Postoperative Recovery of Balance Function in Lumbar Spinal Stenosis: A 12-Month Longitudinal Study Using the Brief BESTest and Its Association with Patient-Reported Outcomes
Abstract
1. Introduction
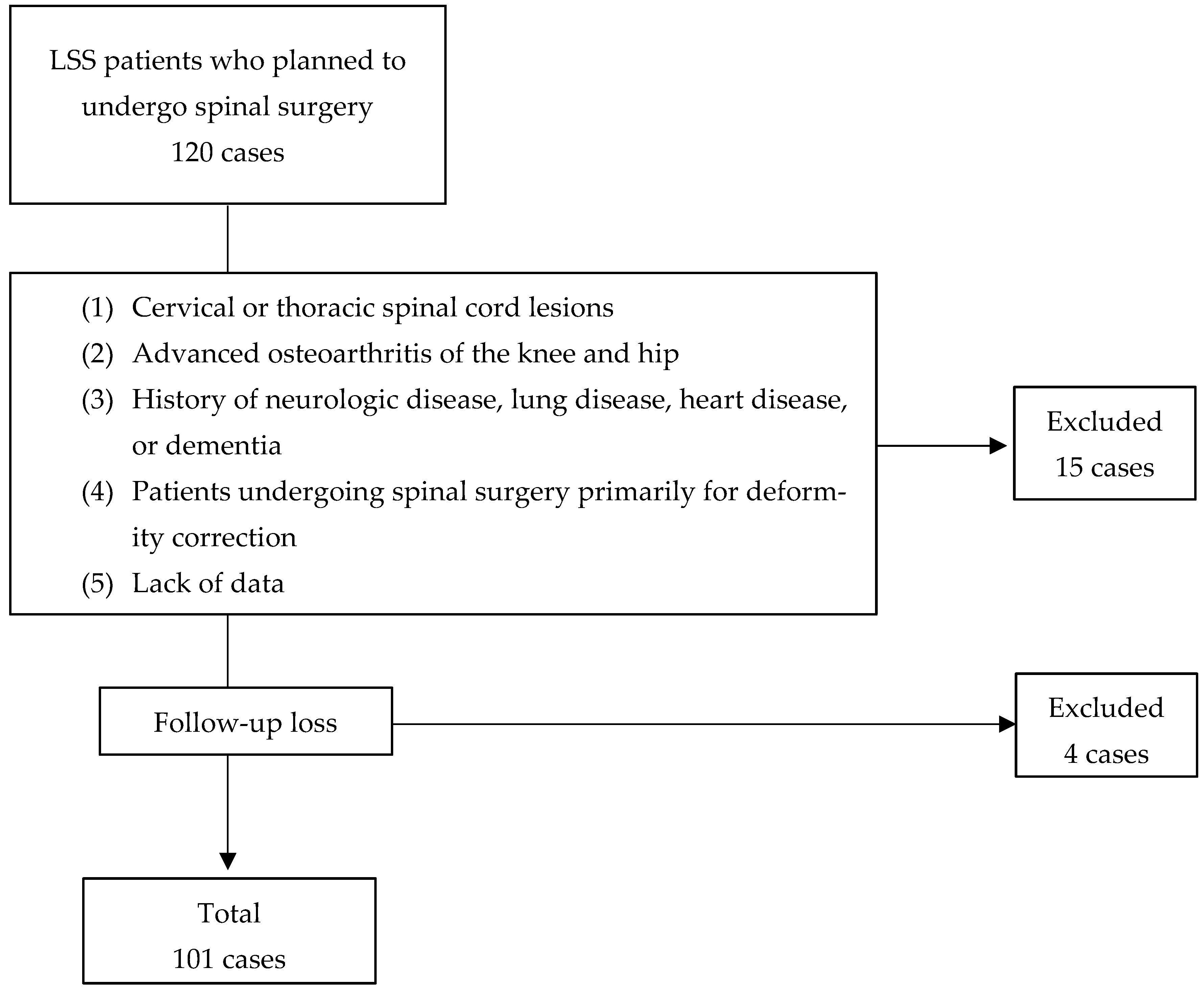
2. Materials and Methods
2.1. Patient and Surgical Factors
2.2. Brief Balance Evaluation Systems Test (BESTest)
2.3. Oswestry Disability Index (ODI)
2.4. Modified Falls Efficacy Scale (MFES)
2.5. Zurich Claudication Questionnaire (ZCQ)
2.6. Pain Assessment
2.7. Statistical Analysis
3. Results
3.1. Patient and Surgical Factors
3.2. Changes in Brief BESTest
3.3. Changes in ODI
3.4. Changes in MFES
3.5. Changes in ZCQ
3.6. Changes in Pain and Numbness
3.7. Correlation Between Brief BESTest and Patient-Reported Outcome Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdou, A.; Kades, S.; Masri-zada, T.; Asim, S.; Bany-Mohammed, M.; Agrawal, D.K. Lumbar Spinal Stenosis: Pathophysiology, Biomechanics, and Innovations in Diagnosis and Management. J. Spine Res. Surg. 2025, 7, 1–17. [Google Scholar] [CrossRef]
- Steurer, J.; Nydegger, A.; Held, U.; Brunner, F.; Hodler, J.; Porchet, F.; Min, K.; Mannion, A.F.; Michel, B. LumbSten Research Collaboration LumbSten: The lumbar spinal stenosis outcome study. BMC Musculoskelet. Disord. 2010, 11, 254. [Google Scholar] [CrossRef]
- Kim, H.-J.; Chun, H.-J.; Han, C.-D.; Moon, S.-H.; Kang, K.-T.; Kim, H.-S.; Park, J.-O.; Moon, E.-S.; Kim, B.-R.; Sohn, J.-S.; et al. The risk assessment of a fall in patients with lumbar spinal stenosis. Spine 2011, 36, E588–E592. [Google Scholar] [CrossRef]
- Gautschi, O.P.; Joswig, H.; Corniola, M.V.; Smoll, N.R.; Schaller, K.; Hildebrandt, G.; Stienen, M.N. Pre- and postoperative correlation of patient-reported outcome measures with standardized Timed Up and Go (TUG) test results in lumbar degenerative disc disease. Acta Neurochir. 2016, 158, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Uysal, İ.; Özden, F.; Tümtürk, İ.; Şimşek, M. Does physical performance demonstrate patient-reported outcomes after lumbar spine surgery? BMC Musculoskelet. Disord. 2024, 25, 1000. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-I.; Lin, R.