Survival and Prognostic Factors in Unresectable Head and Neck Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
- -
- Oral cavity: This includes invasion of the masticator space, skull base, pterygoid, or internal carotid artery.
- -
- Oropharynx: This includes invasion of the lateral pterygoid muscle, pterygoid, lateral nasopharynx, skull base, or carotid artery.
- -
- Hypopharynx: This includes invasion of the paratracheal fascia, carotid artery, or mediastinal structures.
- -
- Larynx: This includes invasion of the paravertebral space, carotid artery, or mediastinal structures.
- -
- Cancer of unknown primary tumours (CUPs): This includes cases of metastatic cervical adenopathy involving more than 270 degrees of circumferential involvement of the common and internal carotid artery, and invasion of the prevertebral space, mediastinum, or skull base.
Statistical Analysis
- Kaplan–Meier (KM) analysis: This is a non-parametric method for estimating the survival function over time. It shows the proportion of patients surviving (or remaining event-free) at each time point, generating a survival curve that reflects how survival probability changes over time.
- Log-rank test: This is used to compare survival between two or more groups. It evaluates whether Kaplan–Meier curves differ significantly by comparing observed versus expected events across groups. The test yields a chi-squared statistic and a p-value. If p < 0.05, there is evidence of a statistically significant difference in survival between groups; if p > 0.05, no such difference can be concluded.
- Cox proportional hazards regression model: This is a semi-parametric regression model used to estimate the effect of covariates on the time to event. It provides hazard ratios (HRs), which quantify the relative likelihood of the event occurring in one group compared to another:
