Early Positive Fluid Balance Associates with Increased Mortality in Neurological Critically Ill Patients: A 10-Year Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Definitions and Outcomes
2.3. Statistical Analyses
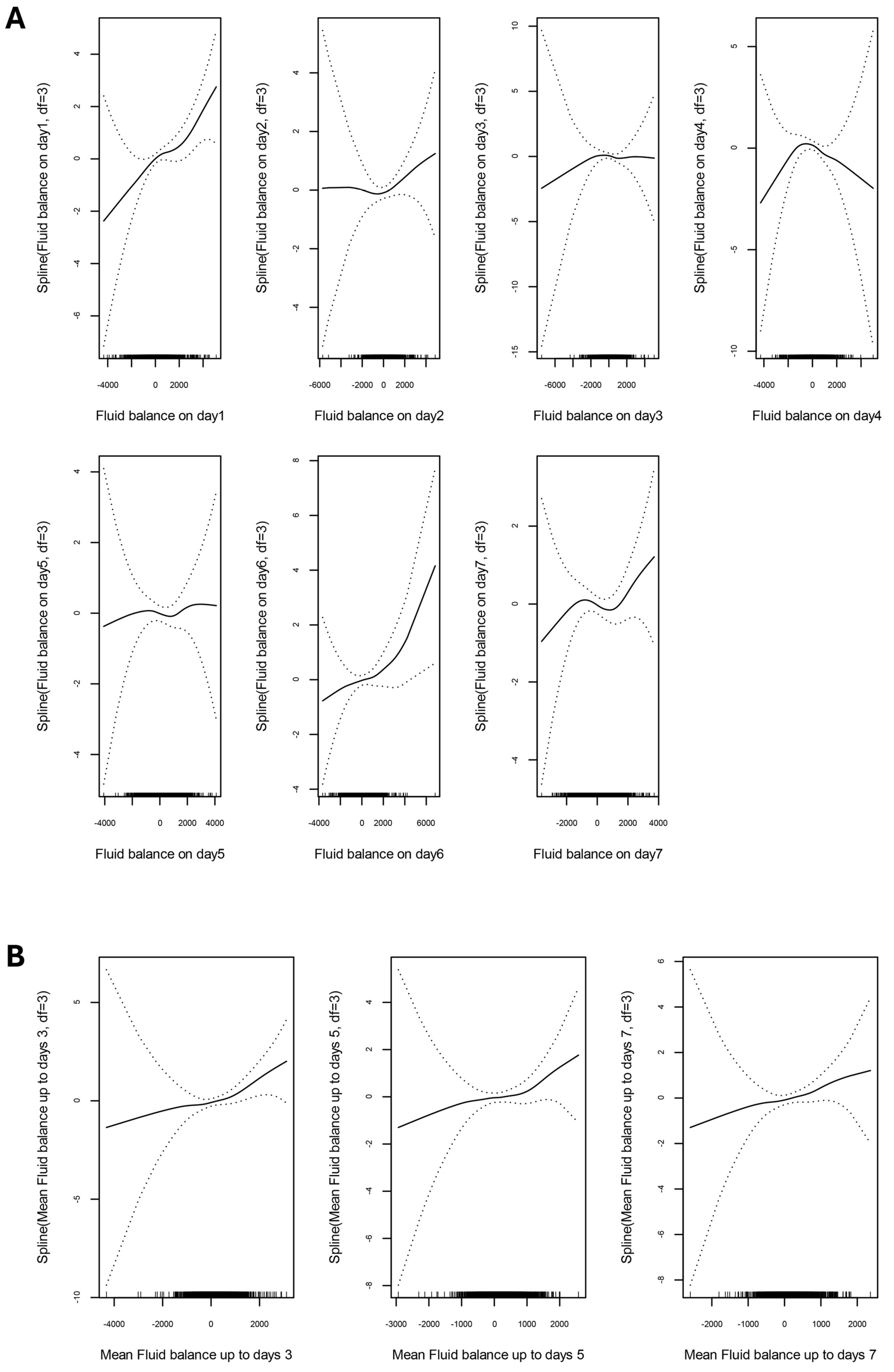
3. Results
3.1. Baseline Characteristics of the Study Population
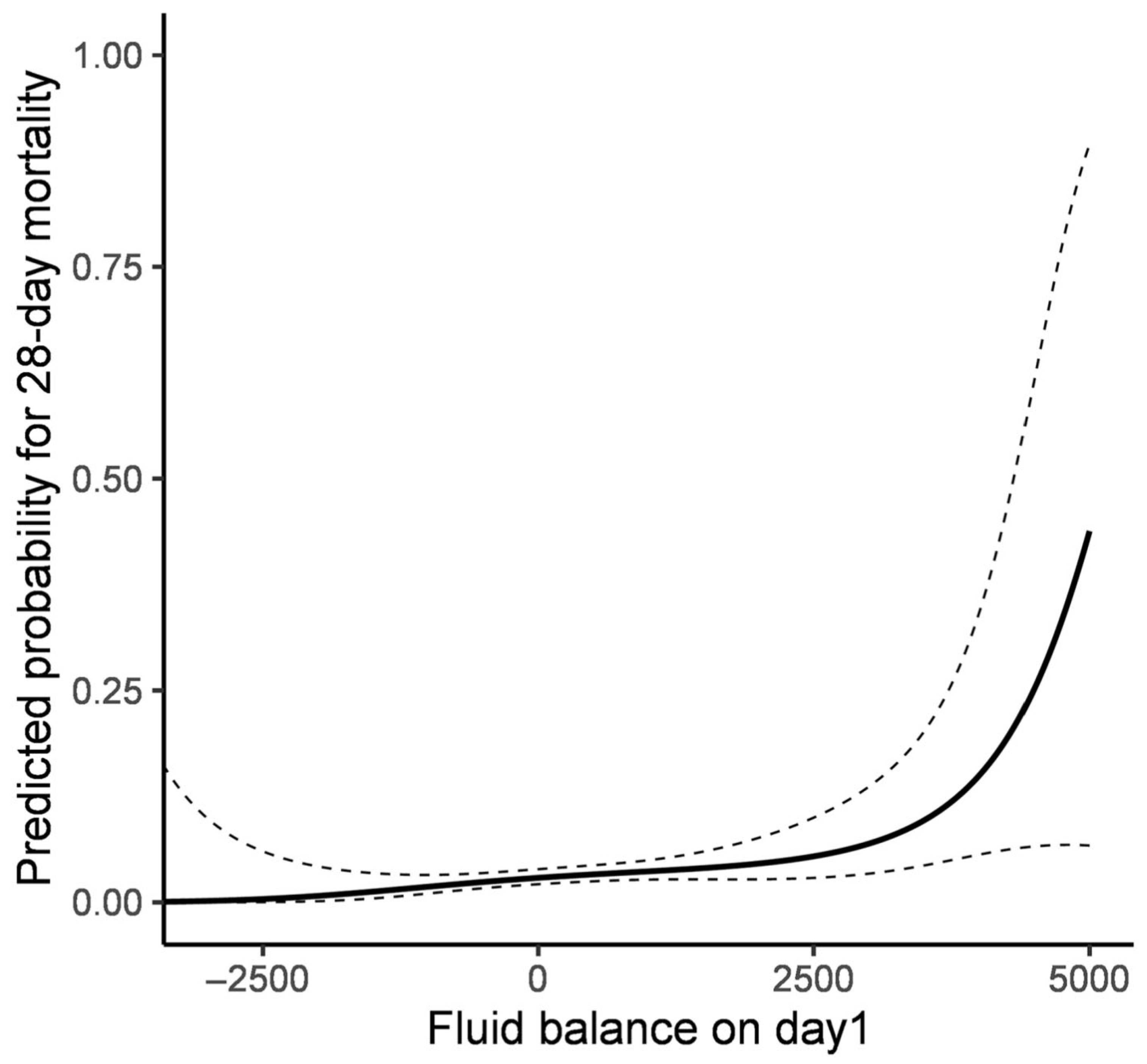
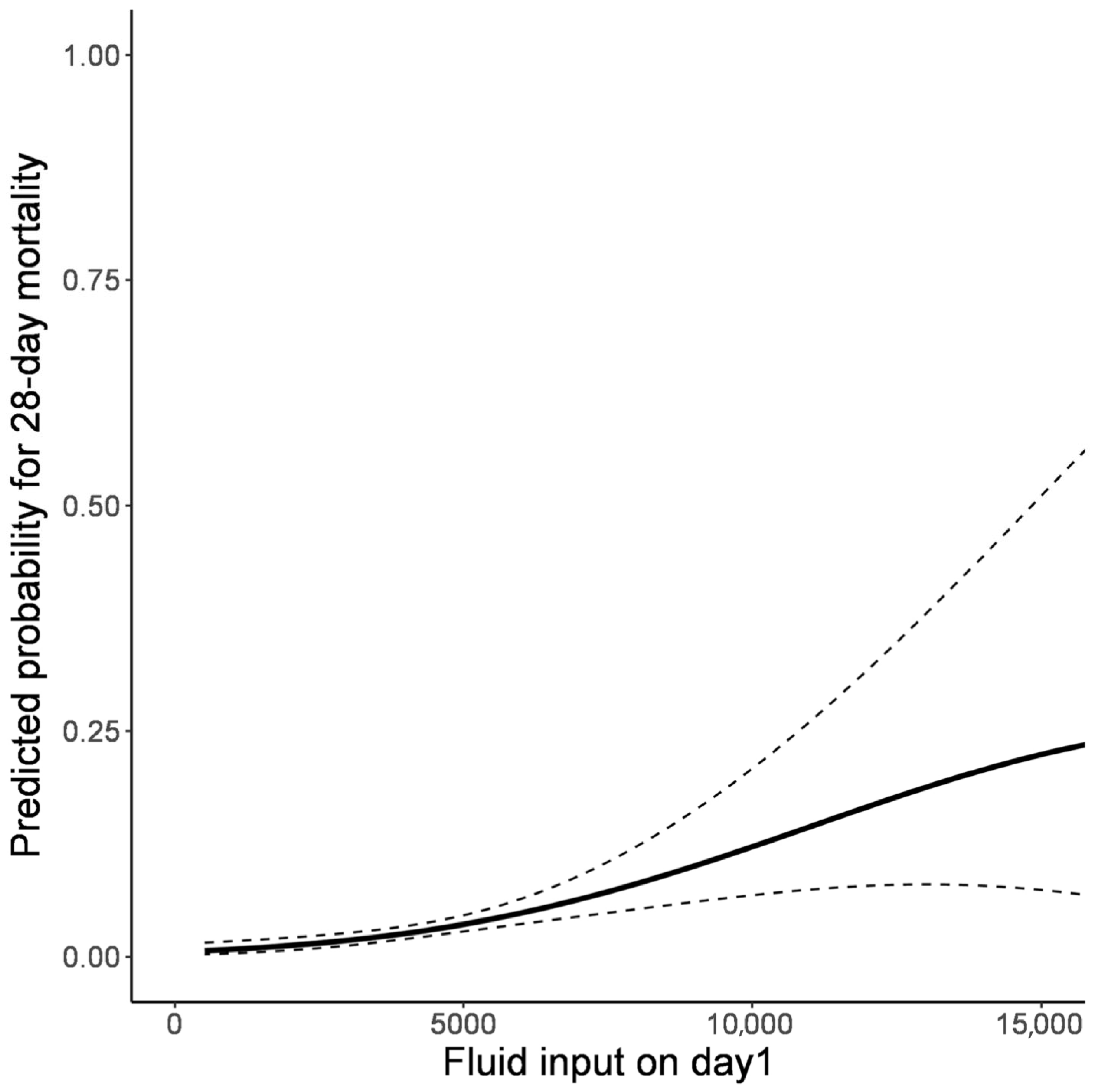
3.2. Association Between Fluid Balance and Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICU | intensive care unit |
| ICP | intracranial pressure |
| GAM | generalized additive model |
| OR | odds ratio |
| CI | confidence interval |
| APACHE | Acute Physiology and Chronic Health Evaluation |
| GCS | Glasgow Coma Scale |
References
- Mayer, S.A.; Chong, J.Y. Critical Care Management of Increased Intracranial Pressure. J. Intensive Care Med. 2002, 17, 55–67. [Google Scholar] [CrossRef]
- Zornow, M.H.; Prough, D.S. Fluid management in patients with traumatic brain injury. New Horiz. 1995, 3, 488–498. [Google Scholar]
- Treggiari, M.M.; Schutz, N.; Yanez, N.D.; Romand, J.A. Role of intracranial pressure values and patterns in predicting outcome in traumatic brain injury: A systematic review. Neurocrit. Care 2007, 6, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Clifton, G.L.; Miller, E.R.; Choi, S.C.; Levin, H.S. Fluid thresholds and outcome from severe brain injury. Crit. Care Med. 2002, 30, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K. Traumatic brain injury: Pathophysiology for neurocritical care. J. Intensive Care 2016, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Oddo, M.; Poole, D.; Helbok, R.; Meyfroidt, G.; Stocchetti, N.; Bouzat, P.; Cecconi, M.; Geeraerts, T.; Martin-Loeches, I.; Quintard, H.; et al. Fluid therapy in neurointensive care patients: ESICM consensus and clinical practice recommendations. Intensive Care Med. 2018, 44, 449–463. [Google Scholar] [CrossRef]
