Application of Augmented Reality in Reverse Total Shoulder Arthroplasty: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Study Design and Protocol
2.2. Eligibility Criteria
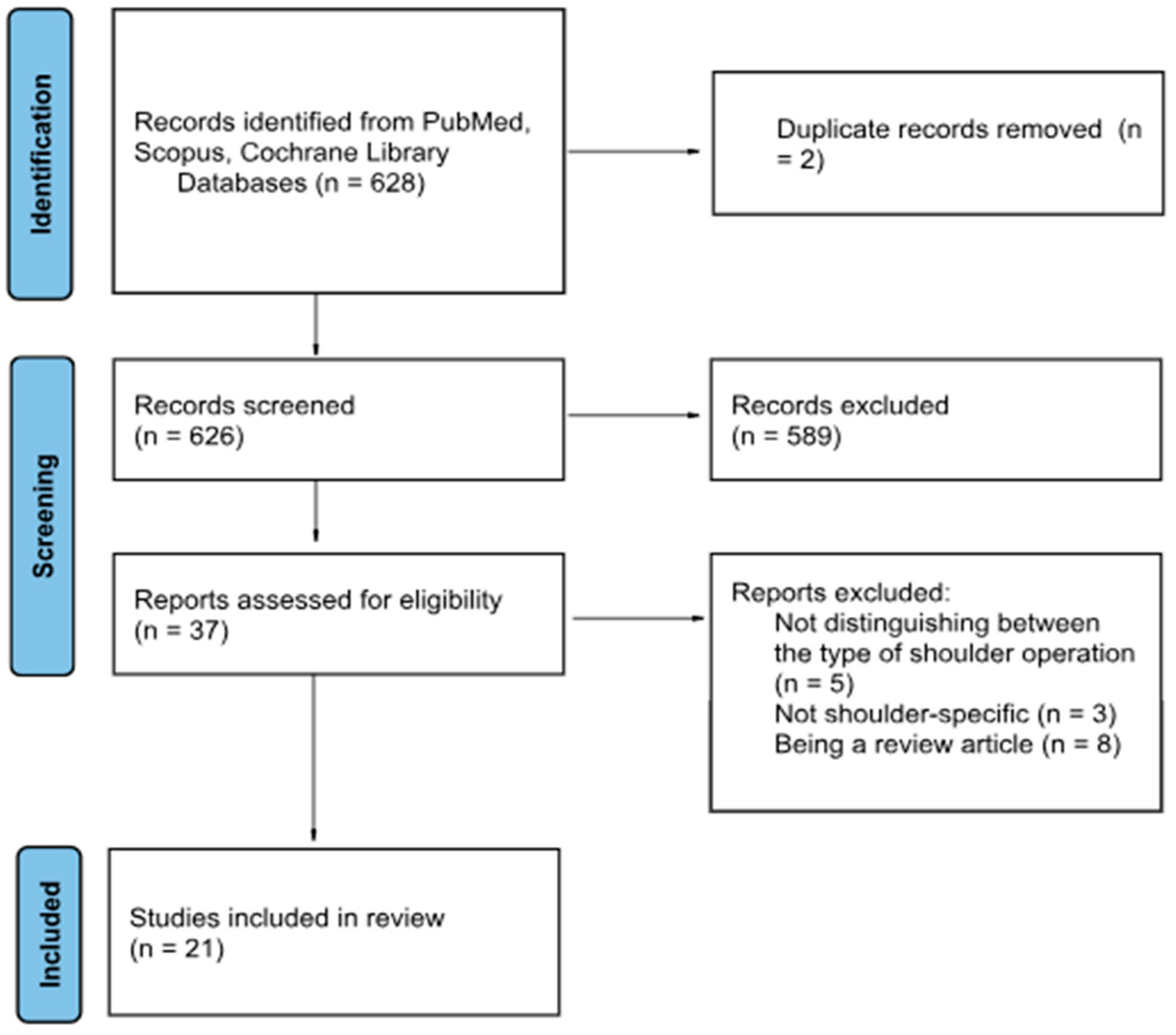
2.3. Search Strategy and Study Selection
2.4. Data Extraction
- Study characteristics (author, year, study design, number of participants)
- Application setting (training and skill acquiring or intraoperative guiding and navigation)
- Comparison group (if any)
- Outcome measures and key findings such as baseplate position (entry point at glenoid, version, inclination, medialization, outliers) and surgical time
2.5. Data Synthesis
- Skill acquisition and training
- Intraoperative planning and navigation
3. Results
3.1. Training and Skill Acquisition
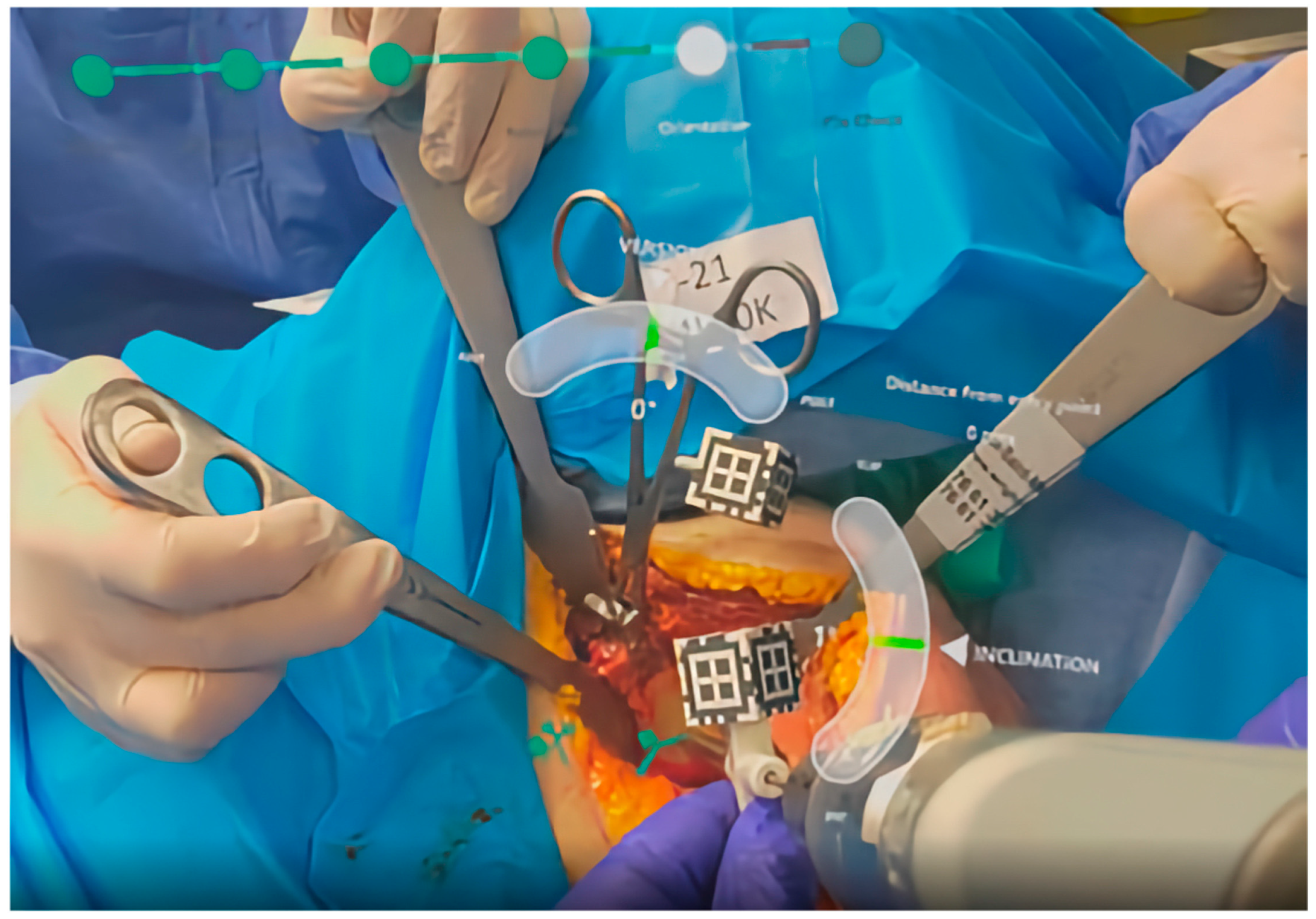
3.2. Intraoperative Navigation and Guiding
4. Discussion
4.1. Orthopedic Training
4.2. Clinical Application
4.3. General Outlook
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ek, E.T.; Catanzaro, S.; Gerber, C. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: Results after five to fifteen years. J. Should. Elb. Surg. 2013, 22, 1199–1208. [Google Scholar] [CrossRef]
- Favard, L.; Levigne, C.; Nerot, C.; Gerber, C.; De Wilde, L.; Mole, D. Reverse prostheses in arthropathies with cuff tear: Are survivorship and function maintained over time? Clin. Orthop. Relat. Res. 2011, 469, 2469–2475. [Google Scholar] [CrossRef]
- McFarland, E.G.; Huri, G.; Hyun, Y.S.; Petersen, S.A.; Srikumaran, U. Reverse total shoulder arthroplasty without bone-grafting for severe glenoid bone loss in patients with osteoarthritis and intact rotator cuff. J. Bone Jt. Surg. Am. 2016, 98, 1801–1807. [Google Scholar] [CrossRef]
- Kelly, B.J.; Myeroff, C.M. Reverse Shoulder Arthroplasty for Proximal Humerus Fracture. Curr. Rev. Musculoskelet. Med. 2020, 13, 186–199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boileau, P.; Melis, B.; Duperron, D.; Moineau, G.; Rumian, A.P.; Han, Y. Revision surgery of reverse shoulder arthroplasty. J. Should. Elb. Surg. 2013, 22, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Klug, A.; Herrmann, E.; Fischer, S.; Hoffmann, R.; Gramlich, Y. Projections of primary and revision shoulder arthroplasty until 2040: Facing a massive rise in fracture-related procedures. J. Clin. Med. 2021, 10, 5123. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.R.; Farley, K.X.; Higgins, I.; Wilson, J.M.; Daly, C.A.; Gottschalk, M.B. The incidence of shoulder arthroplasty: Rise and future projections compared with hip and knee arthroplasty. J. Should. Elb. Surg. 2020, 29, 2601–2609. [Google Scholar] [CrossRef]