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers. 2020, 6, 92, Erratum in Nat. Rev. Dis. Primers 2023, 9, 4. https://doi.org/10.1038/s41572-023-00418-5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chaturvedi, A.K.; Engels, E.A.; Anderson, W.F.; Gillison, M.L. Incidence trends for human papillomavirus-related and-unrelated oral squamous cell carcinomas in the United States. J. Clin. Oncol. 2008, 26, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Marur, S.; D’Souza, G.; Westra, W.H.; Forastiere, A.A. HPV-associated head and neck cancer: A virus-related cancer epidemic. Lancet Oncol. 2010, 11, 781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yousem, D.M.; Gad, K.; Tufano, R.P. Resectability issues with head and neck cancer. Am. J. Neuroradiol. 2006, 27, 2024–2036. [Google Scholar] [PubMed] [PubMed Central]
- Xu, P.; Liu, D.; Zhou, J.; Tang, Z.; Wang, S.; Huang, Y.; Feng, M.; Lu, S.; Lang, J.; Orlandini, L.C. Survival analysis of patients with metastatic head and neck squamous cell carcinoma treated with metastasis-directed radiotherapy and immunotherapy. Radiat. Oncol. 2025, 20, 31. [Google Scholar] [CrossRef] [PubMed]
- Hauswald, H.; Simon, C.; Hecht, S.; Debus, J.; Lindel, K. Long-term outcome and patterns of failure in patients with advanced head and neck cancer. Radiat. Oncol. 2011, 6, 70. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zolkind, P.; Lee, J.J.; Jackson, R.S.; Pipkorn, P.; Massa, S.T. Untreated head and neck cancer: Natural history and associated factors. Head Neck 2021, 43, 89–97. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shahid Iqbal, M.; Kelly, C.; Kovarik, J.; Goranov, B.; Shaikh, G.; Morgan, D.; Dobrowsky, W.; Paleri, V. Palliative radiotherapy for locally advanced non-metastatic head and neck cancer: A systematic review. Radiother. Oncol. 