- Wiegers, E.J.A.; Lingsma, H.F.; Huijben, J.A.; Cooper, D.J.; Citerio, G.; Frisvold, S.; Helbok, R.; Maas, A.I.R.; Menon, D.K.; Moore, E.M.; et al. Fluid balance and outcome in critically ill patients with traumatic brain injury (CENTER-TBI and OzENTER-TBI): A prospective, multicentre, comparative effectiveness study. Lancet Neurol. 2021, 20, 627–638. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Capuzzo, M.; Valpondi, V.; Sgarbi, A.; Bortolazzi, S.; Pavoni, V.; Gilli, G.; Candini, G.; Gritti, G.; Alvisi, R. Validation of severity scoring systems SAPS II and APACHE II in a single-center population. Intensive Care Med. 2000, 26, 1779–1785. [Google Scholar] [CrossRef]
- Meredith, W.; Rutledge, R.; Fakhry, S.M.; Emery, S.; Kromhout-Schiro, S. The conundrum of the Glasgow Coma Scale in intubated patients: A linear regression prediction of the Glasgow verbal score from the Glasgow eye and motor scores. J. Trauma Inj. Infect. Crit. Care 1998, 44, 839–844, discussion 844–835. [Google Scholar] [CrossRef]
- Schoenfeld, D.A.; Bernard, G.R. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit. Care Med. 2002, 30, 1772–1777. [Google Scholar] [CrossRef] [PubMed]
- Lausen, B.; Schumacher, M. Maximally Selected Rank Statistics. Biometrics 1992, 48, 73–85. [Google Scholar] [CrossRef]
- Malbrain, M.L.; Marik, P.E.; Witters, I.; Cordemans, C.; Kirkpatrick, A.W.; Roberts, D.J.; Van Regenmortel, N. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: A systematic review with suggestions for clinical practice. Anaesthesiol. Intensive Ther. 2014, 46, 361–380. [Google Scholar] [CrossRef]
