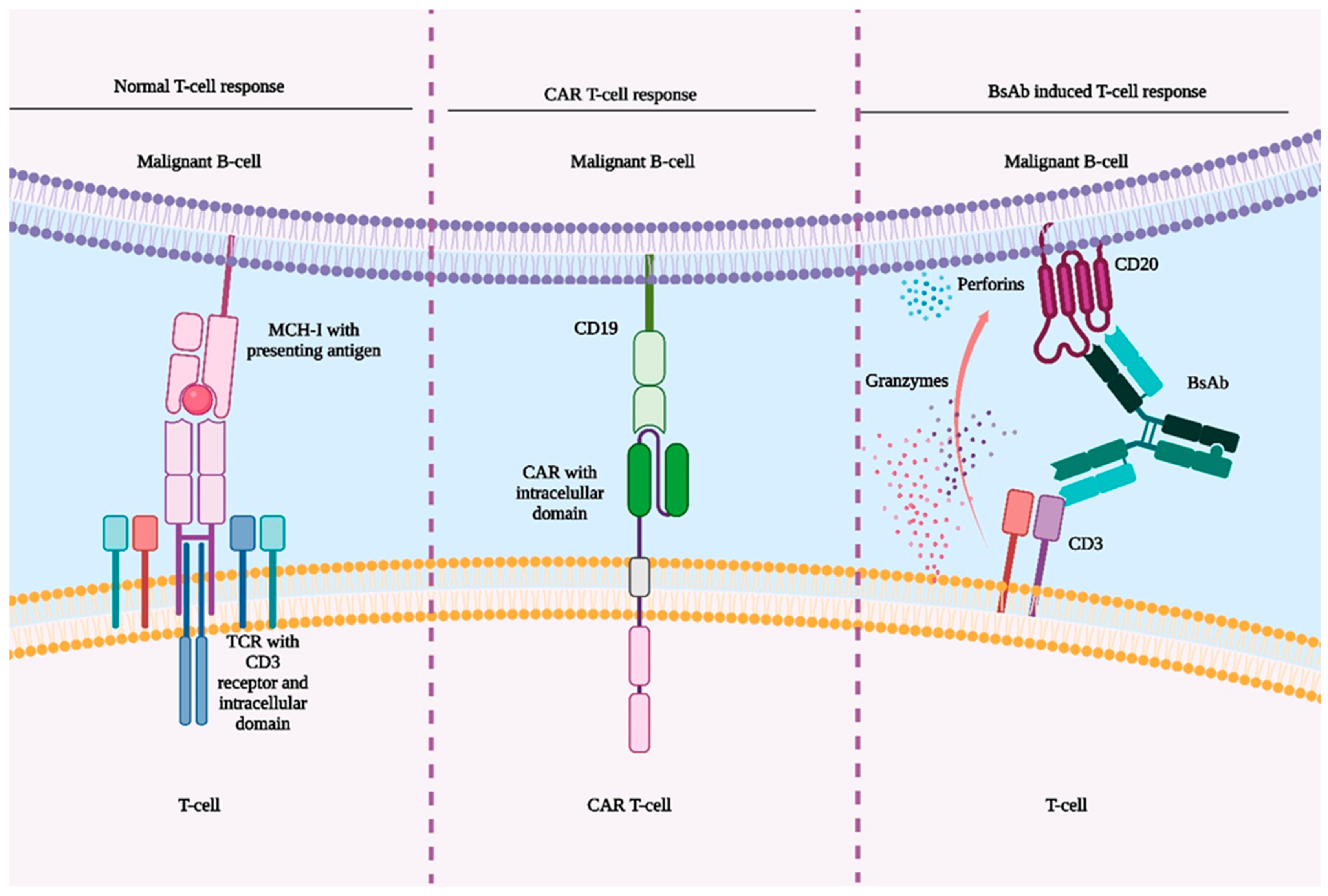
4. Comparison of CAR T-Cell Therapy and BsAbs
5. Advantages and Limits of Bispecific Antibodies
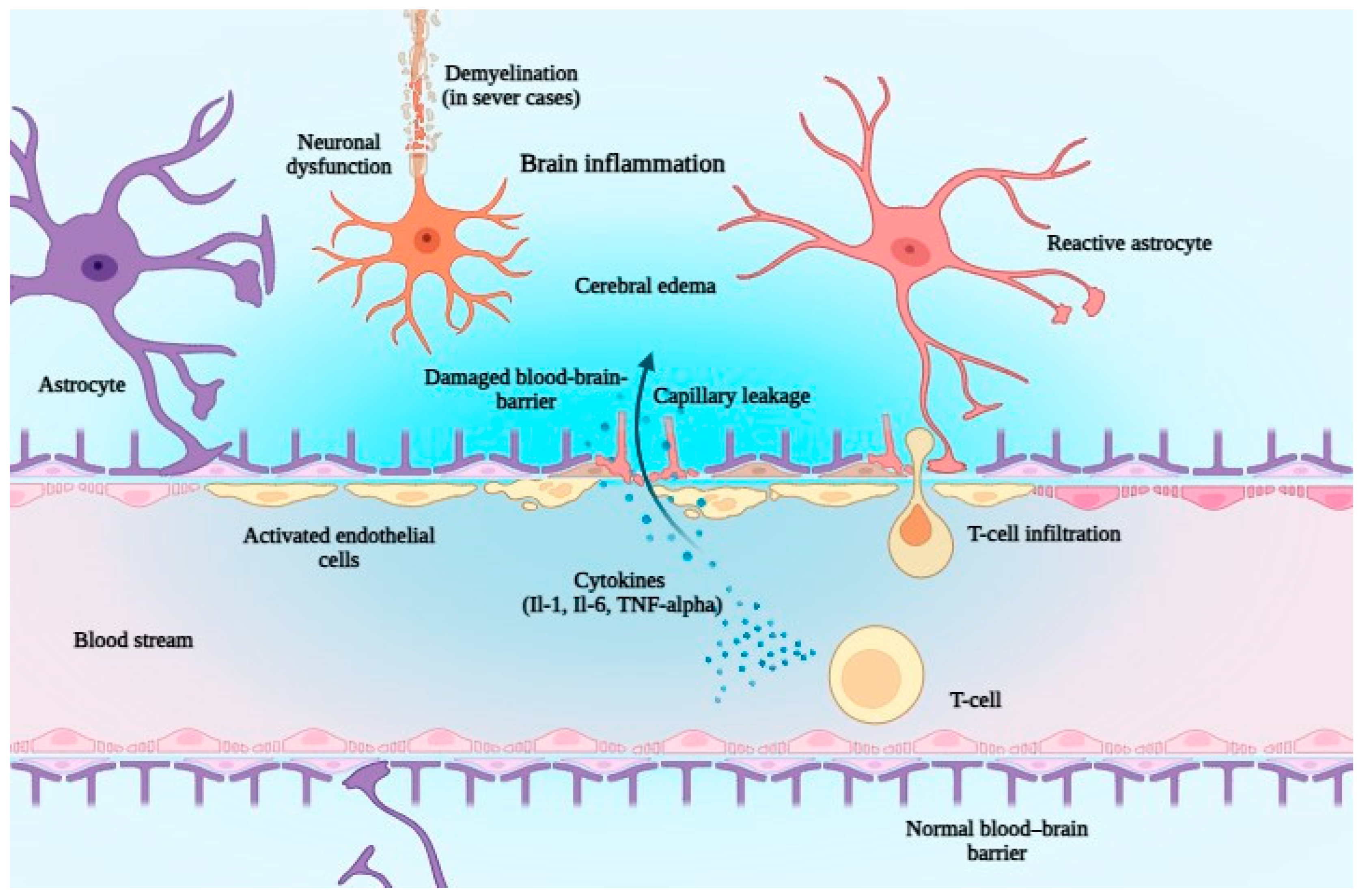
6. The Safety of Bispecific Antibodies
7. Conclusions and Future Developments
Author Contributions
Funding
Conflicts of Interest
References
- Barraclough, A.; Hawkes, E.A. Antibody and Immunotherapy in Diffuse Large B-Cell Lymphoma. Semin. Hematol. 2023, 60, 338–345. [Google Scholar] [CrossRef]
- D’Alò, F.; Bellesi, S.; Maiolo, E.; Alma, E.; Bellisario, F.; Malafronte, R.; Viscovo, M.; Campana, F.; Hohaus, S. Novel Targets and Advanced Therapies in Diffuse Large B Cell Lymphomas. Cancers 2024, 16, 2243. [Google Scholar] [CrossRef]
- Bennett, R.; Dickinson, M. SOHO State of the Art Updates and Next Questions|Current Evidence and Future Directions for Bispecific Antibodies in Large B-Cell Lymphoma. Clin. Lymphoma Myeloma Leuk. 2024, 24, S2152265024001812. [Google Scholar] [CrossRef]
- Polgarova, K.; Trneny, M. An Evaluation of Glofitamab, the First Fixed-Duration Bispecific Antibody for Relapsed or Refractory Large B-Cell Lymphomas. Expert Opin. Biol. Ther. 2024, 24, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Zhang, J.; Xu-Monette, Z.Y.; Young, K.H. The Progress of Novel Strategies on Immune-Based Therapy in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Exp. Hematol. Oncol. 2023, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Saleh, K.; Khoury, R.; Khalife, N.; Chahine, C.; Ibrahim, R.; Tikriti, Z.; Le Cesne, A. The Evolving Role of Bispecific Antibodies in Diffuse Large B-Cell Lymphoma. J. Pers. Med. 2024, 14, 666. [Google Scholar] [CrossRef] [PubMed]
- Dogliotti, I.; Peri, V.; Clerico, M.; Vassallo, F.; Musto, D.; Mercadante, S.; Ragaini, S.; Botto, B.; Levis, M.; Novo, M.; et al. Real Life Clinical Outcomes of Relapsed/Refractory Diffuse Large B Cell Lymphoma in the Rituximab Era: The STRIDER Study. Cancer Med. 2024, 13, e7448. [Google Scholar] [CrossRef]
- Ide, D.; Fujino, T.; Kobayashi, T.; Egashira, A.; Miyashita, A.; Mizuhara, K.; Isa, R.; Tsukamoto, T.; Mizutani, S.; Uchiyama, H.; et al. Prognostic Model for Relapsed/Refractory Transplant-Ineligible Diffuse Large B-Cell Lymphoma Utilizing the Lymphocyte-to-Monocyte Ratio. Int. J. Hematol. 2024, 119, 697–706. [Google Scholar] [CrossRef]
- Fabbri, N.; Mussetti, A.; Sureda, A. Second-Line Treatment of Diffuse Large B-cell Lymphoma: Evolution of Options. Semin. Hematol. 2023, 60, 305–312. [Google Scholar] [CrossRef]
- Bücklein, V.; Von Tresckow, B.; Subklewe, M. T-Zell-rekrutierende Immuntherapien des B-Zell-Lymphoms—Bald in allen Therapielinien? Dtsch. Med. Wochenschr. 2024, 149, 630–637. [Google Scholar] [CrossRef]