-M. Disability and walking capacity in patients with lumbar spinal stenosis: Association with sensorimotor function, balance, and functional performance. J. Orthop. Sports Phys. Ther. 2005, 35, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Magnani, P.E.; Porto, J.M.; Genovez, M.B.; Zanellato, N.F.G.; Alvarenga, I.C.; Dos Santos, P.F.; de Abreu, D.C.C. What is the best clinical assessment tool for identification of adults aged ≥80 years at high risk of falls? Physiotherapy 2021, 110, 63–69. [Google Scholar] [CrossRef]
- Papadakis, N.C.; Christakis, D.G.; Tzagarakis, G.N.; Chlouverakis, G.I.; Kampanis, N.A.; Stergiopoulos, K.N.; Katonis, P.G. Gait variability measurements in lumbar spinal stenosis patients: Part B. Preoperative versus postoperative gait variability. Physiol. Meas. 2009, 30, 1187–1195. [Google Scholar] [CrossRef]
- Wong, W.-J.; Lai, D.-M.; Wang, S.-F.; Wang, J.-L.; Hsu, W.-L. Changes of balance control in individuals with lumbar degenerative spine disease after lumbar surgery: A longitudinal study. Spine J. 2019, 19, 1210–1220. [Google Scholar] [CrossRef]
- Horak, F.B.; Wrisley, D.M.; Frank, J. The Balance Evaluation Systems Test (BESTest) to differentiate balance deficits. Phys. Ther. 2009, 89, 484–498. [Google Scholar] [CrossRef]
- Chan, A.C.M.; Pang, M.Y.C.; Ouyang, H.; Jehu, D.A.M. Minimal Clinically Important Difference of Four Commonly Used Balance Assessment Tools in Individuals after Total Knee Arthroplasty: A Prospective Cohort Study. PM R 2020, 12, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Kondo, Y.; Bando, K.; Hara, T.; Takahashi, Y. Structural Validity of the Mini-Balance Evaluation Systems Test in Individuals With Spinocerebellar Ataxia: A Rasch Analysis Study. Arch. Phys. Med. Rehabil. 2024, 105, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Padgett, P.K.; Jacobs, J.V.; Kasser, S.L. Is the BESTest at its best? A suggested brief version based on interrater reliability, validity, internal consistency, and theoretical construct. Phys. Ther. 2012, 92, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, T.; Heyder, A.; Tanaka, M.; Uotani, K.; Omori, T.; Kodama, Y.; Takamatsu, K.; Yasuda, Y.; Sugyo, A.; Takeda, M.; et al. Rehabilitation to Improve Outcomes after Cervical Spine Surgery: Narrative Review. J. Clin. Med. 2024, 13, 5363. [Google Scholar] [CrossRef]
- Fujiwara, A.; Kobayashi, N.; Saiki, K.; Kitagawa, T.; Tamai, K.; Saotome, K. Association of the Japanese Orthopaedic Association score with the Oswestry Disability Index, Roland-Morris Disability Questionnaire, and short-form 36. Spine 2003, 28, 1601–1607. [Google Scholar] [CrossRef]