2018, 126, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Knegjens, J.L.; Hauptmann, M.; Pameijer, F.A.; Balm, A.J.; Hoebers, F.J.; de Bois, J.A.; Kaanders, J.H.; van Herpen, C.M.; Verhoef, C.G.; Wijers, O.B.; et al. Tumor volume as prognostic factor in chemoradiation for advanced head and neck cancer. Head Neck 2011, 33, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Stordeur, S.; Schillemans, V.; Savoye, I.; Vanschoenbeek, K.; Leroy, R.; Macq, G.; Verleye, L.; De Gendt, C.; Nuyts, S.; Vermorken, J.; et al. Comorbidity in head and neck cancer: Is it associated with therapeutic delay, post-treatment mortality and survival in a population-based study? Oral Oncol. 2020, 102, 104561. [Google Scholar] [CrossRef] [PubMed]
- Schimansky, S.; Lang, S.; Beynon, R.; Penfold, C.; Davies, A.; Waylen, A.; Thomas, S.; Pring, M.; Pawlita, M.; Waterboer, T.; et al. Association between comorbidity and survival in head and neck cancer: Results from Head and Neck 5000. Head Neck 2019, 41, 1053–1062. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williamson, A.; Lim, A.E.; Green, F.; Lee, Y.K.; Li, L.; Moen, C.; Vasanthan, R.; Wharf, O.; Wong, J.; Paleri, V. The Burden of Recurrent Head and Neck Squamous Cell Carcinoma Across the United Kingdom: Results From a National Snapshot Study. Head Neck 2025, 47, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, G.; Amin, N.; Herberg, M.E.; Maroun, C.A.; Wang, H.; Guller, M.; Gourin, C.G.; Rooper, L.M.; Vosler, P.S.; Tan, M.; et al. Association of Tumor Site with the Prognosis and Immunogenomic Landscape of Human Papillomavirus-Related Head and Neck and Cervical Cancers. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 70–79. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, E.; Mazul, A.L.; Farquhar, D.; Brennan, P.; Anantharaman, D.; Abedi-Ardekani, B.; Weissler, M.C.; Hayes, D.N.; Olshan, A.F.; Zevallos, J.P. Long-term Survival in Head and Neck Cancer: Impact of Site, Stage, Smoking, and Human Papillomavirus Status. Laryngoscope 2019, 129, 2506–2513. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Razavian, N.B. Advances in Systemic and Immunotherapies for Human Papillomavirus-Mediated Oropharyngeal Cancers. Cancer J. 2025, 31, e0766. [Google Scholar] [CrossRef] [PubMed]
- O’Rorke, M.A.; Ellison, M.V.; Murray, L.J.; Moran, M.; James, J.; Anderson, L.A. Human papillomavirus related head and neck cancer survival: A systematic review and meta-analysis. Oral Oncol. 2012, 48, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Gormley, M.; Creaney, G.; Schache, A.; Ingarfield, K.; Conway, D.I. Reviewing the epidemiology of head and neck cancer: Definitions, trends and risk factors. Br. Dent. J. 2022, 233, 780–786. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hobbs, C.G.; Sterne, J.A.; Bailey, M.; Heyderman, R.S.; Birchall, M.A.; Thomas, S.J. Human papillomavirus and head and neck cancer: A systematic review and meta-analysis. Clin. Otolaryngol. 2006, 31, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heck, J.E.; Berthiller, J.; Vaccarella, S.; Winn, D.M.; Smith, E.M.; Shan’gina, O.; Schwartz, S.M.; Purdue, M.P.; Pilarska, A.; Eluf-Neto, J.; et al. Sexual behaviours and the risk of head and neck cancers: A pooled analysis in the International Head and Neck Cancer Epidemiology (INHANCE) consortium. Int. J. Epidemiol. 2010, 39, 166–181. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schache, A.G.; Powell, N.G.; Cuschieri, K.S.; Robinson, M.; Leary, S.; Mehanna, H.; Rapozo, D.; Long, A.; Cubie, H.; Junor, E.; et al. HPV-Related Oropharynx Cancer in the United Kingdom: An Evolution in the Understanding of Disease Etiology. Cancer Res. 2016, 76, 6598–6606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehanna, H.; Franklin, N.; Compton, N.; Robinson, M.; Powell, N.; Biswas-Baldwin, N.; Paleri, V.; Hartley, A.; Fresco, L.; Al-Booz, H.; et al. Geographic variation in human papillomavirus-related oropharyngeal cancer: Data from 4 multinational randomized trials. Head Neck 2016, 38, E1863–E1869. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anantharaman, D.; Abedi-Ardekani, B.; Beachler, D.C.; Gheit, T.; Olshan, A.F.; Wisniewski, K.; Wunsch-Filho, V.; Toporcov, T.N.; Tajara, E.H.; Levi, J.E.; et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int. J. Cancer 2017, 140, 1968–1975. [Google Scholar] [CrossRef] [PubMed]
- Anantharaman, D.; Muller, D.C.; Lagiou, P.; Ahrens, W.; Holcátová, I.; Merletti, F.; Kjærheim, K.; Polesel, J.; Simonato, L.; Canova, C.; et al. Combined effects of smoking and HPV16 in oropharyngeal cancer. Int. J. Epidemiol. 2016, 45, 752–761. [Google Scholar] [CrossRef]
- Genden, E.M.; Sambur, I.M.; de Almeida, J.R.; Posner, M.; Rinaldo, A.; Rodrigo, J.P.; Strojan, P.; Takes, R.P.; Ferlito, A. Human papillomavirus and oropharyngeal squamous cell carcinoma: What the clinician should know. Eur. Arch. Otorhinolaryngol. 2013, 270, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, J.N.; Zhao, S.J.; Peng, L.P.; Mo, D.C.; Yin, S.H. Efficacy and safety of PD-1/PD-L1 inhibitors combined with standard of care for locally advanced head and neck squamous cell carcinoma: A meta-analysis of randomized controlled trials. Crit. Rev. Oncol. Hematol. 2025, 209, 104668. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Shi, K.; Chen, G.; Xu, W.; Qiu, L.; Fei, Y.; Zhu, Y.; Wu, M.; Li, Y.; Sun, X.; et al. A Network Meta-Analysis of the Systemic Therapies in Unresectable Head and Neck Squamous Cell Carcinoma. Cancer Control. 