- Stulce, C.; Reisner, A.; Kane, J.M.; Shin, H.S.; McCracken, C.; Williamson, J.; Walson, K.; Paden, M. Fluid Overload in Pediatric Severe Traumatic Brain Injury. Pediatr. Crit. Care Med. 2020, 21, 164–169. [Google Scholar] [CrossRef] [PubMed]
- van der Jagt, M. Fluid management of the neurological patient: A concise review. Crit. Care 2016, 20, 126. [Google Scholar] [CrossRef]
- Munroe, E.S.; Weinstein, J.; Gershengorn, H.B.; Karlic, K.J.; Seelye, S.; Sjoding, M.W.; Valley, T.S.; Prescott, H.C. Understanding How Clinicians Personalize Fluid and Vasopressor Decisions in Early Sepsis Management. JAMA Netw. Open 2024, 7, e247480. [Google Scholar] [CrossRef]
- Chesnut, R.; Aguilera, S.; Buki, A.; Bulger, E.; Citerio, G.; Cooper, D.J.; Arrastia, R.D.; Diringer, M.; Figaji, A.; Gao, G.; et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: The Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2020, 46, 919–929. [Google Scholar] [CrossRef]
- Le Roux, P.; Menon, D.K.; Citerio, G.; Vespa, P.; Bader, M.K.; Brophy, G.M.; Diringer, M.N.; Stocchetti, N.; Videtta, W.; Armonda, R.; et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: A statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Intensive Care Med. 2014, 40, 1189–1209. [Google Scholar] [CrossRef]
- Stocchetti, N.; Maas, A.I. Traumatic intracranial hypertension. N. Engl. J. Med. 2014, 370, 2121–2130. [Google Scholar] [CrossRef]
- Rangel-Castilla, L.; Gasco, J.; Nauta, H.J.; Okonkwo, D.O.; Robertson, C.S. Cerebral pressure autoregulation in traumatic brain injury. Neurosurg. Focus. 2008, 25, E7. [Google Scholar] [CrossRef]
- Donnelly, J.; Budohoski, K.P.; Smielewski, P.; Czosnyka, M. Regulation of the cerebral circulation: Bedside assessment and clinical implications. Crit. Care 2016, 20, 129. [Google Scholar] [CrossRef]