- Moon, D.; Tae, N.; Park, Y.; Lee, S.-W.; Kim, D.H. Development of Bispecific Antibody for Cancer Immunotherapy: Focus on T Cell Engaging Antibody. Immune Netw. 2022, 22, e4. [Google Scholar] [CrossRef]
- Melody, M.; Gordon, L.I. Sequencing of Cellular Therapy and Bispecific Antibodies for the Management of Diffuse Large B-Cell Lymphoma. Haematologica 2024, 109, 3138–3145. [Google Scholar] [CrossRef] [PubMed]
- Cosenza, M.; Sacchi, S.; Pozzi, S. Cytokine Release Syndrome Associated with T-Cell-Based Therapies for Hematological Malignancies: Pathophysiology, Clinical Presentation, and Treatment. Int. J. Mol. Sci. 2021, 22, 7652. [Google Scholar] [CrossRef] [PubMed]
- Trabolsi, A.; Arumov, A.; Schatz, J.H. Bispecific Antibodies and CAR-T Cells: Dueling Immunotherapies for Large B-Cell Lymphomas. Blood Cancer J. 2024, 14, 27. [Google Scholar] [CrossRef]
- Frampton, J.E. Epcoritamab: First Approval. Drugs 2023, 83, 1331–1340. [Google Scholar] [CrossRef]
- Takaura, K.; Ando, H.; Ganoza, E.R. Pharmacological Characteristics and Clinical Outcomes of Epcoritamab (Recombinant) (Epkinly® Subcutaneous Injection) for Malignant Lymphoma. Folia Pharmacol. Jpn. 2024, 159, 61–68. [Google Scholar] [CrossRef]
- Shirouchi, Y.; Maruyama, D. Recent Advances and Future Perspectives of T-Cell Engagers in Lymphoid Malignancies. Jpn. J. Clin. Oncol. 2024, 54, 376–385. [Google Scholar] [CrossRef]
- Minson, A.G.; Dickinson, M.J. New Bispecific Antibodies in Diffuse Large B-Cell Lymphoma. Haematologica 2025, 110, 1483. [Google Scholar] [CrossRef] [PubMed]
- Thieblemont, C.; Karimi, Y.H.; Ghesquieres, H.; Cheah, C.Y.; Clausen, M.R.; Cunningham, D.; Jurczak, W.; Do, Y.R.; Gasiorowski, R.; Lewis, D.J.; et al. Epcoritamab in Relapsed/Refractory Large B-Cell Lymphoma: 2-Year Follow-up from the Pivotal EPCORE NHL-1 Trial. Leukemia 2024, 38, 2653–2662. [Google Scholar] [CrossRef]
- Brody, J.D.; Jørgensen, J.; Belada, D.; Costello, R.; Trněný, M.; Vitolo, U.; Lewis, D.J.; Karimi, Y.H.; Sureda, A.; André, M.; et al. Epcoritamab plus GemOx in Transplant-Ineligible Relapsed/Refractory DLBCL: Results from the EPCORE NHL-2 Trial. Blood 2025, 145, 1621–1631. [Google Scholar] [CrossRef]
- Hutchings, M.; Morschhauser, F.; Iacoboni, G.; Carlo-Stella, C.; Offner, F.C.; Sureda, A.; Salles, G.; Martínez-Lopez, J.; Crump, M.; Thomas, D.N.; et al. Glofitamab, a Novel, Bivalent CD20-Targeting T-Cell–Engaging Bispecific Antibody, Induces Durable Complete Remissions in Relapsed or Refractory B-Cell Lymphoma: A Phase I Trial. J. Clin. Oncol. 2021, 39, 1959–1970. [Google Scholar] [CrossRef]