- Hussain, Z.B.; Wagner, E.R. Revolutionizing Shoulder Arthroplasty: The Transformative Impact of Extended Reality, Robotics, and Artificial Intelligence on Training, Planning, and Execution. Oper. Tech. Orthop. 2024, 34, 101153. [Google Scholar] [CrossRef]
- Matsen, F.A., III; Clinton, J.M.; Lynch, J.M.; Bertelsen, A.P.; Richardson, M.L. Glenoid Component Failure in Total Shoulder Arthroplasty. J. Bone Jt. Surg. 2008, 90, 885–896. [Google Scholar] [CrossRef]
- Berhouet, J.; Gulotta, L.V.; Dines, D.M.; Craig, E.; Warren, R.F.; Choi, D.; Chen, X.; Kontaxis, A. Preoperative planning for accurate glenoid component positioning in reverse shoulder arthroplasty. Orthop. Traumatol. Surg. Res. 2017, 103, 407–413. [Google Scholar] [CrossRef]
- Mao, R.Q.; Lan, L.; Kay, J.; Lohre, R.; Ayeni, O.R.; Goel, D.P.; de Sa, D. Immersive Virtual Reality for Surgical Training: A Systematic Review. J. Surg. Res. 2021, 268, 40–58. [Google Scholar] [CrossRef]
- Jud, L.; Fotouhi, J.; Andronic, O.; Aichmair, A.; Osgood, G.; Navab, N.; Farshad, M. Applicability of augmented reality in orthopedic surgery—A systematic review. BMC Musculoskelet. Disord. 2020, 21, 103. [Google Scholar] [CrossRef]
- Chen, J.; Or, C.K.; Chen, T. Effectiveness of Using Virtual Reality-Supported Exercise Therapy for Upper Extremity Motor Rehabilitation in Patients With Stroke: Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Med. Internet Res. 2022, 24, e24111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Longo, U.G.; Lalli, A.; Gobbato, B.; Nazarian, A. Metaverse, virtual reality and augmented reality in total shoulder arthroplasty: A systematic review. BMC Musculoskelet. Disord. 2024, 25, 396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Lohre, R.; Bois, A.J.; Athwal, G.S.; Goel, D.P. Improved Complex Skill Acquisition by Immersive Virtual Reality Training: A Randomized Controlled Trial. J. Bone Jt. Surg Am. 2020, 102, e26. [Google Scholar] [CrossRef]
- Lohre, R.; Bois, A.J.; Pollock, J.W.; Lapner, P.; McIlquham, K.; Athwal, G.S.; Goel, D.P. Effectiveness of Immersive Virtual Reality on Orthopedic Surgical Skills and Knowledge Acquisition among Senior Surgical Residents: A Randomized Clinical Trial. JAMA Network Open 2020, 3, e2031217. [Google Scholar] [CrossRef]