- Andrews, P.J.; Sinclair, H.L.; Rodriguez, A.; Harris, B.A.; Battison, C.G.; Rhodes, J.K.; Murray, G.D.; Eurotherm Trial Collaborators. Hypothermia for Intracranial Hypertension after Traumatic Brain Injury. N. Engl. J. Med. 2015, 373, 2403–2412. [Google Scholar] [CrossRef]
- Wijayatilake, D.S.; Shepherd, S.J.; Sherren, P.B. Updates in the management of intracranial pressure in traumatic brain injury. Curr. Opin. Anaesthesiol. 2012, 25, 540–547. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Picetti, E.; Zoerle, T.; Carbonara, M.; Zanier, E.R.; Stocchetti, N. Fluid Management in Acute Brain Injury. Curr. Neurol. Neurosci. Rep. 2018, 18, 74. [Google Scholar] [CrossRef]
- Hutchinson, P.J.; Kolias, A.G.; Tajsic, T.; Adeleye, A.; Aklilu, A.T.; Apriawan, T.; Bajamal, A.H.; Barthélemy, E.J.; Devi, B.I.; Bhat, D.; et al. Consensus statement from the International Consensus Meeting on the Role of Decompressive Craniectomy in the Management of Traumatic Brain Injury: Consensus statement. Acta Neurochir. 2019, 161, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Ertmer, C.; Zwissler, B.; Van Aken, H.; Christ, M.; Spohr, F.; Schneider, A.; Deisz, R.; Jacob, M. Fluid therapy and outcome: A prospective observational study in 65 German intensive care units between 2010 and 2011. Ann. Intensive Care 2018, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Chalifoux, N.; Ko, T.; Slovis, J.; Spelde, A.; Kilbaugh, T.; Mavroudis, C.D. Cerebral Autoregulation: A Target for Improving Neurological Outcomes in Extracorporeal Life Support. Neurocrit. Care 2024, 41, 1055–1072. [Google Scholar] [CrossRef]

| Negative Fluid Balance (n = 932) | Positive Fluid Balance (n = 1254) | p-Value | |
|---|---|---|---|
| Patient demographics | |||
| Age (year) | 56.0 (44.0–66.0) | 58.0 (46.0–69.0) | 0.003 |
| Sex, male | 456 (48.9) | 615 (49.0) | 0.992 |
| Comorbidities | |||