- Dickinson, M.J.; Carlo-Stella, C.; Morschhauser, F.; Bachy, E.; Corradini, P.; Iacoboni, G.; Khan, C.; Wróbel, T.; Offner, F.; Trněný, M.; et al. Glofitamab for Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 387, 2220–2231. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.S.; Ku, M.; Hertzberg, M.; Huang, H.-Q.; Fox, C.P.; Zhang, H.; Yoon, D.H.; Kim, W.-S.; Abdulhaq, H.; Townsend, W.; et al. Glofitamab plus Gemcitabine and Oxaliplatin (GemOx) versus Rituximab-GemOx for Relapsed or Refractory Diffuse Large B-Cell Lymphoma (STARGLO): A Global Phase 3, Randomised, Open-Label Trial. Lancet 2024, 404, 1940–1954. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, A.; Foley, N.C.; Cohen, J.; Rettig, M.P.; Cashen, A.F.; Gehrs, L.; Christ, S.; Street, E.; Wallace, N.; Ritchey, J.; et al. Blinatumomab Consolidation Post–Autologous Stem Cell Transplantation in Patients with Diffuse Large B-Cell Lymphoma. Blood Adv. 2024, 8, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Viardot, A.; Goebeler, M.-E.; Hess, G.; Neumann, S.; Pfreundschuh, M.; Adrian, N.; Zettl, F.; Libicher, M.; Sayehli, C.; Stieglmaier, J.; et al. Phase 2 Study of the Bispecific T-Cell Engager (BiTE) Antibody Blinatumomab in Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Blood 2016, 127, 1410–1416. [Google Scholar] [CrossRef]
- Guièze, R.; Ysebaert, L.; Roos-Weil, D.; Fornecker, L.-M.; Ferrant, E.; Molina, L.; Aurran, T.; Clavert, A.; De Guibert, S.; Michallet, A.-S.; et al. Blinatumomab after R-CHOP Bridging Therapy for Patients with Richter Transformation: A Phase 2 Multicentre Trial. Nat. Commun. 2024, 15, 6822. [Google Scholar] [CrossRef]
- Matasar, M.; Bartlett, N.L.; Shadman, M.; Budde, L.E.; Flinn, I.; Gregory, G.P.; Kim, W.S.; Hess, G.; El-Sharkawi, D.; Diefenbach, C.S.; et al. Mosunetuzumab Safety Profile in Patients with Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma: Clinical Management Experience from a Pivotal Phase I/II Trial. Clin. Lymphoma Myeloma Leuk. 2024, 24, 240–253. [Google Scholar] [CrossRef]
- Budde, L.E.; Assouline, S.; Sehn, L.H.; Schuster, S.J.; Yoon, S.-S.; Yoon, D.H.; Matasar, M.J.; Bosch, F.; Kim, W.S.; Nastoupil, L.J.; et al. Single-Agent Mosunetuzumab Shows Durable Complete Responses in Patients with Relapsed or Refractory B-Cell Lymphomas: Phase I Dose-Escalation Study. J. Clin. Oncol. 2022, 40, 481–491. [Google Scholar] [CrossRef]
- Budde, L.E.; Olszewski, A.J.; Assouline, S.; Lossos, I.S.; Diefenbach, C.; Kamdar, M.; Ghosh, N.; Modi, D.; Sabry, W.; Naik, S.; et al. Mosunetuzumab with Polatuzumab Vedotin in Relapsed or Refractory Aggressive Large B Cell Lymphoma: A Phase 1b/2 Trial. Nat. Med. 2024, 30, 229–239. [Google Scholar] [CrossRef]
- Westin, J.; Phillips, T.J.; Mehta, A.; Hoffmann, M.S.; Gonzalez-Barca, E.; Thieblemont, C.; Bastos-Oreiro, M.; Greil, R.; Giebel, S.; Wei, M.C.; et al. Mosunetuzumab plus Pola-CHP Compared with Pola-R-CHP in Previously Untreated DLBCL: Final Results from a Phase 2 Study. Blood Adv. 2025, 9, 2461–2472. [Google Scholar] [CrossRef]