- Crockatt, W.K.; Confino, J.E.; Kopydlowski, N.J.; Jobin, C.M.; Levine, W.N. Comparing Skill Acquisition and Validity of Immersive Virtual Reality with Cadaver Laboratory Sessions in Training for Reverse Total Shoulder Arthroplasty. JBJS Open Access 2023, 8, e22. [Google Scholar] [CrossRef]
- Erickson, J.; Biron, D.; Berg, A.; Mattern, P.; Rai, R.; Genovese, N. Preoperative mixed reality training improves trainee performance of glenoid guidewire positioning in shoulder arthroplasty in Walch B2 glenoid model. Semin. Arthroplast. JSES 2024, 34, 171–175. [Google Scholar] [CrossRef]
- Bischofreiter, M.; Gabauer, N.; Gruber, M.S.; Gattringer, M.; Breulmann, F.L.; Kindermann, H.; Mattiassich, G.; Ortmaier, R. Learning curve of augmented reality surgical application for reverse shoulder arthroplasty. Arch. Orthop. Trauma Surg. 2025, 145, 252. [Google Scholar] [CrossRef]
- Gregory, T.; Gregory, J.; Charles, D.; Simon Alexander, H. Surgeon experience of mixed reality headset technology during the COVID-19 pandemic: A multicenter international case series in orthopedic surgery. BMJ Surg. Interv. Health Technol. 2022, 4, e000127. [Google Scholar] [CrossRef] [PubMed]
- Kopriva, J.M.; McKissack, H.M.; Griswold, B.G.; Hussain, Z.B.; Cooke, H.L.; Gottschalk, M.B.; Wagner, E.R. Mixed-reality improves execution of templated glenoid component positioning in shoulder arthroplasty: A CT imaging analysis. J. Should. Elb. Surg. 2024, 33, 1789–1798. [Google Scholar] [CrossRef]
- Rojas, J.T.; Menzemer, J.; Rashid, M.S.; Hayoz, A.; Lädermann, A.; Zumstein, M.A. Navigated augmented reality through a head-mounted display leads to low deviation between planned, intra- and postoperative parameters during glenoid component placement of reverse shoulder arthroplasty: A proof-of-concept case series. J. Should. Elb. Surg. 2025, 34, 567–576. [Google Scholar] [CrossRef]
- Kriechling, P.; Loucas, R.; Loucas, M.; Casari, F.; Fürnstahl, P.; Wieser, K. Augmented reality through head-mounted display for navigation of baseplate component placement in reverse total shoulder arthroplasty: A cadaveric study. Arch. Orthop. Trauma Surg. 2023, 143, 169–175. [Google Scholar] [CrossRef]
- Rojas, J.T.; Jost, B.; Zipeto, C.; Budassi, P.; Zumstein, M.A. Glenoid component placement in reverse shoulder arthroplasty assisted with augmented reality through a head-mounted display leads to low deviation between planned and postoperative parameters. J. Should. Elb. Surg. 2023, 32, e587–e596. [Google Scholar] [CrossRef]