| Malignancy | 602 (64.6) | 713 (56.9) | <0.001 |
| Cerebrovascular disease | 360 (38.6) | 584 (46.6) | <0.001 |
| Hypertension | 154 (16.5) | 218 (17.4) | 0.637 |
| Dyslipidemia | 114 (12.2) | 154 (12.3) | 0.999 |
| Diabetes mellitus | 91 (9.8) | 121 (9.6) | 0.987 |
| Chronic kidney disease | 25 (2.7) | 46 (3.7) | 0.244 |
| Cardiovascular disease | 19 (2.0) | 24 (1.9) | 0.959 |
| Chronic liver disease | 7 (0.8) | 31 (2.5) | 0.004 |
| Behavioral risk factors | |||
| Current alcohol consumption | 208 (22.3) | 281 (22.4) | 0.999 |
| Current smoking | 101 (10.8) | 149 (11.9) | 0.489 |
| Cause of ICU admission | 0.001 | ||
| Brain tumor | 491 (52.7) | 541 (43.1) | |
| Intracerebral hemorrhage | 101 (10.8) | 184 (14.7) | |
| Subarachnoid hemorrhage | 94 (10.1) | 141 (11.2) | |
| Traumatic brain injury | 70 (7.5) | 121 (9.6) | |
| Cerebral infarction | 32 (3.4) | 54 (4.3) | |
| Elective vascular surgery | 49 (5.3) | 98 (7.8) | |
| Central nervous system infection | 23 (2.5) | 27 (2.2) | |
| Epilepsy | 11 (1.2) | 10 (0.8) | |
| Others | 61 (6.5) | 78 (6.2) | |
| APACHE II score on ICU admission | 10.0 (7.0–14.0) | 11.0 (7.0–16.0) | <0.001 |
| GCS score on ICU admission | 14.0 (12.0–15.0) | 14.0 (11.0–15.0) | 0.108 |
| ICU management | |||
| Mechanical ventilation | 426 (45.7) | 618 (49.3) | 0.107 |
| The duration of mechanical ventilation, day | 3.0 (1.0–10.0) | 7.0 (2.0–12.0) | <0.001 |
| Continuous renal replacement therapy | 12 (1.3) | 30 (2.4) | 0.088 |
| The duration of using continuous renal replacement therapy, day | 5.5 (3.0–9.0) | 4.0 (2.0–6.0) | 0.207 |
| ICP monitoring | 318 (34.1) | 404 (32.2) | 0.374 |
| The duration of using ICP monitoring, day | 8.0 (5.0–11.0) | 8.0 (5.0–12.0) | 0.697 |
| Survivor (n = 2119) | Non-Survivor (n = 67) | p-Value | |
|---|---|---|---|
