Aromatase Inhibitor-Induced Carpal Tunnel Syndrome Immunohistochemical Analysis and Clinical Evaluation: An Observational, Cross-Sectional, Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
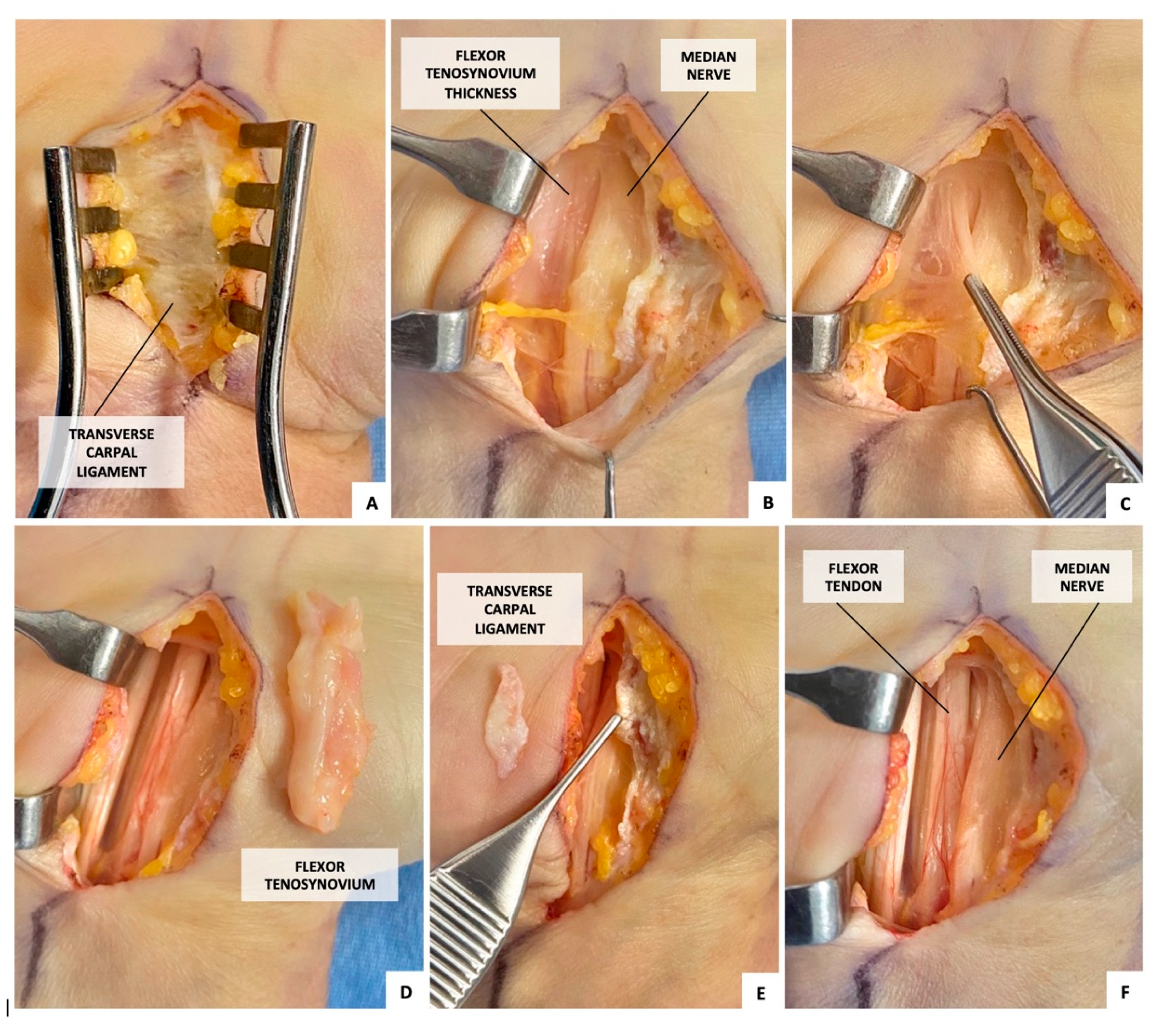
2.1. Surgical Procedure (Figure 1)
2.2. Immunohistochemical Analysis
- Mouse monoclonal anti-ERα (sc-8002, Santa Cruz Biotechnology, Santa Cruz, CA, USA, dilution: 1:25, incubation time: 2 h);
- Rabbit polyclonal anti-ERβ (ab5786, Abcam, Cambridge, UK, dilution: 1:300, incubation time: 30 min).
- Image Preprocessing: WSIs were normalized to ensure consistent background illumination and staining intensity across all samples.
- Tissue Segmentation: Non-tissue areas, artifacts, and backgrounds were automatically excluded using QuPath’s region-of-interest (ROI) selection and thresholding functions.
- Color Deconvolution: The hematoxylin and immunostain signals were separated using QuPath’s color deconvolution algorithm, isolating the diaminobenzidine (DAB)-positive staining corresponding to nuclear ERα and ERβ expression.
- Thresholding and Classification: A customized color threshold was applied to detect and quantify nuclear immunoreactivity. A dynamic intensity range was used to classify cells as positive (above threshold) or negative (below threshold) while minimizing background noise.
- Automated Cell Counting and Labeling Index Calculation: The proportion of ERα positive and ERβ positive cells was calculated relative to the total cell count in the analyzed region. The labeling index was expressed as the percentage of positively stained cells per field of view.