- Sanchez-Sotelo, J.; Berhouet, J.; Chaoui, J.; Freehill, M.T.; Collin, P.; Warner, J.; Walch, G.; Athwal, G.S. Validation of mixed-reality surgical navigation for glenoid axis pin placement in shoulder arthroplasty using a cadaveric model. J. Should. Elb. Surg. 2024, 33, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Dordain, F.; Nourrissat, G.; van Rooij, F.; Ferrand, M.; Petroff, E.; Antoni, M. Augmented reality significantly reduces the absolute error between achieved and planned inclination and version of the glenoid baseplate for reversed shoulder arthroplasty. JSES Int. 2025, 9, 1215–1219. [Google Scholar] [CrossRef]
- Dey Hazra, R.O.; Paksoy, A.; Imiolczyk, J.P.; Gebauer, H.; Hayta, A.; Akgun, D. Augmented reality–assisted intraoperative navigation increases precision of glenoid inclination in reverse shoulder arthroplasty. J. Should. Elb. Surg. 2025, 34, 577–583. [Google Scholar] [CrossRef]
- Berhouet, J.; Slimane, M.; Facomprez, M.; Jiang, M.; Favard, L. Views on a new surgical assistance method for implanting the glenoid component during total shoulder arthroplasty. Part 2: From three-dimensional reconstruction to augmented reality: Feasibility study. Orthop. Traumatol Surg. Res. 2019, 105, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Kriechling, P.; Roner, S.; Liebmann, F.; Casari, F.; Fürnstahl, P.; Wieser, K. Augmented reality for base plate component placement in reverse total shoulder arthroplasty: A feasibility study. Arch. Orthop. Trauma. Surg. 2021, 141, 1447–1453. [Google Scholar] [CrossRef]
- Schlueter-Brust, K.; Henckel, J.; Katinakis, F.; Buken, C.; Opt-Eynde, J.; Pofahl, T.; Baena, F.R.Y.; Tatti, F. Augmented-Reality-Assisted K-Wire Placement for Glenoid Component Positioning in Reversed Shoulder Arthroplasty: A Proof-of-Concept Study. J. Pers. Med. 2021, 11, 777. [Google Scholar] [CrossRef] [PubMed]
- Fleet, C.T.; Gao, R.; Johnson, J.A.; Athwal, G.S. An in vitro comparison of mixed-reality navigation to traditional freehand and patient-specific instrumentation techniques for glenoid guide pin insertion during shoulder arthroplasty. J. Should. Elb. Surg. 2024, 33, 2482–2492. [Google Scholar] [CrossRef]