| Fluid balance on day 1 | 156.0 (−432.5–763.5) | 480.0 (−104.0–865.5) | 0.042 |
| Fluid balance on day 2 | 110.0 (−456.0–699.5) | 222.0 (−379.0–942.0) | 0.999 |
| Fluid balance on day 3 | 120.0 (−415.0–656.5) | 57.0 (−387.5–409.0) | 0.999 |
| Fluid balance on day 4 | 150.0 (−380.5–679.6) | 48.0 (−450.6–447.5) | 0.999 |
| Fluid balance on day 5 | 155.3 (−359.5–710.0) | 108.0 (−446.5–607.0) | 0.999 |
| Fluid balance on day 6 | 174.0 (−384.0–713.0) | 323.0 (−265.5–788.0) | 0.999 |
| Fluid balance on day 7 | 110.0 (−440.0–648.0) | −24.0 (−486.9–626.0) | 0.999 |
| Mean fluid balance up to day 3 | 118.2 (−249.8–499.1) | 220.0 (−48.9–620.3) | 0.219 |
| Mean fluid balance up to day 5 | 138.4 (−166.3–437.9) | 205.8 (−82.7–451.1) | 0.996 |
| Mean fluid balance up to day 7 | 128.9 (−126.6–405.4) | 238.6 (−56.9–426.8) | 0.438 |
| Fluid input on day 1 | 3978.0 (3152.5–4896.0) | 4444.0 (3668.5–6491.8) | 0.007 |
| Fluid input on day 2 | 3900.0 (3021.0–4984.0) | 4407.0 (3559.0–5304.0) | 0.112 |
| Fluid input on day 3 | 3689.0 (2816.5–4767.0) | 4476.0 (3390.5–5686.5) | 0.007 |
| Fluid input on day 4 | 3470.0 (2603.5–4555.5) | 4404.0 (3030.0–5690.4) | <0.001 |
| Fluid input on day 5 | 3280.0 (2510.0–4313.9) | 4105.0 (3018.0–5279.0) | 0.007 |
| Fluid input on day 6 | 3145.0 (2350.0–4205.0) | 3986.0 (2561.0–5038.5) | 0.007 |
| Fluid input on day 7 | 2933.0 (2029.0–3959.0) | 3410.0 (2289.5–4394.5) | 0.147 |
| Mean fluid input up to day 3 | 3905.3 (3141.3–4817.8) | 4689.0 (3671.7–5357.4) | <0.001 |
| Mean fluid input up to day 5 | 3705.2 (3006.4–4605.3) | 4644.6 (3498.5–5343.8) | <0.001 |
| Mean fluid input up to day 7 | 3513.2 (2833.6–4417.7) | 4348.6 (3308.4–5210.1) | <0.001 |
| Negative Fluid Balance (n = 932) | Positive Fluid Balance (n = 1254) | p-Value | |
|---|---|---|---|
| Ventilator-free days | 25.0 (17.0–27.0) | 21.0 (9.0–26.0) | <0.001 |