2.3. Statistical Analysis
3. Results
3.1. Study Group
3.1.1. Intraoperative Appearance
3.1.2. Immunohistochemical Analysis (Figure 2 and Figure 3)
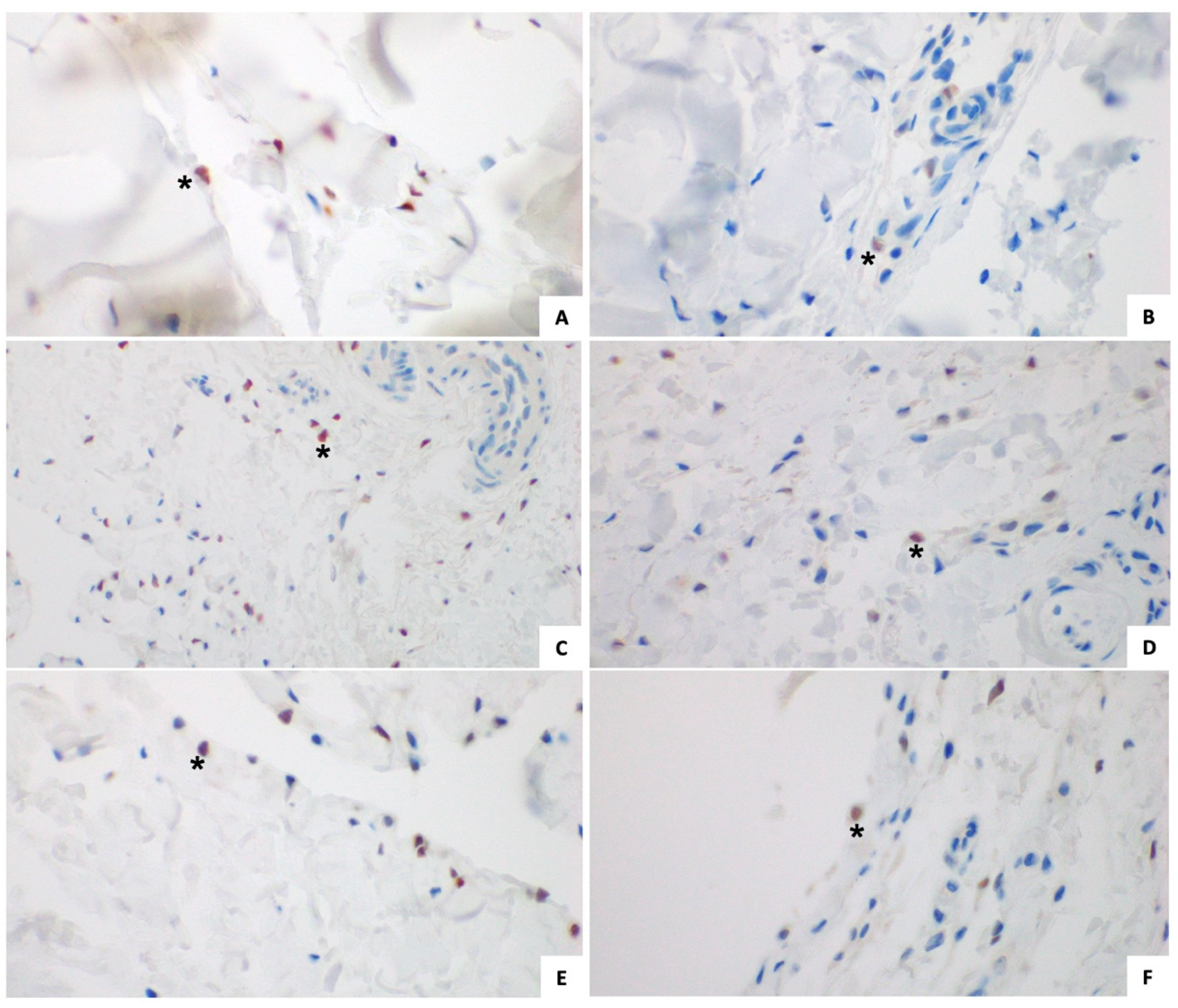
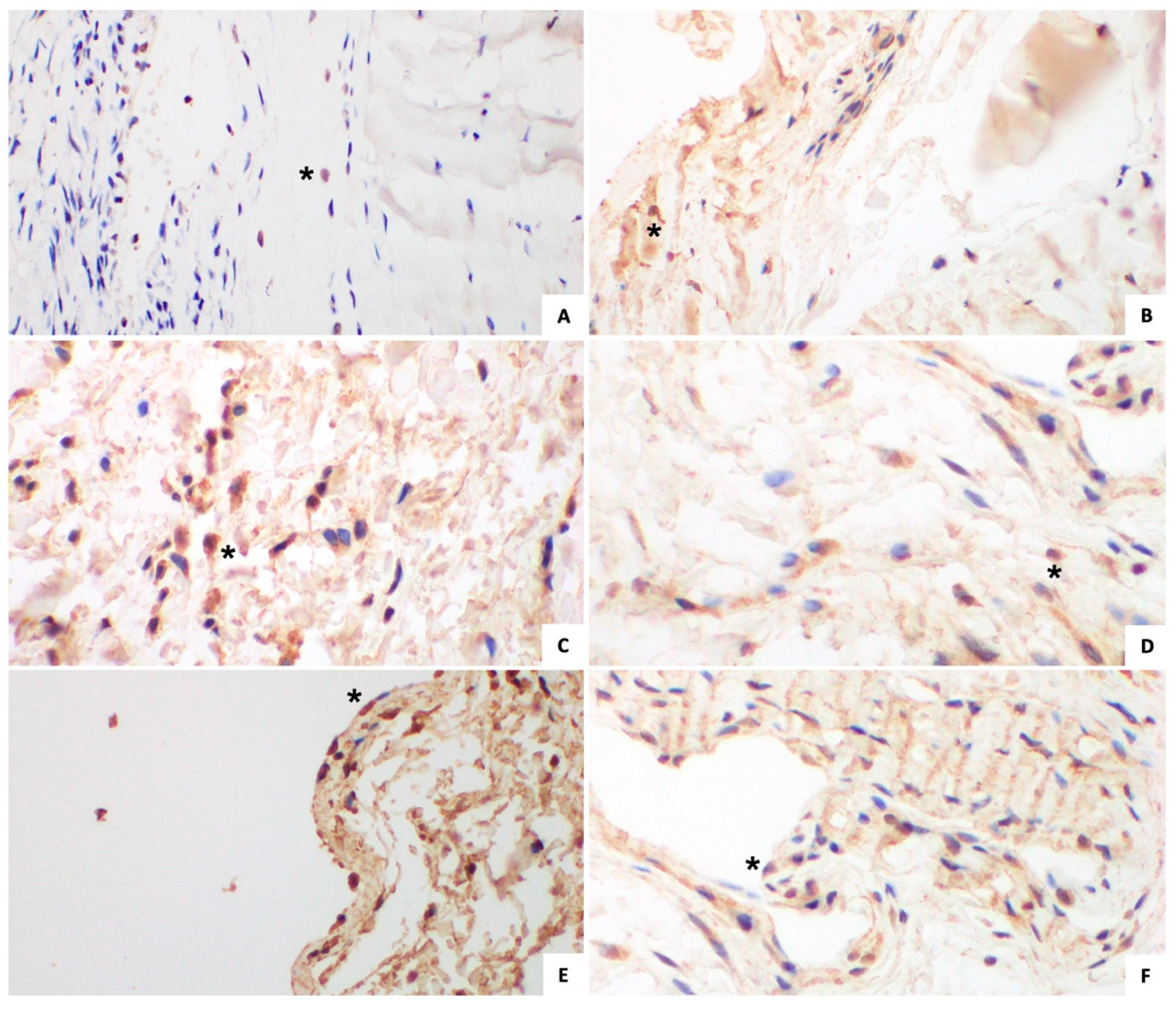
3.2. Control Group
3.2.1. Intraoperative Appearance
3.2.2. Immunohistochemical Analysis (Figure 2 and Figure 3)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Anderson, B.O.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Blair, S.L.; Burstein, H.J.; Dang, C.; Elias, A.D.; et al. Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 452–478. [Google Scholar] [CrossRef]
- Duncan, S.F.M.; Bhate, O.; Mustaly, H. Pathophysiology of carpal tunnel syndrome. In Carpal Tunnel Syndrome and Related Median Neuropathies. Challenges and Complications; Duncan, S.F.M., Kakinoki, R., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 13–29. ISBN 978-3-319-57008-2. [Google Scholar]
- Sestak, I.; Sapunar, F.; Cuzick, J. Aromatase inhibitor-induced carpal tunnel syndrome: Results from the ATAC trial. J. Clin. Oncol. 2009, 27, 4961–4965. [Google Scholar] [CrossRef]
- Colleoni, M.; Giobbie-Hurder, A.; Regan, M.M.; Thürlimann, B.; Mouridsen, H.; Mauriac, L.; Forbes, J.F.; Paridaens, R.; Láng, I.; Smith, I.; et al. Analyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1-98 study. J. Clin. Oncol. 2011, 29, 1117–1124. [Google Scholar] [CrossRef]
- Spagnolo, F.; Sestak, I.; Howell, A.; Forbes, J.F.; Cuzick, J. Anastrozole-Induced Carpal Tunnel Syndrome: Results From the International Breast Cancer Intervention Study II Prevention Trial. J. Clin. Oncol. 2016, 34, 139–143. [Google Scholar] [CrossRef]
- Chien, H.C.; Kao Yang, Y.H.; Kwoh, C.K.; Chalasani, P.; Wilson, D.L.; Lo-Ciganic, W.H. Aromatase Inhibitors and Risk of Arthritis and Carpal Tunnel Syndrome among Taiwanese Women with Breast Cancer: A Nationwide Claims Data Analysis. J. Clin. Med. 2020, 9, 566. [Google Scholar] [CrossRef]
- Rocque, G. What Is the Role of Symptom Management and Patient-Reported Outcomes in Adherence to Aromatase Inhibitors? J. Clin. Oncol. 2018, 36, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Padua, R.; Padua, L.; Romanini, E.; Aulisa, L.; Lupparelli, S.; Sanguinetti, C. Versione italiana del questionario “Boston carpal tunnel”: Valutazione preliminare. GIOT 1998, 24, 121–129. [Google Scholar]
- Ernst, M.; Heath, J.K.; Rodan, G.A. Estradiol effects on proliferation, messenger ribonucleic acid for collagen and insulin-like growth factor-I, and parathyroid hormone-stimulated adenylate cyclase activity in osteoblastic cells from calvariae and long bones. Endocrinology 1989, 125, 825–833. [Google Scholar] [CrossRef]
- Lebrun, C.M. The effect of the phase of the menstrual cycle and the birth control pill on athletic performance. Clin. Sports Med. 1994, 13, 419–441. [Google Scholar] [CrossRef]
- Majeska, R.J.; Ryaby, J.T.; Einhorn, T.A. Direct modulation of osteoblastic activity with estrogen. J. Bone Jt. Surg. Am. 1994, 76, 713–721. [Google Scholar] [CrossRef]