- Italia, K.; Launay, M.; Gilliland, L.; Lane, A.; Nielsen, J.; Stalin, K.A.; Green, N.; Maharaj, J.; Whitehouse, S.; Cutbush, K.; et al. Improving glenoid guidewire placement in shoulder arthroplasty: A comparative study of mixed reality holographic overlay technique with freehand technique and conventional navigation. JSES Int. 2025, 9, 981–987. [Google Scholar] [CrossRef]
- Gu, W.; Knopf, J.; Cast, J.; Higgins, L.D.; Knopf, D.; Unberath, M. Nail it! vision-based drift correction for accurate mixed reality surgical guidance. Int. J. Comput. Assist. Radiol. Surg. 2023, 18, 1235–1243. [Google Scholar] [CrossRef]
- Trehin, A.; Boas, D.; Jouet, V.; Zago, B.; Cariou, D. An accurate scapula registration process in shoulder arthroplasty using mixed reality. Int. J. Comput. Assist. Radiol. Surg. 2023, 18, 1341–1344. [Google Scholar] [CrossRef] [PubMed]
- Abdic, S.; Van Osch, N.J.; Langohr, D.G.; Johnson, J.A.; Athwal, G.S. Mixed reality visualization in shoulder arthroplasty: Is it better than traditional preoperative planning software? Clin. Should. Elb. 2023, 26, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Throckmorton, T.W.; Gulotta, L.V.; Bonnarens, F.O.; Wright, S.A.; Hartzell, J.L.; Rozzi, W.B.; Hurst, J.M.; Frostick, S.P.; Sperling, J.W. Patient-specific targeting guides compared with traditional instrumentation for glenoid component placement in shoulder arthroplasty: A multi-surgeon study in 70 arthritic cadaver specimens. J. Should. Elb. Surg. 2015, 24, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Twomey-Kozak, J.; Hurley, E.; Levin, J.; Anakwenze, O.; Klifto, C. Technological innovations in shoulder replacement: Current concepts and the future of robotics in total shoulder arthroplasty. J. Should. Elb. Surg. 2023, 32, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sotelo, J. Robot-assisted shoulder arthroplasty. JSES Int. 2025, 9, 974–980. [Google Scholar] [CrossRef]
- Giorgini, A.; Tarallo, L.; Porcellini, G.; Micheloni, G.M. GPS Navigation system allows the surgeon to prepare the implant site as planned on preoperative software in reverse shoulder arthroplasty. Orthop. Procs. 2021, 103, 8. [Google Scholar]
- Marescalchi, M.; El Motassime, A.; Andriollo, L.; Polizzi, A.; Niccoli, G.; Morea, V. Computer-Assisted Navigation in Shoulder Arthroplasty: A Narrative Review. J. Clin. Med. 2025, 14, 2763. [Google Scholar] [CrossRef] [PubMed]
- Heany, D. Microsoft Is Discontinuing HoloLens 2, With No Replacement. 2024. Available online: https://www.uploadvr.com/microsoft-discontinuing-hololens-2/ (accessed on 25 June 2025).