- Hill, K.D.; Schwarz, J.A.; Kalogeropoulos, A.J.; Gibson, S.J. Fear of falling revisited. Arch. Phys. Med. Rehabil. 1996, 77, 1025–1029. [Google Scholar] [CrossRef]
- Stucki, G.; Daltroy, L.; Liang, M.H.; Lipson, S.J.; Fossel, A.H.; Katz, J.N. Measurement properties of a self-administered outcome measure in lumbar spinal stenosis. Spine 1996, 21, 796–803. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Güneş, M.; Apaydın, A.S.; Keski̇n, N.K. Investigation of lumbar multifidus muscle, pain, and fear of falling in patients with lumbar spinal stenosis with poor balance. Clin. Neurol. Neurosurg. 2024, 246, 108578. [Google Scholar] [CrossRef]
- Wada, T.; Kitsuda, Y.; Tanishima, S.; Osumi, M.; Takeda, C.; Osaki, M.; Nagashima, H. Association between lumbar spine kinematics and falls in patients with lumbar spinal stenosis: A cross-sectional study. Eur. Spine J. 2025, 34, 1562–1568. [Google Scholar] [CrossRef]
- Chiu, A.Y.Y.; Pang, M.Y.C. Assessment of Psychometric Properties of Various Balance Assessment Tools in Persons With Cervical Spondylotic Myelopathy. J. Orthop. Sports Phys. Ther. 2017, 47, 673–682. [Google Scholar] [CrossRef]
- Thornes, E.; Robinson, H.S.; Vøllestad, N.K. Dynamic balance in patients with degenerative lumbar spinal stenosis; a cross-sectional study. BMC Musculoskelet. Disord. 2018, 19, 192. [Google Scholar] [CrossRef]
- Nascimento, M.B.; Vilarinho, L.G.; Lobato, D.F.M.; Dionisio, V.C. Role of gluteus maximus and medius activation in the lower limb biomechanical control during functional single-leg Tasks: A systematic review. Knee 2023, 43, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Chong, F.; Fan, W.; Liu, L.; Zhang, Y.; Lin, H.; Huang, B. Correlation Between Atrophy of the Gluteus Medius Muscle and Symptoms of Lumbar Spinal Stenosis. World Neurosurg. 2023, 172, e177–e184. [Google Scholar] [CrossRef] [PubMed]
- Pasma, J.H.; Engelhart, D.; Maier, A.B.; Schouten, A.C.; van der Kooij, H.; Meskers, C.G.M. Changes in sensory reweighting of proprioceptive information during standing balance with age and disease. J. Neurophysiol. 2015, 114, 3220–3233. [Google Scholar] [CrossRef] [PubMed]