- Shikata, J.; Sanada, H.; Tamamuro, T.; Takeda, T. Experimental studies of the elastic fiber of the capsular ligament: Influence of ageing and sex hormones on the hip joint capsule of rats. Connect. Tissue Res. 1979, 7, 21–27. [Google Scholar] [CrossRef]
- Toesca, A.; Pagnotta, A.; Specchia, N. Evidence of type II estrogen receptor in human osteoblast-like cells. Cell Biol. Int. 2000, 24, 303–309. [Google Scholar] [CrossRef]
- Tinti, L.; Niccolini, S.; Lamboglia, A.; Pascarelli, N.A.; Cervone, R.; Fioravanti, A. Raloxifene protects cultured human chondrocytes from IL-1β induced damage: A biochemical and morphological study. Eur. J. Pharmacol. 2011, 670, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, S.; Stearns, V. Aromatase inhibitor-associated bone and musculoskeletal effects: New evidence defining etiology and strategies for management. Breast Cancer Res. 2011, 13, 205. [Google Scholar] [CrossRef] [PubMed]
- Lintermans, A.; Neven, P. Safety of aromatase inhibitor therapy in breast cancer. Expert Opin. Drug Saf. 2015, 14, 1201–1211. [Google Scholar] [CrossRef]
- Niravath, P. Aromatase inhibitor-induced arthralgia: A review. Ann. Oncol. 2013, 24, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, H.F.; Mattsson, L.A.; Ohlsson, C.; Nordborg, E.; Carlsten, H. Hormone replacement therapy in rheumatoid arthritis is associated with lower serum levels of soluble IL-6 receptor and higher insulin-like growth factor 1. Arthritis Res. Ther. 2003, 5, R202–R209. [Google Scholar] [CrossRef]
- Yu, W.D.; Panossian, V.; Hatch, J.D.; Liu, S.H.; Finerman, G.A. Combined effects of estrogen and progesterone on the anterior cruciate ligament. Clin. Orthop. Relat. Res. 2001, 383, 268–281. [Google Scholar] [CrossRef]
- Liu, S.H.; Al-Shaikh, R.A.; Panossian, V.; Finerman, G.A.; Lane, J.M. Estrogen affects the cellular metabolism of the anterior cruciate ligament. A potential explanation for female athletic injury. Am. J. Sports Med. 1997, 25, 704–709. [Google Scholar] [CrossRef]
- Fischer, G.M. Comparison of collagen dynamics in different tissues under the influence of estradiol. Endocrinology 1973, 93, 1216–1218. [Google Scholar] [CrossRef]
- Akeson, W.H.; Woo, S.L.; Amiel, D.; Doty, D.H.; Rutherford, L. Value of 17beta-oestradiol in prevention of contracture formation. Ann. Rheum. Dis. 1975, 35, 429–436. [Google Scholar] [CrossRef]
- Hanstein, B.; Djahansouzi, S.; Dall, P.; Beckmann, M.W.; Bender, H.G. Insights into the molecular biology of the estrogen receptor define novel therapeutic targets for breast cancer. Eur. J. Endocrinol. 2004, 150, 243–255. [Google Scholar] [CrossRef]
- Hall, J.M.; McDonnell, D.P. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 1999, 140, 5566–5578. [Google Scholar] [CrossRef]
- Isaacs, C.; Wellstein, A.; Riegel, A.T. Hormones and related agents in the therapy of cancer. In Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 13th ed.; Brunton, L.L., Knollmann, B.C., Hilal-Dandan, R., Eds.; McGraw-Hill Education: New York, NY, USA, 2018; pp. 1237–1247. ISBN 978-1-25-958474-9. [Google Scholar]