- Nam, J.; Koh, Y.-G.; Chung, S.; Kim, P.S.; Jang, J.; Park, J.H.; Kang, K.T. The Application of Virtual Reality in Shoulder Surgery Rehabilitation. Cureus 2024, 16, e58280. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, A.; Mannocchi, I.; Sassi, M.S.H.; Carli, M.; De Luca, G.; Longo, U.G.; Denaro, V.; Schena, E. Virtual Reality for Shoulder Rehabilitation: Accuracy Evaluation of Oculus Quest 2. Sensus 2022, 22, 5511. [Google Scholar] [CrossRef] [PubMed]

| First Author | Year | Study Type | Participants | Outcomes | Key Findings |
|---|---|---|---|---|---|
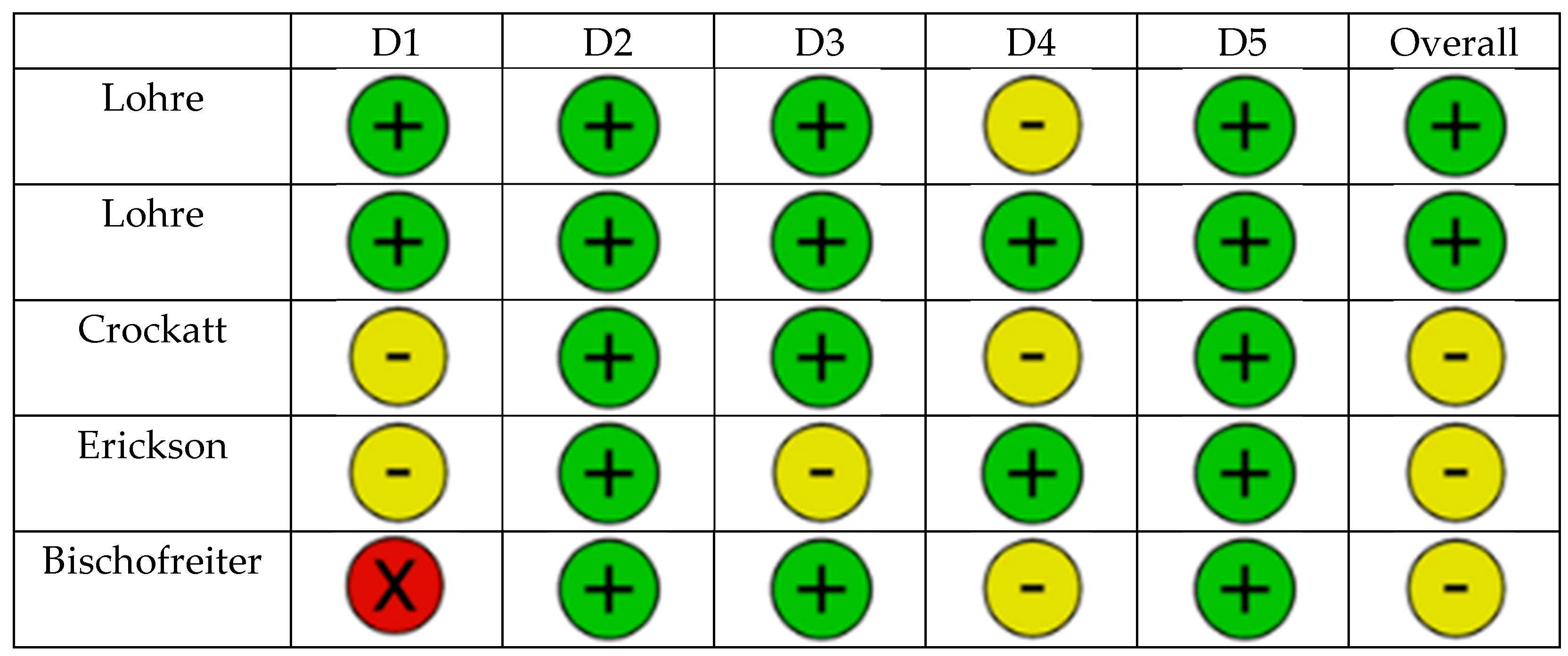
| Lohre [17] | 2020 | RCT | Orthopedic residents | OSATS scores, task time | VR group had faster times for completion of cadaveric procedure (p = 0.13), higher cumulative OSATS scores (p < 0.001) |
| Lohre [18] | 2020 | RCT | Senior orthopedic residents and consultants | OSATS scores, task time | Higher OSATS scores for instrument handling (p = 0.03), faster task time (p = 0.04) |
| Crockatt [19] | 2023 | RCT | Junior orthopedic residents | OSATS, GRS, task time | No significant differences; VR non-inferior to cadaver labs |
| Erickson [20] | 2024 | RCT | Junior orthopedic residents | Entry point, inclination, version | Improved guidewire placement in dysplastic glenoids |
| Bischofreiter [21] | 2025 | Retrospective case series | Single High-volume shoulder surgeon | Surgical time, blood loss | Surgical time reduced (p < 0.001), blood loss reduced (p = 0.005) along learning curve |
| First Author | Year | Study Type | Participants | Technology | Evaluated Items |
|---|---|---|---|---|---|
| Berhouet [30] | 2017 | Feasibility | Printed scapula model | Epson Moverio BT-200 smart glasses | First implementation to superimpose a 3D model |
| Kriechling [31] | 2020 | Feasibility | 10 3D-printed scapula models | MS HoloLens 1 AR-based guidance | Entry point, angular deviation |
| Schlueter-Brust [32] | 2021 | Feasibility | 9 3D-printed scapula models | MS HoloLens 1 AR-based guidance | Entry point, angular deviation |