- Chong, E.A.; Penuel, E.; Napier, E.B.; Lundberg, R.K.; Budde, L.E.; Shadman, M.; Matasar, M.J.; Bartlett, N.L.; Flinn, I.W.; Bosch, F.; et al. Impact of Prior CAR T-Cell Therapy on Mosunetuzumab Efficacy in Patients with Relapsed or Refractory B-Cell Lymphomas. Blood Adv. 2025, 9, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.; Khalid, M.F.; Amin, M.K.; Basharat, A.; Ammad-Ud-Din, M.; Park, R.; Anwar, I.; Faisal, M.S.; Jaglal, M. Geographic and Racial Disparities in Chimeric Antigen Receptor–T Cells and Bispecific Antibodies Trials Access for Diffuse Large B-Cell Lymphoma. Clin. Lymphoma Myeloma Leuk. 2024, 24, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Davila, M.L.; Brentjens, R.; Wang, X.; Rivière, I.; Sadelain, M. How Do CARs Work?: Early Insights from Recent Clinical Studies Targeting CD19. OncoImmunology 2012, 1, 1577–1583. [Google Scholar] [CrossRef]
- Sadelain, M.; Brentjens, R.; Rivière, I. The Basic Principles of Chimeric Antigen Receptor Design. Cancer Discov. 2013, 3, 388–398. [Google Scholar] [CrossRef]
- First-Ever CAR T-Cell Therapy Approved in U.S. Cancer Discov. 2017, 7, OF1. [CrossRef]
- Si Lim, S.J.; Grupp, S.A.; DiNofia, A.M. Tisagenlecleucel for Treatment of Children and Young Adults with Relapsed/Refractory B-cell Acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 2021, 68, e29123. [Google Scholar] [CrossRef] [PubMed]
- Fowler, N.H.; Dickinson, M.; Dreyling, M.; Martinez-Lopez, J.; Kolstad, A.; Butler, J. Tisagenlecleucel Is Safe and Effective in Relapsed/Refractory Follicular Lymphoma. Cancer Discov. 2022, 12, OF4. [Google Scholar] [CrossRef]
- Bouchkouj, N.; Kasamon, Y.L.; De Claro, R.A.; George, B.; Lin, X.; Lee, S.; Blumenthal, G.M.; Bryan, W.; McKee, A.E.; Pazdur, R. FDA Approval Summary: Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma. Clin. Cancer Res. 2019, 25, 1702–1708. [Google Scholar] [CrossRef]
- Bouchkouj, N.; Zimmerman, M.; Kasamon, Y.L.; Wang, C.; Dai, T.; Xu, Z.; Wang, X.; Theoret, M.; Purohit-Sheth, T.; George, B. FDA Approval Summary: Axicabtagene Ciloleucel for Relapsed or Refractory Follicular Lymphoma. Oncol. 2022, 27, 587–594. [Google Scholar] [CrossRef]
- De Ramon Ortiz, C.; Wang, S.; Stathis, A.; Bertoni, F.; Zenz, T.; Novak, U.; Simonetta, F. How to Integrate CD19 Specific Chimeric Antigen Receptor T Cells with Other CD19 Targeting Agents in Diffuse Large B-cell Lymphoma? Hematol. Oncol. 2024, 42, e3237. [Google Scholar] [CrossRef]