- Tenti, S.; Correale, P.; Cheleschi, S.; Fioravanti, A.; Pirtoli, L. Aromatase Inhibitors-Induced Musculoskeletal Disorders: Current Knowledge on Clinical and Molecular Aspects. Int. J. Mol. Sci. 2020, 21, 5625. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chlebowski, R.T.; Shi, J.; Barac, A.; Haque, R. Aromatase inhibitor and tamoxifen use and the risk of venous thromboembolism in breast cancer survivors. Breast Cancer Res. Treat. 2019, 174, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Beckwée, D.; Leysen, L.; Meuwis, K.; Adriaenssens, N. Prevalence of aromatase inhibitor- induced arthralgia in breast cancer: A systematic review and meta-analysis. Support. Care Cancer 2017, 25, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- Henry, N.L.; Giles, J.T.; Ang, D.; Mohan, M.; Dadabhoy, D.; Robarge, J.; Hayden, J.; Lemler, S.; Shahverdi, K.; Powers, P.; et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res. Treat. 2008, 111, 365–372. [Google Scholar] [CrossRef]
- Borrie, A.E.; Kim, R.B. Molecular basis of aromatase inhibitor associated arthralgia: Known and potential candidate genes and associated biomarkers. Expert Opin. Drug Metab. Toxicol. 2017, 13, 149–156. [Google Scholar] [CrossRef]
- Chung, K.Y.; Ho, G.; Novak, C.B.; Baltzer, H.L. Aromatase Inhibitor-Induced Carpal Tunnel Syndrome and Stenosing Tenosynovitis: A Systematic Review. Plast. Reconstr. Surg. 2022, 149, 445e–452e. [Google Scholar] [CrossRef]
- Atroshi, I.; Gummesson, C.; Johnsson, R.; Ornstein, E.; Ranstam, J.; Rosén, I. Prevalence of carpal tunnel syndrome in a general population. JAMA 1999, 282, 153–158. [Google Scholar] [CrossRef]
- De Krom, M.C.; Knipschild, P.G.; Kester, A.D.; Thijs, C.T.; Boekkooi, P.F.; Spaans, F. Carpal tunnel syndrome: Prevalence in the general population. J. Clin. Epidemiol. 1992, 45, 373–376. [Google Scholar] [CrossRef]
- Sheng, J.Y.; Blackford, A.L.; Bardia, A.; Venkat, R.; Rosson, G.; Giles, J.; Hayes, D.F.; Jeter, S.C.; Zhang, Z.; Hayden, J.; et al. Prospective evaluation of finger two-point discrimination and carpal tunnel syndrome among women with breast cancer receiving adjuvant aromatase inhibitor therapy. Breast Cancer Res. Treat. 2019, 176, 617–624. [Google Scholar] [CrossRef]
- Mieog, J.S.; Morden, J.P.; Bliss, J.M.; Coombes, R.C.; van de Velde, C.J.; IES Steering Committee. Carpal tunnel syndrome and musculoskeletal symptoms in postmenopausal women with early breast cancer treated with exemestane or tamoxifen after 2-3 years of tamoxifen: A retrospective analysis of the Intergroup Exemestane Study. Lancet Oncol. 2012, 13, 420–432. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund. Breast Cancer Statistics. Available online: http://www.wcrf.org/dietandcancer/cancer-trends/breast-cancer-statistics (accessed on 23 February 2019).