| Abdic [37] | 2023 | Feasibility, comparative | 128 3D-printed deformed scapula models | MS HoloLens 2 AR-based guidance vs. 3D software planning | Entry point, version, inclination, procedural time, surgeon’s confidence |
| Gu [35] | 2023 | Feasibility | 30 3D-printed scapula models | MS HoloLens 2 AR-based guidance | Entry point, angular deviation |
| Trehin [36] | 2023 | Feasibility | 13 3D-printed scapula models | MS HoloLens 2 AR-based guidance | Entry point antero-inferior and supero-inferior, inclination, version, global vector, rotation, procedural time |
| Fleet [33] | 2024 | Feasibility, comparative | 20 3D-printed scapula models | Traditional vs. PSI vs. MR Stryker navigation | Entry point, version, inclination |
| Italia [34] | 2024 | Feasibility, comparative | 60 3D-printed scapula models | Traditional vs. MR overlay vs. MR navigation | Entry point, version, inclination, outliers |
| Kriechling [25] | 2021 | Cadaveric | 12 Cadaver models | MS HoloLens AR-based guidance | Entry point, angular deviation |
| Rojas [26] | 2023 | Cadaveric | 12 Cadaver models | NextAR HMD | Entry point, version, inclination, rotation, depth, procedural time, outliers, complications |
| Sanchez-Sotelo [27] | 2024 | Cadaveric, 7 surgeons | 14 Cadaver models | Stryker, MS HoloLens 2 | Entry point, version, inclination, superior-inferior, anterior-inferior position, complications |
| Dordain [28] | 2025 | Cadaveric | 10 Cadaver models | Pixee AR-HMD | Entry point as superior-inferior and anterior-posterior, version, inclination, outliers |
| Dey Hazra [29] | 2025 | Cadaveric | 16 Cadaver models | NextAR HMD | Version, Inclination, position anterior-posterior, superior-inferior, lateral-medial |
| Author | Year | Study Type | n | Technology Application | Evaluated Items |
|---|---|---|---|---|---|
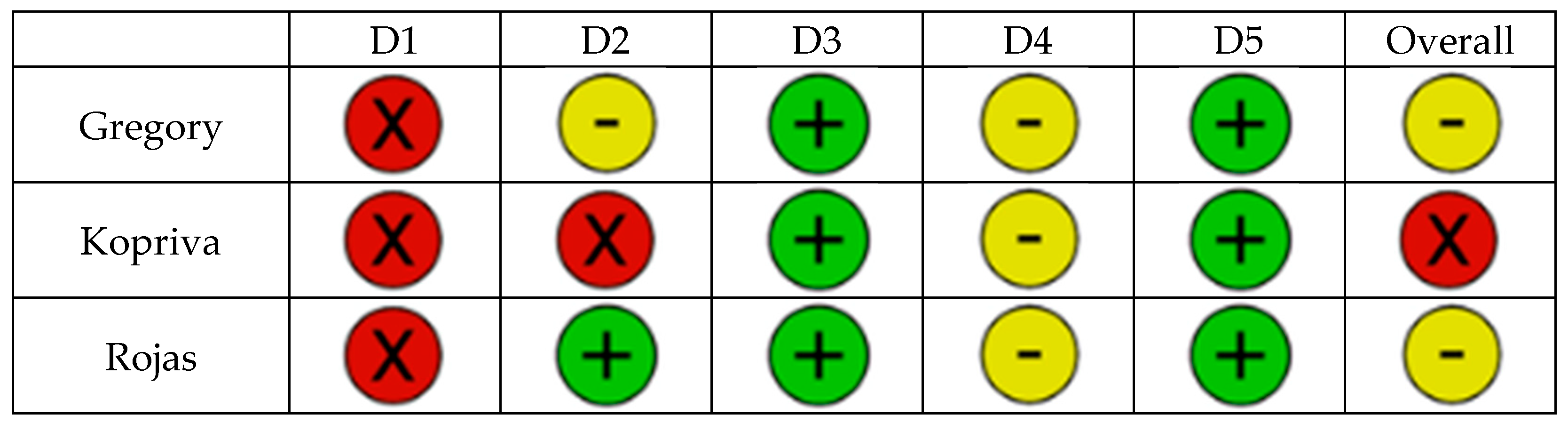
| Gregory [22] | 2022 | Multicenter feasibility | 13 | Visualization, HoloLens for pre-op Plan | Satisfaction of surgeons |
| Kopriva [23] | 2024 | Retrospective comparative | 25/72 | Stryker Holoblueprint Visualization, no navigation | Version, Inclination, Outliers, Surgical time |