- Kneis, S.; Bruetsch, V.; Dalin, D.; Hubbe, U.; Maurer, C. Altered postural timing and abnormally low use of proprioception in lumbar spinal stenosis pre- and post- surgical decompression. BMC Musculoskelet. Disord. 2019, 20, 183. [Google Scholar] [CrossRef]
- Stienen, M.N.; Joswig, H.; Smoll, N.R.; Corniola, M.V.; Schaller, K.; Hildebrandt, G.; Gautschi, O.P. Influence of Body Mass Index on Subjective and Objective Measures of Pain, Functional Impairment, and Health-Related Quality of Life in Lumbar Degenerative Disc Disease. World Neurosurg. 2016, 96, 570–577.e1. [Google Scholar] [CrossRef]
- Gautschi, O.P.; Smoll, N.R.; Corniola, M.V.; Joswig, H.; Chau, I.; Hildebrandt, G.; Schaller, K.; Stienen, M.N. Validity and Reliability of a Measurement of Objective Functional Impairment in Lumbar Degenerative Disc Disease: The Timed Up and Go (TUG) Test. Neurosurgery 2016, 79, 270–278. [Google Scholar] [CrossRef]
- Park, S.; Han, H.S.; Kim, G.-U.; Kang, S.S.; Kim, H.-J.; Lee, M.; Park, S.H.; Choi, K.H.; Kim, S.-H.; Yeom, J.S. Relationships among Disability, Quality of Life, and Physical Fitness in Lumbar Spinal Stenosis: An Investigation of Elderly Korean Women. Asian Spine J. 2017, 11, 256–263. [Google Scholar] [CrossRef]
- Schenkman, M.; Morey, M.; Kuchibhatla, M. Spinal flexibility and balance control among community-dwelling adults with and without Parkinson’s disease. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M441–M445. [Google Scholar] [CrossRef]
- Halvarsson, A.; Dohrn, I.-M.; Ståhle, A. Taking balance training for older adults one step further: The rationale for and a description of a proven balance training programme. Clin. Rehabil. 2015, 29, 417–425. [Google Scholar] [CrossRef]
- Copay, A.G.; Glassman, S.D.; Subach, B.R.; Berven, S.; Schuler, T.C.; Carreon, L.Y. Minimum clinically important difference in lumbar spine surgery patients: A choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. 2008, 8, 968–974. [Google Scholar] [CrossRef]
- Fukushima, M.; Oka, H.; Oshima, Y.; Yuzawa, Y.; Matsudaira, K.; Tanaka, S.; Inanami, H. Evaluation of the Minimum Clinically Important Differences of the Zurich Claudication Questionnaire in Patients With Lumbar Spinal Stenosis. Clin. Spine Surg. 2020, 33, E499–E503. [Google Scholar] [CrossRef]
- Stucki, G.; Liang, M.H.; Fossel, A.H.; Katz, J.N. Relative responsiveness of condition-specific and generic health status measures in degenerative lumbar spinal stenosis. J. Clin. Epidemiol. 1995, 48, 1369–1378. [Google Scholar] [CrossRef]