- Shin, D.J.; Nam, K.E.; Song, D.H.; Im, S.; Won, S.J.; Kim, Y.H.; Lim, S.H.; Lee, J.I. Carpal tunnel syndrome and tenosynovitis in women with breast cancer associated with hormone therapy: A multi-institutional analysis using a clinical data warehouse. Medicine 2022, 101, e28786. [Google Scholar] [CrossRef] [PubMed]
- Luchetti, R. Etiopathogenesis. In Carpal Tunnel Syndrome; Luchetti, R., Amadio, P., Eds.; Springer: New York, NY, USA, 2007; pp. 21–27. ISBN 978-3-5402-2387-0. [Google Scholar]
- Donato, G.; Galasso, O.; Valentino, P.; Conforti, F.; Zuccalà, V.; Russo, E.; Maltese, L.; Perrotta, I.; Tripepi, S.; Amorosi, A. Pathological findings in subsynovial connective tissue in idiopathic carpal tunnel syndrome. Clin. Neuropathol. 2009, 28, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Zhao, C.; Amadio, P.C.; An, K.N.; Zobitz, M.E.; Wold, L.E. Immunolocalization of collagen types in the subsynovial connective tissue within the carpal tunnel in humans. J. Orthop. Res. 2005, 23, 1226–1231. [Google Scholar] [CrossRef]
- Bland, J.D. Carpal tunnel syndrome. Curr. Opin. Neurol. 2005, 18, 581–585. [Google Scholar] [CrossRef]
- Ettema, A.M.; Amadio, P.C.; Zhao, C.; Wold, L.E.; An, K.N. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J. Bone Jt. Surg. Am. 2004, 86, 1458–1466. [Google Scholar] [CrossRef]
- Pagnotta, A.; Formica, V.M.; Marcovici, L.L.; Molayem, I.; Taglieri, E. A novel local adipofascial flap for the management of recalcitrant ulnar tunnel syndrome. Hand Surg. Rehabil. 2021, 40, 377–381. [Google Scholar] [CrossRef]
- Phalen, G.S. The carpal-tunnel syndrome. Seventeen years’ experience in diagnosis and treatment of six hundred fifty-four hands. J. Bone Jt. Surg. Am. 1966, 48, 211–228. [Google Scholar] [CrossRef]
- Song, C.H.; Gong, H.S.; Bae, K.J.; Kim, J.H.; Nam, K.P.; Baek, G.H. Evaluation of female hormone-related symptoms in women undergoing carpal tunnel release. J. Hand Surg. Eur. Vol. 2014, 39, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.C.; Beard, C.M.; O’Fallon, W.M.; Kurland, L.T. Conditions associated with carpal tunnel syndrome. Mayo Clin. Proc. 1992, 67, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Pascual, E.; Giner, V.; Aróstegui, A.; Conill, J.; Ruiz, M.T.; Picó, A. Higher incidence of carpal tunnel syndrome in oophorectomized women. Br. J. Rheumatol. 1991, 30, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, J.M.; Clark, D.I.; Bainbridge, L.C.; Smith, C.; Hubbard, R. Risk factors in carpal tunnel syndrome. J. Hand Surg. Br. 2004, 29, 315–320. [Google Scholar] [CrossRef]
- Toesca, A.; Pagnotta, A.; Zumbo, A.; Sadun, R. Estrogen and progesterone receptors in carpal tunnel syndrome. Cell Biol. Int. 2008, 32, 75–79. [Google Scholar] [CrossRef]
- Kim, J.K.; Hann, H.J.; Kim, M.J.; Kim, J.S. The expression of estrogen receptors in the tenosynovium of postmenopausal women with idiopathic carpal tunnel syndrome. J. Orthop. Res. 2010, 28, 1469–1474. [Google Scholar] [CrossRef]
- Yamanaka, Y.; Menuki, K.; Tajima, T.; Okada, Y.; Kosugi, K.; Zenke, Y.; Sakai, A. Effect of estradiol on fibroblasts from postmenopausal idiopathic carpal tunnel syndrome patients. J. Cell Physiol. 2018, 233, 8723–8730. [Google Scholar] [CrossRef]
- Mohammadi, A.; Naseri, M.; Namazi, H.; Ashraf, M.J.; Ashraf, A. Correlation between Female Sex Hormones and Electrodiagnostic Parameters and Clinical Function in Post-menopausal Women with Idiopathic Carpal Tunnel Syndrome. J. Menopausal Med. 2016, 22, 80–86. [Google Scholar] [CrossRef]
- Elmlinger, M.W.; Kühnel, W.; Ranke, M.B. Reference ranges for serum concentrations of lutropin (LH), follitropin (FSH), estradiol (E2), prolactin, progesterone, sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), cortisol and ferritin in neonates, children and young adults. Clin. Chem. Lab. Med. 2002, 40, 1151–1160. [Google Scholar] [CrossRef]