2024, 31, 10732748241255535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Michelon, I.; Nachtigal, G.C.; Dacoregio, M.I.; Moraes, A.C.B.K.; Moraes, M.; Piva, L.S.; da Costa, C.T.; Lund, R.G.; Michelon, D. Treatment options for cisplatin-ineligible patients with locally advanced head and neck squamous cell carcinoma: A systematic review. J. Cancer Res. Clin. Oncol. 2024, 150, 379. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kessoku, H.; Mizunari, Y. Outcomes and Prognostic Factors in Patients With Untreated Head and Neck Squamous Cell Carcinoma. Cureus 2025, 17, e77141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheraghlou, S.; Kuo, P.; Mehra, S.; Yarbrough, W.G.; Judson, B.L. Untreated oral cavity cancer: Long-term survival and factors associated with treatment refusal. Laryngoscope 2018, 128, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Sjöstedt, S.; Garset-Zamani, M.; Korsholm, K.; Agander, T.K.; Todsen, T.; Wessel, I. HPV-Positive Squamous Cell Carcinoma Metastasis Without Clinical Evidence of Primary: The Posterior Oropharyngeal Wall May Be an Overlooked Location. Head Neck 2025. [Google Scholar] [CrossRef] [PubMed]
- Hardman, J.C.; Constable, J.; Dobbs, S.; Hogan, C.; Hulse, K.; Khosla, S.; Milinis, K.; Tudor-Green, B.; Williamson, A.; Paleri, V.; et al. Survival outcomes in head and neck squamous cell carcinoma of unknown primary: A national cohort study. Clin. Otolaryngol. 2024, 49, 604–620. [Google Scholar] [CrossRef] [PubMed]
| Overall | ||
|---|---|---|
| n | 39 | |
| Sex | Male | 32 (82.1%) |
| Female | 7 (17.9%) | |
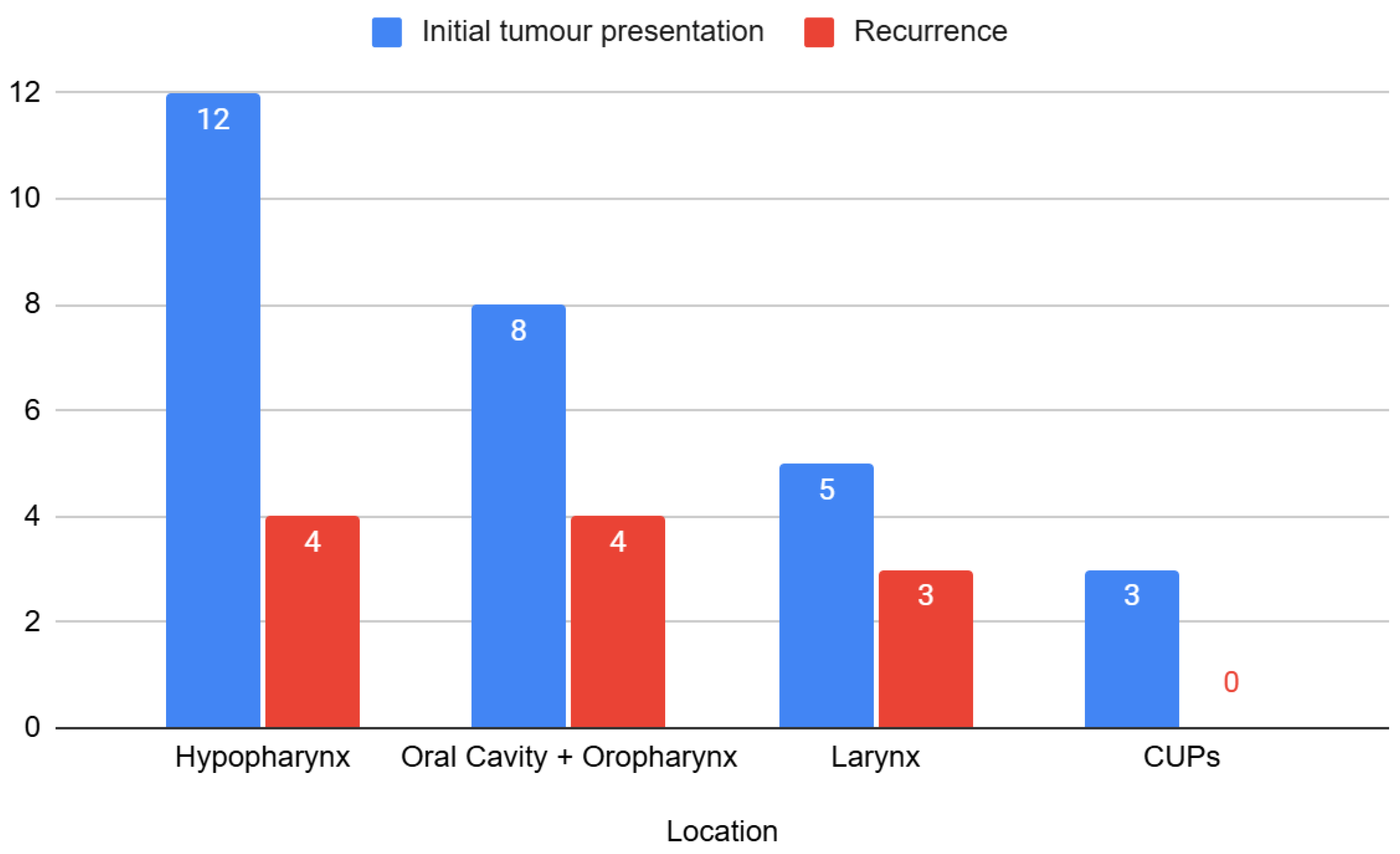
| Tumour location | Hypopharynx | 16 (41.0%) |
| Oral cavity + oropharynx | 12 (30.8%) | |
| CUPs | 3 (7.7%) | |
| Larynx | 8 (20.5%) | |
| Initial tumour presentation or recurrence | Initial | 27 (69.2%) |
| Recurrence | 12 (30.8%) | |
| HPV-positive | Yes | 8 (20.5%) |
| No | 31 (79.5%) | |
| Radiotherapy | Yes | 31 (79.5%) |
| No | 8 (20.5%) | |
| Chemotherapy | Yes | 29 (74.4%) |