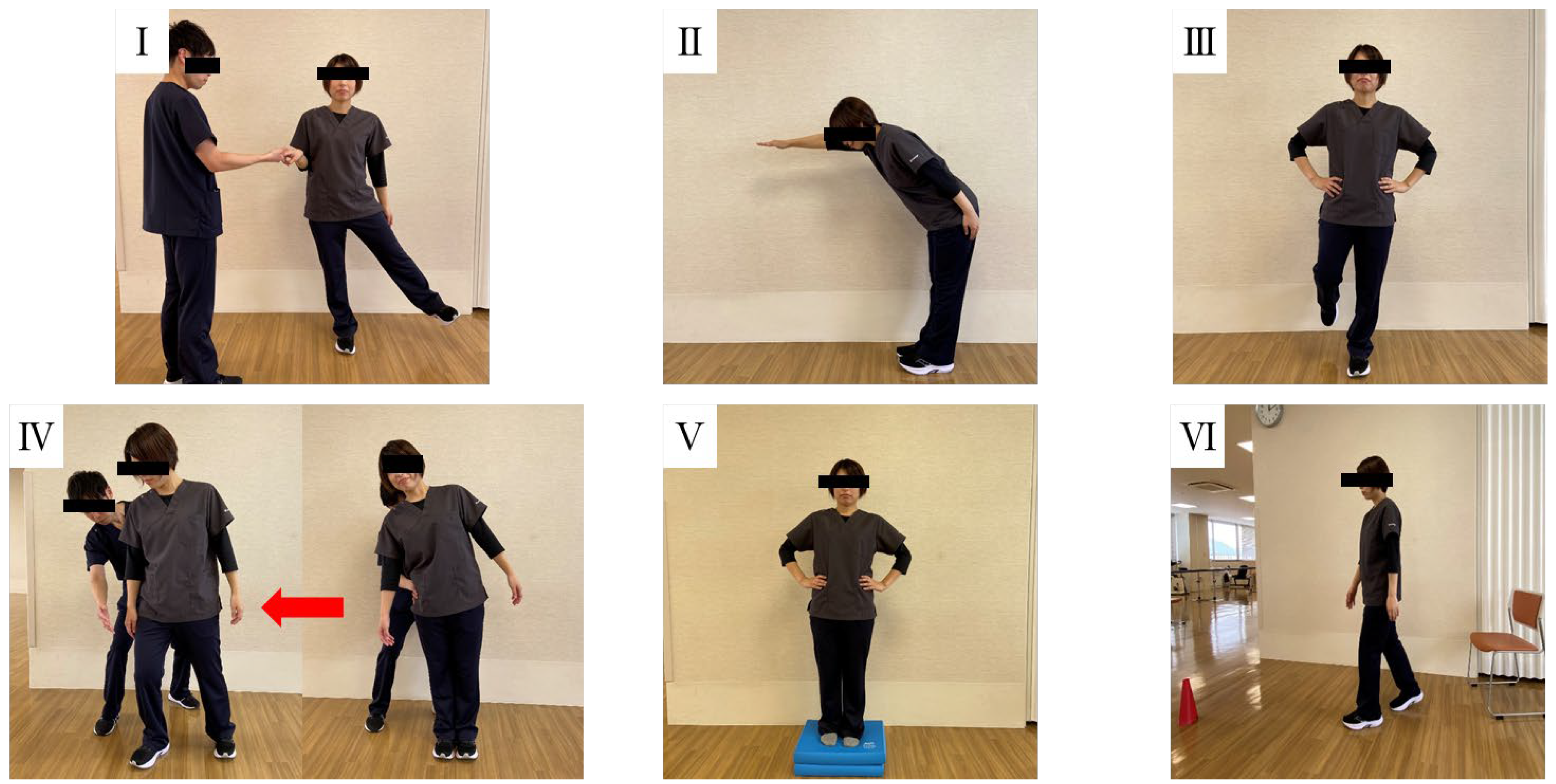
| Domain No. | Domain Name | Task Description |
|---|---|---|
| (I) | Biomechanical constraints | Hip/trunk lateral strength |
| (II) | Stability limits | Functional reach forward |
| (III) | Anticipatory postural adjustments | Stand on one leg (left and right) |
| (IV) | Postural responses | Compensatory stepping correction, lateral (left and right) |
| (V) | Sensory orientation | Stance on foam, eyes closed |
| (VI) | Stability in gait | Timed “Get Up and Go” test |
| Mean ± SD; n | |
|---|---|
| Age (years) | 74.9 ± 6.9 |
| Sex | |
| Male | 60 |
| Female | 41 |
| Body Mass Index (kg/m2) | 24.6 ± 3.4 |
| Surgical Type | |
| Decompression | 64 |
| Fusion | 37 |
| Surgical segment | |
| 1 | 65 |
| ≥2 | 36 |
| Surgical levels | |
| L1/2 | 2 |
| L2/3 | 8 |
| L3/4 | 33 |
| L4/5 | 79 |
| L5/S | 14 |
| Operative time (min) | 112.7 ± 45.9 |
| Blood loss (mL) | 120.8 ± 121.4 |
| Pre-Op | 6 M Post-Op | 12 M Post-Op | |
|---|---|---|---|
| Brief BESTest (Total Score) | 13.3 ± 5.3 | 16.1 ± 5.1 ** | 16.0 ± 5.1 ** |
| I. Biomechanical constraints (pt) | 1.3 ± 1.1 | 1.6 ± 1.1 ** | 1.6 ± 1.2 * |
| II. Stability limits (pt) | 2.1 ± 0.5 | 2.1 ± 0.4 | 2.1 ± 0.4 |
| III. Anticipatory postural adjustments (pt) | 3.5 ± 2.1 | 4.6 ± 1.6 ** | 4.4 ± 1.8 * |
| IV. Postural responses (pt) | 2.8 ± 1.7 | 3.4 ± 1.7 ** | 3.2 ± 1.8 * |
| V. Sensory orientation (pt) | 1.6 ± 1.0 | 1.9 ± 1.1 | 2.0 ± 1.0 ** |
| VI. Stability in gait (pt) | 2.1 ± 1.0 | 2.7 ± 0.6 ** | 2.7 ± 0.5 ** |
| LBP VAS (0–100) | 36.3 ± 30.1 | 17.5 ± 23.1 ** | 19.2 ± 23.6 ** |
| LLP VAS (0–100) | 50.5 ± 30.1 | 21.9 ± 26.6 ** | 24.0 ± 29.2 ** |
| LLN VAS (0–100) | 45.3 ± 33.2 | 18.7 ± 26.3 ** | 25.8 ± 32.3 ** |
| MFES (pt) | 97.8 ± 32.6 | 115.6 ± 28.8 ** | 115.9 ± 27.1 ** |
| ODI (%) | 42.2 ± 15.9 | 19.9 ± 17.6 ** | 23.1 ± 17.5 ** |
| ZCQ SSS (pt) | 3.1 ± 0.5 | 2.2 ± 0.7 ** | 2.3 ± 1.3 ** |
| ZCQ PFS (pt) | 2.7 ± 0.6 | 1.8 ± 0.6 ** | 1.8 ± 0.6 ** |
| ZCQ SFS (pt) | NA | 1.8 ± 0.6 | 2.0 ± 0.8 |
| Time Point | Outcome Measure | Spearman’s ρ | p-Value |
|---|---|---|---|
| Preoperative | MFES | 0.439 | <0.001 |
| ODI | −0.301 | 0.163 | |
| ZCQ SSS | −0.172 | 0.153 | |
| ZCQ PFS | −0.419 | <0.001 | |
| LBP VAS | −0.012 | 0.906 | |