- Fiet, J.; Hermano, M.; Witte, J.; Villette, J.M.; Haimart, M.; Gourmel, B.; Tabuteau, F.; Rouffy, J.; Dreux, C. Post-menopausal concentrations of plasma oestradiol, oestrone, FSH and LH and of total urinary oestradiol and oestrone after a single sublingual dose of oestradiol-17 beta. Acta Endocrinol. 1982, 101, 93–97. [Google Scholar] [CrossRef]
- Esqueda, M.E.; Craig, T.; Hinojosa-Laborde, C. Effect of ovariectomy on renal estrogen receptor-alpha and estrogen receptor-beta in young salt-sensitive and -resistant rats. Hypertension 2007, 50, 768–772. [Google Scholar] [CrossRef]
- Ihionkhan, C.E.; Chambliss, K.L.; Gibson, L.L.; Hahner, L.D.; Mendelsohn, M.E.; Shaul, P.W. Estrogen causes dynamic alterations in endothelial estrogen receptor expression. Circ. Res. 2002, 91, 814–820. [Google Scholar] [CrossRef]
| CASE | AGE AT SYMPTOM ONSET (yy) | HORMONE REPLACEMENT THERAPY (PRIOR) | SURGERY | LYMPH NODE (POSITIVE) | CHT | RDT | AROMATASE INHIBITOR | TAMOXIFEN | SYMPTOM ONSET - AI ADMINISTRATION (TIME ELAPSED, mm) | DOMINANT HAND | CONCOMITANT AIA DISEASES |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AI-CTS 1 | 53 | NO | Q, M | 0, 1 | YES | YES | LETROZOLE | YES | 0 | YES | NO |
| AI-CTS 2 | 56 | NO | Q | 1 | NO | YES | LETROZOLE | NO | 1 | NO | YES |
| AI-CTS 3 | 60 | NO | M | 0 | YES | YES | LETROZOLE | NO | 3 | YES | NO |
| AI-CTS 4 | 52 | NO | M | 0 | YES | NO | EXEMESTANE | YES | 1 | YES | YES |
| AI-CTS 5 | 58 | NO | Q | 0 | NO | YES | LETROZOLE | NO | 3 | NO | YES |
| AI-CTS 6 | 60 | NO | Q | 0 | NO | YES | LETROZOLE | NO | 4 | NO | YES |
| AI-CTS 7 | 50 | NO | M | 2 | YES | NO | EXEMESTANE | NO | 1 | YES | YES |
| SYMPTOM ONSET - MENOPAUSE (TIME ELAPSED, mm) | CONCOMITANT AIA-LIKE DISEASES | ||||||||||
| PI-CTS 1 | 54 | NO | - | - | - | - | - | - | 6 | YES | NO |
| PI-CTS 2 | 53 | NO | - | - | - | - | - | - | 3 | NO | NO |
| PI-CTS 3 | 56 | YES | - | - | - | - | - | - | 1 | NO | YES |
| PI-CTS 4 | 57 | NO | - | - | - | - | - | - | 1 | YES | YES |
| PI-CTS 5 | 60 | NO | - | - | - | - | - | - | 6 | YES | NO |
| PI-CTS 6 | 53 | NO | - | - | - | - | - | - | 3 | YES | NO |
| PI-CTS 7 | 55 | YES | - | - | - | - | - | - | 2 | YES | NO |
| CASE | SENSORY NCS, VELOCITY (m/s, nv > 45) | MOTOR NCS, LATENCY (ms, nv < 4.0) | SERUM, ESTRONE (nv 30.92–99.82 pg/mL) | SERUM, 17BESTRADIOL (nv 0–66 pg/mL) | BCTQ, PREOPERATIVE (pv 19–95) | BCTQ, POSTOPERATIVE (pv 19–95) |
|---|---|---|---|---|---|---|
| AI-CTS 1 | 29.7 | 5.25 | IN | IN | 53 | 19 |
| AI-CTS 2 | 0 | 5.17 | IN | IN | 62 | 34 |
| AI-CTS 3 | 24.2 | 5.50 | IN | IN | 47 | 19 |
| AI-CTS 4 | 29 | 5 | OUT (↑) | IN | 53 | 19 |
| AI-CTS 5 | 0 | 10.5 | IN | OUT (↓) | 69 | 19 |
| AI-CTS 6 | 0 | 6.2 | IN | IN | 66 | 29 |
| AI-CTS 7 | 39.3 | 3.6 | OUT (↑) | NULL | 88 | 19 |
| PI-CTS 1 | 38.7 | 4.4 | IN | IN | 50 | 19 |
| PI-CTS 2 | 42.3 | 4.7 | OUT (↓) | OUT (↓) | 48 | 21 |
| PI-CTS 3 | 33 | 4.5 | IN | IN | 77 | 19 |
| PI-CTS 4 | 32 | 4.4 | OUT (↑) | IN | 86 | 27 |
| PI-CTS 5 | 0 | 17.1 | OUT (↑) | IN | 79 | 27 |
| PI-CTS 6 | 32 | 5.04 | IN | IN | 66 | 26 |
| PI-CTS 7 | 0 | 8.19 | NULL | NULL | 82 | 23 |
| CASE | TCL (CONSISTENCE) | FT (THICKENING) | MEDIAN NERVE (APPEARANCE) |
|---|---|---|---|
| AI-CTS 1 | NORMAL | EXTENSIVE | NORMAL |
| AI-CTS 2 | MILDLY STIFF | EXTENSIVE | NORMAL |
| AI-CTS 3 | MILDLY STIFF | EXTENSIVE | NEUROMA |
| AI-CTS 4 | STIFF | EXTENSIVE | NORMAL |
| AI-CTS 5 | MILDLY STIFF | MILD | NORMAL |
| AI-CTS 6 | NORMAL | EXTENSIVE | NORMAL |
| AI-CTS 7 | NORMAL | EXTENSIVE | NORMAL |
| PI-CTS 1 | STIFF | EXTENSIVE | NORMAL |
| PI-CTS 2 | MILDLY STIFF | EXTENSIVE | NORMAL |
| PI-CTS 3 | STIFF | EXTENSIVE | NEUROMA |