- Valery, M.; Saleh, K.; Ecea, R.; Michot, J.M.; Ribrag, V.; Fizazi, K.; Hollebecque, A.; Lecesne, A.; Ponce, S.; Loriot, Y.; et al. Infections Occurring Following IL6 Blockade for the Management of Cytokine Release Syndrome in Onco-Hematology Patients. Cancer Chemother. Pharmacol. 2023, 92, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Tumuluru, S.; Godfrey, J.K.; Cooper, A.; Yu, J.; Chen, X.; MacNabb, B.W.; Venkataraman, G.; Zha, Y.; Pelzer, B.; Song, J.; et al. Integrative Genomic Analysis Identifies Unique Immune Environments Associated with Immunotherapy Response in Diffuse Large B Cell Lymphoma. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Brooks, T.R.; Caimi, P.F. A Paradox of Choice: Sequencing Therapy in Relapsed/Refractory Diffuse Large B-Cell Lymphoma. Blood Rev. 2024, 63, 101140. [Google Scholar] [CrossRef]
- Park, J.H.; Nath, K.; Devlin, S.M.; Sauter, C.S.; Palomba, M.L.; Shah, G.; Dahi, P.; Lin, R.J.; Scordo, M.; Perales, M.-A.; et al. CD19 CAR T-Cell Therapy and Prophylactic Anakinra in Relapsed or Refractory Lymphoma: Phase 2 Trial Interim Results. Nat. Med. 2023, 29, 1710–1717. [Google Scholar] [CrossRef]
- Kim, J.; Cho, J.; Lee, M.H.; Yoon, S.E.; Kim, W.S.; Kim, S.J. CAR T Cells vs Bispecific Antibody as Third- or Later-Line Large B-Cell Lymphoma Therapy: A Meta-Analysis. Blood 2024, 144, 629–638. [Google Scholar] [CrossRef]
- Topp, M.S.; Matasar, M.; Allan, J.N.; Ansell, S.M.; Barnes, J.A.; Arnason, J.E.; Michot, J.-M.; Goldschmidt, N.; O’Brien, S.M.; Abadi, U.; et al. Odronextamab Monotherapy in R/R DLBCL after Progression with CAR T-Cell Therapy: Primary Analysis of the ELM-1 Study. Blood 2025, 145, 1498–1509. [Google Scholar] [CrossRef]
- Sousa-Pimenta, M.; Martins, Â.; Mariz, J.M.; Berraondo, P. Response to Therapy in Richter Syndrome: A Systematic Review with Meta-Analysis of Early Clinical Trials. Front. Immunol. 2023, 14, 1295293. [Google Scholar] [CrossRef]
- Carlo-Stella, C. Relapse after Glofitamab, a Novel Unmet Medical Need with High Rates of CD20 Loss. Br. J. Haematol. 2024, 205, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Burt, R.; Warcel, D.; Fielding, A.K. Blinatumomab, a Bispecific B-Cell and T-Cell Engaging Antibody, in the Treatment of B-Cell Malignancies. Hum. Vaccines Immunother. 2019, 15, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Gaballa, S.; Hou, J.-Z.; Devata, S.; Cho, S.-G.; Nair, R.; Yoon, D.H.; Jacobs, R.; Izutsu, K.; Stevens, D.A.; Maruyama, D.; et al. Evaluation of AZD0486, a Novel CD19xCD3 T-Cell Engager, in Relapsed/Refractory Diffuse Large B-Cell Lymphoma in an Ongoing First-in-Human Phase 1 Study: High Complete Responses Seen in CAR-T-Naive and CAR-T-Exposed Patients. Blood 2024, 144, 868. [Google Scholar] [CrossRef]
- Leclercq-Cohen, G.; Steinhoff, N.; Albertí Servera, L.; Nassiri, S.; Danilin, S.; Piccione, E.; Yángüez, E.; Hüsser, T.; Herter, S.; Schmeing, S.; et al. Dissecting the Mechanisms Underlying the Cytokine Release Syndrome (CRS) Mediated by T-Cell Bispecific Antibodies. Clin. Cancer Res. 2023, 29, 4449–4463. [Google Scholar] [CrossRef]