| LLP VAS | −0.141 | 0.172 | |
| LLN VAS | 0.013 | 0.902 | |
| 6 months postoperatively | MFES | 0.409 | <0.001 |
| ODI | −0.336 | 0.126 | |
| ZCQ SSS | −0.346 | 0.014 | |
| ZCQ PFS | −0.313 | 0.025 | |
| ZCQ SFS | −0.409 | 0.004 | |
| LBP VAS | −0.299 | 0.010 | |
| LLP VAS | −0.202 | 0.086 | |
| LLN VAS | −0.228 | 0.053 | |
| 12 months postoperatively | MFES | 0.451 | <0.001 |
| ODI | −0.442 | 0.027 | |
| ZCQ SSS | −0.481 | <0.001 | |
| ZCQ PFS | −0.295 | 0.013 | |
| ZCQ SFS | −0.299 | 0.013 | |
| LBP VAS | −0.331 | <0.001 | |
| LLP VAS | −0.275 | 0.006 | |
| LLN VAS | −0.206 | 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakaguchi, T.; Tanaka, M.; Arataki, S.; Komatsubara, T.; Miyamoto, A.; Borde, M.; Arvind, U.; Takamatsu, K.; Yasuda, Y.; Doană-Prodan, A.; et al. Postoperative Recovery of Balance Function in Lumbar Spinal Stenosis: A 12-Month Longitudinal Study Using the Brief BESTest and Its Association with Patient-Reported Outcomes. J. Clin. Med. 2025, 14, 5520. https://doi.org/10.3390/jcm14155520
Sakaguchi T, Tanaka M, Arataki S, Komatsubara T, Miyamoto A, Borde M, Arvind U, Takamatsu K, Yasuda Y, Doană-Prodan A, et al. Postoperative Recovery of Balance Function in Lumbar Spinal Stenosis: A 12-Month Longitudinal Study Using the Brief BESTest and Its Association with Patient-Reported Outcomes. Journal of Clinical Medicine. 2025; 14(15):5520. https://doi.org/10.3390/jcm14155520
Chicago/Turabian StyleSakaguchi, Tomoyoshi, Masato Tanaka, Shinya Arataki, Tadashi Komatsubara, Akiyoshi Miyamoto, Mandar Borde, Umarani Arvind, Kazuhiko Takamatsu, Yosuke Yasuda, Adrian Doană-Prodan, and et al. 2025. "Postoperative Recovery of Balance Function in Lumbar Spinal Stenosis: A 12-Month Longitudinal Study Using the Brief BESTest and Its Association with Patient-Reported Outcomes" Journal of Clinical Medicine 14, no. 15: 5520. https://doi.org/10.3390/jcm14155520
APA StyleSakaguchi, T., Tanaka, M., Arataki, S., Komatsubara, T., Miyamoto, A., Borde, M., Arvind, U., Takamatsu, K., Yasuda, Y., Doană-Prodan, A., & Ishihara, K. (2025). Postoperative Recovery of Balance Function in Lumbar Spinal Stenosis: A 12-Month Longitudinal Study Using the Brief BESTest and Its Association with Patient-Reported Outcomes. Journal of Clinical Medicine, 14(15), 5520. https://doi.org/10.3390/jcm14155520