| PI-CTS 4 | STIFF | EXTENSIVE | NEUROMA |
| PI-CTS 5 | MILDLY STIFF | EXTENSIVE | NORMAL |
| PI-CTS 6 | STIFF | EXTENSIVE | NORMAL |
| PI-CTS 7 | MILDLY STIFF | EXTENSIVE | NORMAL |
| TCL | TCL | FT | FT | FT | FT | |
|---|---|---|---|---|---|---|
| CASE | FIBROBLASTS ERα | FIBROBLASTS ERβ | FIBROBLASTS ERα | FIBROBLASTS ERβ | SYNOVIAL LINING CELLS ERα | SYNOVIAL LINING CELLS ERβ |
| AI-CTS 1 | 6.40 | 15.46 | 7.48 | 94.09 | 8.15 | 96.72 |
| AI-CTS 2 | 10.53 | 93.04 | 2.54 | 78.06 | 0.47 | 95.07 |
| AI-CTS 3 | 4.20 | 75.23 | 1.12 | 9.05 | 2.54 | 92.76 |
| AI-CTS 4 | 0.15 | 69.05 | 8.34 | 80.59 | 30.00 | 95.56 |
| AI-CTS 5 | 9.43 | 89.74 | 12.54 | 93.57 | 5.43 | 85.23 |
| AI-CTS 6 | 7.31 | 70.34 | 6.89 | 80.59 | 4.71 | 92.76 |
| AI-CTS 7 | 8.34 | 70.34 | 14.61 | 85.43 | 6.21 | 89.34 |
| PI-CTS 1 | 0.20 | 93.50 | 3.50 | 99.23 | 1.24 | 99.56 |
| PI-CTS 2 | 0.00 | 91.34 | 63.09 | 97.04 | 53.43 | 95.61 |
| PI-CTS 3 | 12.65 | 85.70 | 12.68 | 99.50 | 7.68 | 95.34 |
| PI-CTS 4 | 16.14 | 83.45 | 12.41 | 98.30 | 8.45 | 99.45 |
| PI-CTS 5 | 0.85 | 94.76 | 4.69 | 98.61 | 0.34 | 100.00 |
| PI-CTS 6 | 2.61 | 87.85 | 0.07 | 99.26 | 0.34 | 99.20 |
| PI-CTS 7 | 1.23 | 43.12 | 2.57 | 76.59 | 1.32 | 85.98 |
| STUDY | TOESCA ET AL. [50] | KIM ET AL. [51] | MOHAMMADI ET AL. [53] | YAMANAKA ET AL. [52] |
|---|---|---|---|---|
| STUDY GROUP CONTROL GROUP | idiopathic CTS 23W, 7M no CTS 2W, 2M | postmenopausal idiopathic CTS 12W postmenopausal no CTS 6W | postmenopausal idiopathic CTS 12W postmenopausal no CTS 10W | postmenopausal idiopathic CTS 10W - |
| INVESTIGATIONS (TISSUES, CELLS, RECEPTORS, OTHER) | TCL fibroblasts synovial tissue fibroblasts, lining cells ERα, PR | FT fibroblasts, synovial lining cells ERα, ERβ | TCL fibroblasts ER serum estradiol | subsynovial connective tissue fibroblasts ERα, ERβ, Col1A1, Col3A1, CTGF, VEGF |
| MAIN CONCLUSIONS | ERα was expressed in TCL fibroblasts (and vascular walls) and in synovial tissue fibroblasts and lining cells PR was expressed in TCL fibroblasts (and vascular walls) ERα and PR were more commonly expressed in the study group than in the control group ERα and PR were more commonly expressed in the group of 50–70 year-old women with idiopathic CTS than in the other age groups of women with idiopathic CTS ERα and PR were more commonly expressed in the group of 50–70 year-old women with idiopathic CTS than in the group of men with idiopathic CTS Estrogen plays a larger role (compared to progesterone) in CTS etiopathogenesis TCL plays a larger role (compared to synovial tissue) as a tissue target of hormonal action | ERα and ERβ were expressed in fibroblasts and synovial lining cells (and vessel endothelial cells) ERβ was more commonly expressed than ERα ERα and ERβ were more commonly expressed in the fibroblasts and synovial lining cells of the study group than in the control group there was no correlation between ERα or ERβ and age, symptom duration, or symptom severity | there was no difference in ER expression between the study group and control group there was no difference in estrogen levels between the study group and control group there was no correlation between ER expression and electrodiagnostic parameters or the Boston score there was no correlation between estrogen levels and electrodiagnostic parameters or the Boston score | ERα downregulates Col1A1 and Col3A1 expression reducing collagen I and collagen III synthesis A low concentration of estradiol is unable to act on ERs There is no correlation between ERβ expression and collagen I or collagen III synthesis There is no correlation between the percentage of Erα and Erβ expression and collagen I or collagen III synthesis |
| p-VALUE | R | EXACT CI 95% (HL) | OR | CI 95% | |
|---|---|---|---|---|---|
| STUDY GROUP | |||||