| Rojas [24] | 2025 | Prospective multicenter study | 17 | NextAR HMD Navigation | Entry point, version, inclination, rotation, depth, procedural time, outliers, complications, blood loss |
| Study | Entry (mm) | Version (°) | Inclination (°) | Outliers |
|---|---|---|---|---|
| Kriechling [31] | 2.3 ± 1.1 | 2.7 ± 1.3 | - | |
| Schlueter-Brust [32] | 2.4 ± 0.7 | 3.9 ± 2.4 | - | |
| Abdic [37] | 2.1 ± 0.1 | 9 ± 1 | 8 ± 1 | |
| Gu [35] | 1.5 ± 1.0 | 2.4 ± 0.9 | ||
| Trehin [36] | 0.5 ± 0.4 (vert.) 0.8 ± 0.6 (horiz.) | 0.97 ± 0.8 | 0.89 ± 0.6 | - |
| Fleet [33] | 2 ± 1 | 1 ± 1 | 2 ± 1 | - |
| Italia [34] | 3.3 ± 2.0 | 4 ± 3 | 7 ± 5 | 1 |
| Kriechling [25] | 3.5 ± 1.7 | 3.8 ± 1.7 | - | |
| Rojas [26] | 1.1 ± 0.4 | 1.8 ± 1.3 | 1.0 ± 0.7 | 0/10 |
| Sanchez-Sotelo [27] | 1.7 ± 0.8 | 1.6 ± 1.2 | 1.7 ± 1.5 | - |
| Dordain [28] | 1.1 ± 1.7 (vert.) 0.5 ± 0.9 (horiz.) | 0.7 ± 0.5 | 0.9 ± 1.6 | 1/10 |
| Dey Hazra [29] | - | - | 3 ± 2 | 0/12 |
| Gregory [22] | - | - | - | - |
| Kopriva [23] | - | 2.3 ± 2.1 | 2.4 ± 1.7 | 1/25 |
| Rojas [24] | 2.0 ± 2.5 | 3.4 ± 4.6 | 2.5 ± 3.2 | 3/17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlewski, J.; Hochreiter, B.; Wieser, K.; Kriechling, P. Application of Augmented Reality in Reverse Total Shoulder Arthroplasty: A Systematic Review. J. Clin. Med. 2025, 14, 5533. https://doi.org/10.3390/jcm14155533
Orlewski J, Hochreiter B, Wieser K, Kriechling P. Application of Augmented Reality in Reverse Total Shoulder Arthroplasty: A Systematic Review. Journal of Clinical Medicine. 2025; 14(15):5533. https://doi.org/10.3390/jcm14155533
Chicago/Turabian StyleOrlewski, Jan, Bettina Hochreiter, Karl Wieser, and Philipp Kriechling. 2025. "Application of Augmented Reality in Reverse Total Shoulder Arthroplasty: A Systematic Review" Journal of Clinical Medicine 14, no. 15: 5533. https://doi.org/10.3390/jcm14155533
APA StyleOrlewski, J., Hochreiter, B., Wieser, K., & Kriechling, P. (2025). Application of Augmented Reality in Reverse Total Shoulder Arthroplasty: A Systematic Review. Journal of Clinical Medicine, 14(15), 5533. https://doi.org/10.3390/jcm14155533