- Moreau, P.; Touzeau, C. T-Cell–Redirecting Bispecific Antibodies in Multiple Myeloma: A Revolution? Blood 2022, 139, 3681–3687. [Google Scholar] [CrossRef]
- Juluri, K.R.; Wu, Q.V.; Voutsinas, J.; Hou, J.; Hirayama, A.V.; Mullane, E.; Miles, N.; Maloney, D.G.; Turtle, C.J.; Bar, M.; et al. Severe Cytokine Release Syndrome Is Associated with Hematologic Toxicity Following CD19 CAR T-Cell Therapy. Blood Adv. 2022, 6, 2055–2068. [Google Scholar] [CrossRef] [PubMed]
- Bannerji, R.; Arnason, J.E.; Advani, R.H.; Brown, J.R.; Allan, J.N.; Ansell, S.M.; Barnes, J.A.; O’Brien, S.M.; Chávez, J.C.; Duell, J.; et al. Odronextamab, a Human CD20×CD3 Bispecific Antibody in Patients with CD20-Positive B-Cell Malignancies (ELM-1): Results from the Relapsed or Refractory Non-Hodgkin Lymphoma Cohort in a Single-Arm, Multicentre, Phase 1 Trial. Lancet Haematol. 2022, 9, e327–e339. [Google Scholar] [CrossRef]
- Gurumurthi, A.; Westin, J.; Subklewe, M. The Race Is on: Bispecifics vs CAR T Cells in B-Cell Lymphoma. Blood Adv. 2023, 7, 5713–5716. [Google Scholar] [CrossRef]
- Budde, L.E.; Sehn, L.H.; Matasar, M.; Schuster, S.J.; Assouline, S.; Giri, P.; Kuruvilla, J.; Canales, M.; Dietrich, S.; Fay, K.; et al. Safety and Efficacy of Mosunetuzumab, a Bispecific Antibody, in Patients with Relapsed or Refractory Follicular Lymphoma: A Single-Arm, Multicentre, Phase 2 Study. Lancet Oncol. 2022, 23, 1055–1065. [Google Scholar] [CrossRef]
- Morschhauser, F.; Dahiya, S.; Palomba, M.L.; Martin Garcia-Sancho, A.; Reguera Ortega, J.L.; Kuruvilla, J.; Jäger, U.; Cartron, G.; Izutsu, K.; Dreyling, M.; et al. Lisocabtagene Maraleucel in Follicular Lymphoma: The Phase 2 TRANSCEND FL Study. Nat. Med. 2024, 30, 2199–2207. [Google Scholar] [CrossRef]
- Falchi, L.; Hutchings, M.; Carlo-Stella, C.; Morschhauser, F.; Dickinson, M.; Cartron, G.; Khan, C.; Tani, M.; Martinez-Lopez, J.; Bartlett, N.L.; et al. Dexamethasone Is Associated with Reduced Frequency and Intensity of Cytokine Release Syndrome Compared with Alternative Corticosteroid Regimens as Premedication for Glofitamab in Patients with Relapsed/Refractory Large B-Cell Lymphoma. Haematologica 2024, 110, 999–1004. [Google Scholar] [CrossRef]
- Izutsu, K.; Kumode, T.; Yuda, J.; Nagai, H.; Mishima, Y.; Suehiro, Y.; Yamamoto, K.; Fujisaki, T.; Ishitsuka, K.; Ishizawa, K.; et al. Subcutaneous Epcoritamab Monotherapy in Japanese Adults with Relapsed/Refractory Diffuse Large B-cell Lymphoma. Cancer Sci. 2023, 114, 4643–4653. [Google Scholar] [CrossRef] [PubMed]
- Thieblemont, C.; Phillips, T.; Ghesquieres, H.; Cheah, C.Y.; Clausen, M.R.; Cunningham, D.; Do, Y.R.; Feldman, T.; Gasiorowski, R.; Jurczak, W.; et al. Epcoritamab, a Novel, Subcutaneous CD3xCD20 Bispecific T-Cell–Engaging Antibody, in Relapsed or Refractory Large B-Cell Lymphoma: Dose Expansion in a Phase I/II Trial. J. Clin. Oncol. 2023, 41, 2238–2247. [Google Scholar] [CrossRef]