| ERα TCL fibroblasts vs. ERα FT fibroblasts | 0.6875 | ||||
| ERα FT fibroblasts vs. ERα FT synovial lining cells | 0.5625 | ||||
| ERβ TCL fibroblasts vs. ERβ FT fibroblasts | 0.5781 | ||||
| ERβ FT fibroblasts vs. ERβ FT synovial lining cells | 0.0781 | ||||
| ERα TCL fibroblasts vs. ERβ TCL fibroblasts | 0.0313 * | ||||
| ERα FT fibroblasts vs. ERβ FT fibroblasts | 0.0313 * | ||||
| ERα FT synovial lining cells vs. ERβ FT synovial lining cells | 0.0313 * | ||||
| BCTQ preoperative vs. BCTQ postoperative | 0.0156 * | ||||
| CONTROL GROUP | |||||
| ERα TCL fibroblasts vs. ERα FT fibroblasts | 0.3750 | ||||
| ERα FT fibroblasts vs. ERα FT synovial lining cells | 0.0313 * | ||||
| ERβ TCL fibroblasts vs. ERβ FT fibroblasts | 0.0156 * | ||||
| ERβ FT fibroblasts vs. ERβ FT synovial lining cells | 0.8125 | ||||
| ERα TCL fibroblasts vs. ERβ TCL fibroblasts | 0.0156 * | ||||
| ERα FT fibroblasts vs. ERβ FT fibroblasts | 0.0156 * | ||||
| ERα FT synovial lining cells vs. ERβ FT synovial lining cells | 0.0156 * | ||||
| BCTQ preoperative vs. BCTQ postoperative | 0.0156 * | ||||
| STUDY GROUP VS. CONTROL GROUP | |||||
| ERα TCL fibroblasts | 0.5203 | 0.178 | (−6.71–8.58) | ||
| ERβ TCL fibroblasts | 0.1594 | 0.376 | (−5.19–24.42) | ||
| ERα FT fibroblasts | 1.0000 | 0 | (−11.56–9.04) | ||
| ERβ FT fibroblasts | 0.0213 * | 0.615 | (4.52–21.17) | ||
| ERα FT synovial lining cells | 0.7206 | 0.099 | (−7.98–5.87) | ||
| ERβ FT synovial lining cells | 0.0550 | 0.513 | (0.05–9.86) | ||
| Symptom onset-AI administration interval vs. Symptom onset-menopause interval | 0.2896 | 0.283 | (−1.00–3.00) | ||
| BCTQ preoperative | 0.4812 | 0.188 | (−11.00–26.00) | ||
| BCTQ postoperative | 0.4945 | 0.183 | (−7.00–8.00) | ||
| NCS sensitive velocity | 0.2390 | 0.315 | (−7.30–32.00) | ||
| NCS motor latency | 0.5224 | 0.171 | (−1.70–3.19) | ||
| AIA/AIA-like disease concomitance | 0.1696 | 6.2500 | (0.61–63.54) | ||
| estrone serum | 0.5582 | 2.5000 | (0.25–24.72) | ||
| 17estradiol serum | 0.8865 | 1.0000 | (0.05–20.83) | ||
| STUDY GROUP VS. KIM ET AL. CONTROL GROUP | |||||
| ERα FT fibroblasts | 0.2873 | 0.162 | (−11.66–5.44) | ||
| ERβ FT fibroblasts | 0.0133 * | 0.615 | (−72.57–40.06) | ||
| ERα FT synovial lining cells | 0.1490 | 0.300 | (−20.02–6.00) | ||
| ERβ FT synovial lining cells | 0.0017 ** | 0.813 | (−84.76–67.56) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molayem, I.; Marcovici, L.L.; Gradini, R.; Mancini, M.; Taccogna, S.; Pagnotta, A. Aromatase Inhibitor-Induced Carpal Tunnel Syndrome Immunohistochemical Analysis and Clinical Evaluation: An Observational, Cross-Sectional, Case–Control Study. J. Clin. Med. 2025, 14, 5513. https://doi.org/10.3390/jcm14155513
Molayem I, Marcovici LL, Gradini R, Mancini M, Taccogna S, Pagnotta A. Aromatase Inhibitor-Induced Carpal Tunnel Syndrome Immunohistochemical Analysis and Clinical Evaluation: An Observational, Cross-Sectional, Case–Control Study. Journal of Clinical Medicine. 2025; 14(15):5513. https://doi.org/10.3390/jcm14155513
Chicago/Turabian StyleMolayem, Iakov, Lucian Lior Marcovici, Roberto Gradini, Massimiliano Mancini, Silvia Taccogna, and Alessia Pagnotta. 2025. "Aromatase Inhibitor-Induced Carpal Tunnel Syndrome Immunohistochemical Analysis and Clinical Evaluation: An Observational, Cross-Sectional, Case–Control Study" Journal of Clinical Medicine 14, no. 15: 5513. https://doi.org/10.3390/jcm14155513
APA StyleMolayem, I., Marcovici, L. L., Gradini, R., Mancini, M., Taccogna, S., & Pagnotta, A. (2025). Aromatase Inhibitor-Induced Carpal Tunnel Syndrome Immunohistochemical Analysis and Clinical Evaluation: An Observational, Cross-Sectional, Case–Control Study. Journal of Clinical Medicine, 14(15), 5513. https://doi.org/10.3390/jcm14155513