| No | 10 (25.6%) | |
| Immunotherapy | No | 35 (89.7%) |
| Yes | 4 (10.3%) | |
| Event (death) | No | 17 (43.6%) |
| Yes | 22 (56.4%) | |
| Cause of death | Tumour progression | 15 (38.5%) |
| Opportunistic diseases/comorbidities | 3 (7.7%) | |
| Not Applicable | 21 (53.8%) | |
| Time (median [iqr]) | 16.05 [7.30, 40.20] | |
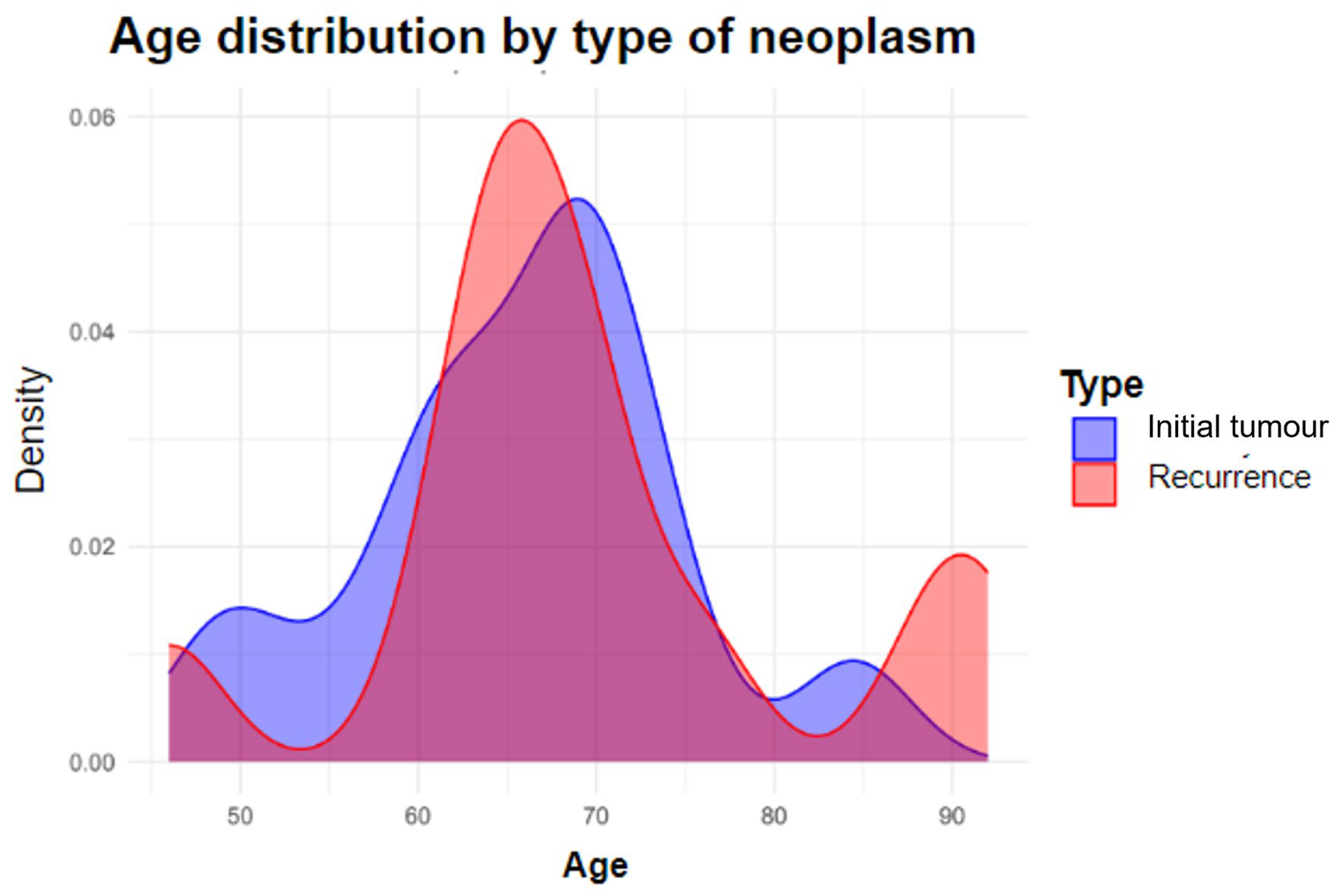
| Age (mean (sd)) | 66.87 (10.08) |
| Overall | No | Yes | p | ||
|---|---|---|---|---|---|
| n (%) | 39 | 17 | 22 | ||
| Sex | Male | 32 (82.1) | 16 (94.1) | 16 (72.7) | 0 |
| Female | 7 (17.9) | 1 (5.9) | 6 (27.3) | ||
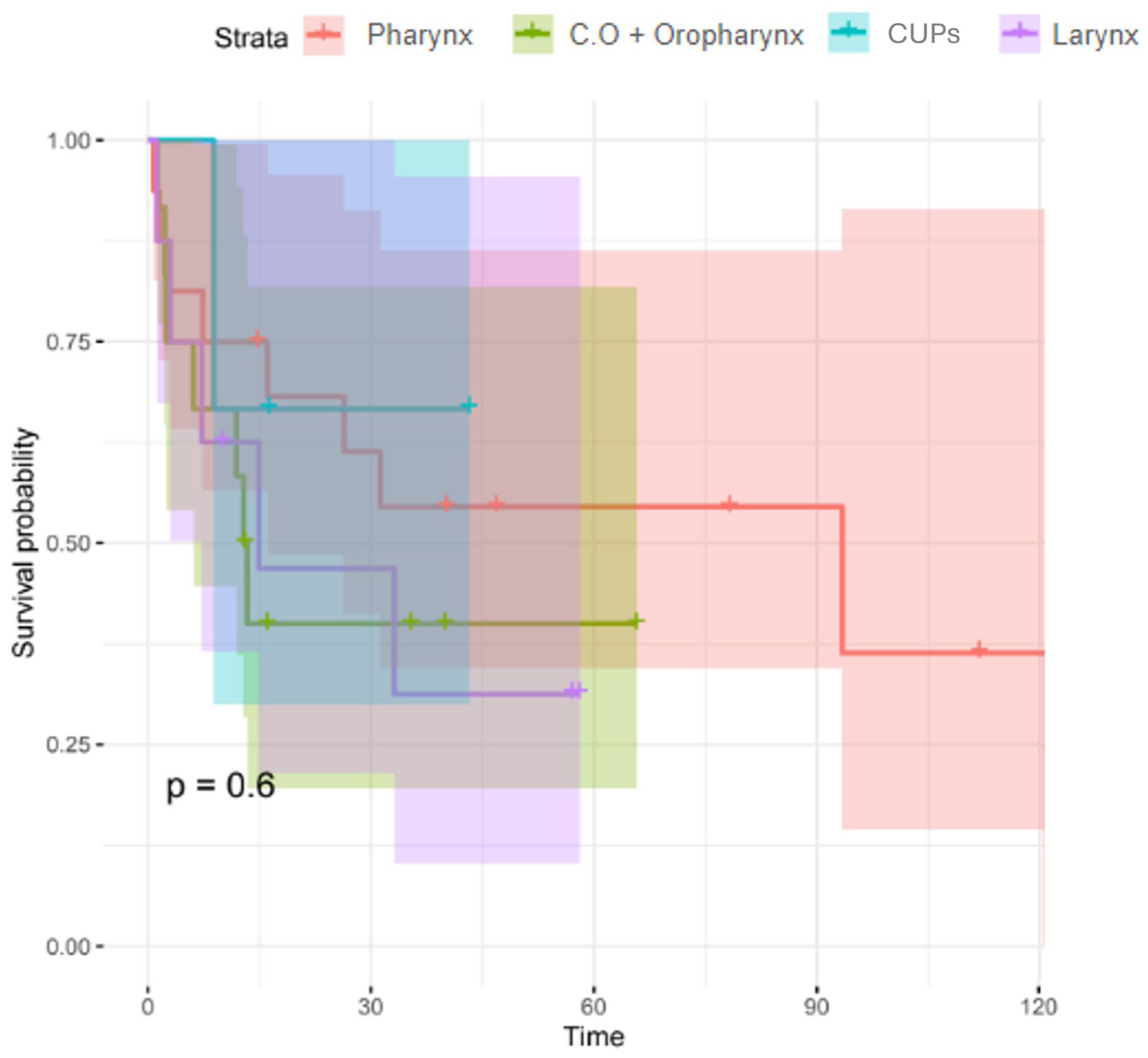
| Location of neoplasm | Hypopharynx | 16 (41) | 7 (41.2) | 9 (41) | 0.864 |
| Oral cavity + Oropharynx | 12 (30.8) | 5 (29.4) | 7 (31.8) | ||
| CUPs | 3 (7.7) | 2 (11.8) | 1 (4.5) | ||
| Larynx | 8 (20.5) | 3 (17.6) | 5 (22.7) | ||
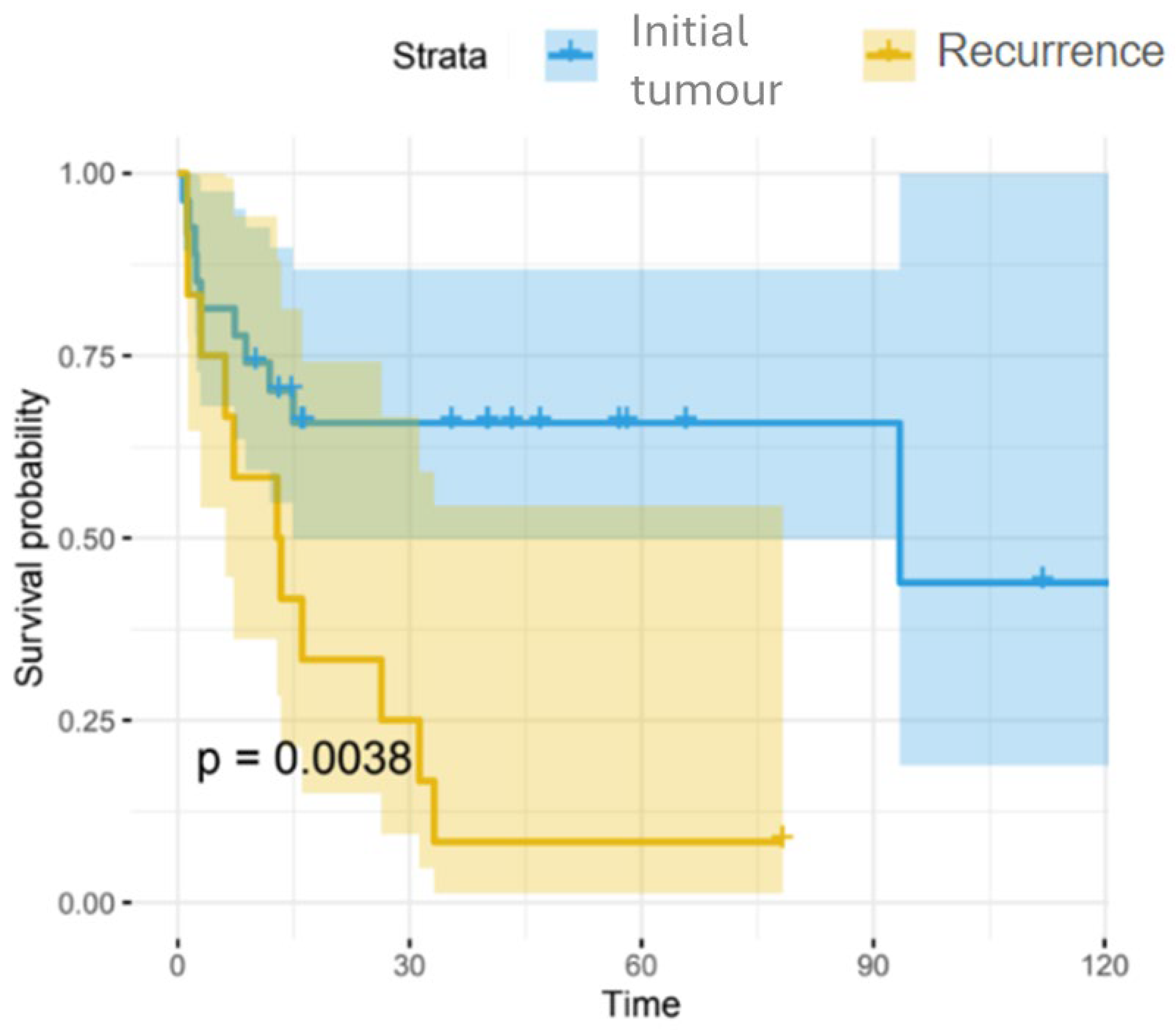
| Tumour presentation | Initial | 27 (69.2) | 16 (94.1) | 11 (50) | 0.004 |
| Recurrence | 12 (30.8) | 1 (5.9) | 11 (50) | ||
| HPV status | Yes | 8 (20.5) | 7 (41.2) | 1 (4.5) | 0.013 |
| No | 31 (79.5) | 10 (58.8) | 21 (95.5) | ||
| Event of death | Yes | 22 (56.4) | 0 (0) | 22 (100) | <0.001 |
| No | 17 (43.6) | 17 (100) | 0 (0) | ||
| Cause of death | Tumour progression | 15 (83.3) | 0 (0) | 15 (83.3) | 1 |
| Opportunistic or concomitant diseases | 3 (16.7) | 0 (nan) | 3 (16.7) | ||
| Time (median [iqr]) | 16.05 [7.30, 40.20] | 40.20 [16.28, 57.11] | 8.09 [2.57, 15.80] | <0.001 | |
| Age (mean (sd)) | 66.87 (10.08) | 63.29 (9.41) | 69.64 (9.91) | 0.05 |
| Overall | No | Yes | p | ||
|---|---|---|---|---|---|
| n | 12 | 1 | 11 | ||
| Sex | Male | 7 (58.3) | 1 (100.0) | 6 (54.5) | 1.0001 |
| Female | 5 (41.7) | 0 (0.0) | 5 (45.5) | ||
| HPV-positive | No | 12 (100.0) | 1 (100.0) | 11 (100.0) | NA |
| Event of death | No | 1 (8.3) | 1 (100.0) | 0 (0.0) | 0.083 |