- Kim, W.-S.; Kim, T.M.; Cho, S.-G.; Jarque, I.; Iskierka-Jażdżewska, E.; Poon, M.L.; Prince, H.M.; Oh, S.Y.; Lim, F.; Carpio, C.; et al. Odronextamab in Patients with Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL): Results from a Prespecified Analysis of the Pivotal Phase II Study ELM-2. Blood 2022, 140, 1070–1071. [Google Scholar] [CrossRef]
- Van De Donk, N.W.C.J.; Zweegman, S. T-Cell-Engaging Bispecific Antibodies in Cancer. Lancet 2023, 402, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Danish, H.; Santomasso, B.D. Neurotoxicity Biology and Management. Cancer J. 2021, 27, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Omer, M.H.; Shafqat, A.; Ahmad, O.; Alkattan, K.; Yaqinuddin, A.; Damlaj, M. Bispecific Antibodies in Hematological Malignancies: A Scoping Review. Cancers 2023, 15, 4550. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Xu, G. Nephrotoxicity in Bispecific Antibodies Recipients: Focus on T-Cell-Engaging Bispecific Antibodies. OncoTargets Ther. 2024, 17, 545–556. [Google Scholar] [CrossRef]
- Uckun, F.M.; Lin, T.L.; Mims, A.S.; Patel, P.; Lee, C.; Shahidzadeh, A.; Shami, P.J.; Cull, E.; Cogle, C.R.; Watts, J. A Clinical Phase 1B Study of the CD3xCD123 Bispecific Antibody APVO436 in Patients with Relapsed/Refractory Acute Myeloid Leukemia or Myelodysplastic Syndrome. Cancers 2021, 13, 4113. [Google Scholar] [CrossRef]
- Sun, H.; Xing, H.; Han, L.; Song, Y.; Jiang, Z.; Liu, Y.; Yu, J. Bispecific Antibodies Targeting CD20xCD3 in Immunotherapy for Adult B-Cell Lymphoma: Insights from the 65th American Society of Hematology 2023 Annual Meeting. Expert Opin. Biol. Ther. 2024, 24, 321–326. [Google Scholar] [CrossRef]
| Drug | Study Group/Target | Patients | Follow-Up | Outcomes | Reference |
|---|---|---|---|---|---|
| Epcoritamab | R/R LBCL (61.1% primary refractory, 38.9% post-CAR-T) | 157 | 10.7 months | ORR, 63.1%; CR, 38.9%; DoR, 12 mo; CR DoR, NR. | [19] |
| Epcoritamab + GemOx | R/R DLBCL (transplant ineligible) | 103 | 13.2 months | ORR, 85%; CR, 61%; CR DoR, 23.6 mo; OS, 21.6 mo. | [20] |
| Glofitamab | R/R B-cell NHL (incl. DLBCL) | 171 | Up to 27.4 months | ORR, 53.8%; CR, 36.8%. At RP2D: ORR, 65.7%; CR, 57.1%; 84.1% CRs ongoing. | [21] |
| Glofitamab | R/R DLBCL | 154 | 12 months | CR, 39%; 35% post-CAR-T; median CR, 42d; 78% CRs ongoing; PFS, 37%. | [22] |
| Glofitamab + GemOx | R/R DLBCL (transplant ineligible) | 274 | 20.7 months | OS: 25.5 vs. 12.9 mo (HR 0.62). | [23] |
| Blinatumomab | Post-auto-HSCT consolidation (DLBCL/FL) | 14 | 1 year | 86% CR at day 100; 50% CR at 1 year. | [24] |
| Blinatumomab | R/R DLBCL (median 3 prior therapies) | 25 | NR | ORR, 43%; CR, 19%; neurotoxicity with flat dosing. | [25] |