| ICU length of stay | 3.9 (2.1–7.6) | 4.0 (2.0–9.0) | 0.793 |
| Hospital length of stay | 29.9 (16.8–47.5) | 26.8 (14.5–46.0) | 0.007 |
| ICU mortality | 24 (2.6) | 56 (4.5) | 0.027 |
| 28-day mortality | 20 (2.1) | 47 (3.7) | 0.043 |
| In-hospital mortality | 41 (4.4) | 99 (7.9) | 0.001 |
| Variables | Adjusted OR (95% CI) | p-Value |
|---|---|---|
| Fluid balance on day1 | ||
| Positive value group | 1.738 (1.013–2.981) | 0.045 |
| Zero or Negative value group | Ref | |
| Age | 1.002 (0.986–1.0118) | 0.802 |
| Malignancy | 0.732 (0.419–1.278) | 0.272 |
| Cerebrovascular disease | 0.616 (0.349–1.087) | 0.095 |
| GCS ≤ 12 | 12.048 (5.682–25.641) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.Y.; Lee, S.-J.; Woo, S.-Y.; Ryu, J.-A. Early Positive Fluid Balance Associates with Increased Mortality in Neurological Critically Ill Patients: A 10-Year Cohort Study. J. Clin. Med. 2025, 14, 5518. https://doi.org/10.3390/jcm14155518
Kim DY, Lee S-J, Woo S-Y, Ryu J-A. Early Positive Fluid Balance Associates with Increased Mortality in Neurological Critically Ill Patients: A 10-Year Cohort Study. Journal of Clinical Medicine. 2025; 14(15):5518. https://doi.org/10.3390/jcm14155518
Chicago/Turabian StyleKim, Dae Yeon, Sung-Jin Lee, Sook-Young Woo, and Jeong-Am Ryu. 2025. "Early Positive Fluid Balance Associates with Increased Mortality in Neurological Critically Ill Patients: A 10-Year Cohort Study" Journal of Clinical Medicine 14, no. 15: 5518. https://doi.org/10.3390/jcm14155518
APA StyleKim, D. Y., Lee, S.-J., Woo, S.-Y., & Ryu, J.-A. (2025). Early Positive Fluid Balance Associates with Increased Mortality in Neurological Critically Ill Patients: A 10-Year Cohort Study. Journal of Clinical Medicine, 14(15), 5518. https://doi.org/10.3390/jcm14155518