| Yes | 11 (91.7) | 0 (0.0) | 11 (100.0) | ||
| Time (median [IQR]) | 13.11 [5.36, 27.57] | 78.26 [78.26, 78.26] | 12.86 [4.57, 21.22] | 0.111 | |
| Age (mean (SD) | 69.25 (12.24) | 65.00 (NA) | 69.64 (12.76) | NA |
| Overall | No | Yes | p | ||
|---|---|---|---|---|---|
| n | 24 | 14 | 10 | ||
| Sex (%) | Male | 22 (91.7) | 13 (92.9) | 9 (90.0) | 1.000 |
| Female | 2 (8.3) | 1 (7.1) | 1 (10.0) | ||
| HPV-positive (%) | Yes | 8 (33.3) | 7 (50.0) | 1 (10.0) | 0.079 |
| No | 16 (66.7) | 7 (50.0) | 9 (90.0) | ||
| Event of death (%) | No | 14 (58.3) | 14 (100.0) | 0 (0.0) | <0.001 |
| Yes | 10 (41.7) | 0 (0.0) | 10 (100.0) | ||
| Time (median [IQR]) | 25.72 [9.39, 49.46] | 40.20 [20.89, 54.56] | 5.16 [2.29, 14.18] | 0.022 | |
| Age (mean (SD) | 66.21 (9.42) | 63.14 (10.27) | 70.50 (6.26) | 0.057 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oishi, N.; Orozco-Núñez, S.; Alba-García, J.R.; Gimeno-Coret, M.; Zapater, E. Survival and Prognostic Factors in Unresectable Head and Neck Cancer Patients. J. Clin. Med. 2025, 14, 5517. https://doi.org/10.3390/jcm14155517
Oishi N, Orozco-Núñez S, Alba-García JR, Gimeno-Coret M, Zapater E. Survival and Prognostic Factors in Unresectable Head and Neck Cancer Patients. Journal of Clinical Medicine. 2025; 14(15):5517. https://doi.org/10.3390/jcm14155517
Chicago/Turabian StyleOishi, Natsuki, Sara Orozco-Núñez, José Ramón Alba-García, Mar Gimeno-Coret, and Enrique Zapater. 2025. "Survival and Prognostic Factors in Unresectable Head and Neck Cancer Patients" Journal of Clinical Medicine 14, no. 15: 5517. https://doi.org/10.3390/jcm14155517
APA StyleOishi, N., Orozco-Núñez, S., Alba-García, J. R., Gimeno-Coret, M., & Zapater, E. (2025). Survival and Prognostic Factors in Unresectable Head and Neck Cancer Patients. Journal of Clinical Medicine, 14(15), 5517. https://doi.org/10.3390/jcm14155517