| Blinatumomab | Richter transformation (post-RCHOP non-CR) | 25 | NR | ORR, 46%; CR, 36%. | [26] |
| Mosunetuzumab | R/R B-NHL (aggressive and indolent) | 197 | NR | ORR, 34.9%/66.2%; CR, 19.4%/48.5%; CR DoR > 20 mo. | [28] |
| Mosunetuzumab + Polatuzumab | R/R LBCL | 59 | NR | ORR, 59.2%; CR, 45.9%; PFS, 11.4 mo; OS, 23.3 mo. | [29] |
| Mosunetuzumab + Pola-CHP | First-line DLBCL | 120 | 24 months | CR: 72.5% vs. 77.3% (Pola-R-CHP); PFS: 70.8% vs. 81.8%. | [30] |
| Mosunetuzumab (post-CAR-T) | R/R LBCL post-CAR-T | 30 | NR | Better when given later; ↑ CD4/CD8/activated CD8 in responders. | [31] |
| Adverse Effect | Frequency/Severity | Mechanism/Notes |
|---|---|---|
| CRS | Common (up to 73.2%), grade ≥ 3: ~2% | Triggered by T-cell activation and cytokine cascade (IL-6, IL-1β, TNF-α); manageable with step-up dosing. |
| ICANS | Rare (~1%), lower than CAR T-cell (11%) | Related to endothelial activation and blood–brain barrier disruption; cerebral edema may occur. |
| Tremor | Rare, low grade (~7%) | Mild, self-limiting; less frequent than in CAR T-cell therapy. |
| Aphasia | Not reported with BsAbs | Frequently seen with CAR T-cells, but absent in BsAbs-treated patients. |
| Cytopenias | Common | Likely from cytokine-induced impaired hematopoiesis. |
| Febrile Neutropenia | <5% | Secondary to neutropenia. |
| Infections (general) | ~10% grade ≥ 3 | Due to lymphopenia, B/T-cell dysfunction, and hypogammaglobulinemia. |
| COVID-19, UTI, Pneumonia | Documented in clinical trials | Immunosuppression related. |
| TLS | Rare but serious | Rapid tumor breakdown → electrolyte imbalances, AKI. |
| AKI | Underreported, emerging concern | Cytokine effects, TLS, or comorbidities. |
| Hypogammaglobulinemia | Variable | Due to B-cell or plasma cell depletion (e.g., CD19/CD20/BCMA targets). |
| T-cell Exhaustion | Associated with infection risk | From sustained immune activation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihaila, R.G.; Todor, S.B. Bispecific Antibodies—A New Hope for Patients with Diffuse Large B-Cell Lymphoma. J. Clin. Med. 2025, 14, 5534. https://doi.org/10.3390/jcm14155534
Mihaila RG, Todor SB. Bispecific Antibodies—A New Hope for Patients with Diffuse Large B-Cell Lymphoma. Journal of Clinical Medicine. 2025; 14(15):5534. https://doi.org/10.3390/jcm14155534
Chicago/Turabian StyleMihaila, Romeo Gabriel, and Samuel B. Todor. 2025. "Bispecific Antibodies—A New Hope for Patients with Diffuse Large B-Cell Lymphoma" Journal of Clinical Medicine 14, no. 15: 5534. https://doi.org/10.3390/jcm14155534
APA StyleMihaila, R. G., & Todor, S. B. (2025). Bispecific Antibodies—A New Hope for Patients with Diffuse Large B-Cell Lymphoma. Journal of Clinical Medicine, 14(15), 5534. https://doi.org/10.3390/jcm14155534






