Real-World Effectiveness of Rosuvastatin–Ezetimibe Single Pill (Rovazet®) in Korean Dyslipidemia Patients
Abstract
1. Introduction
2. Materials and Methods
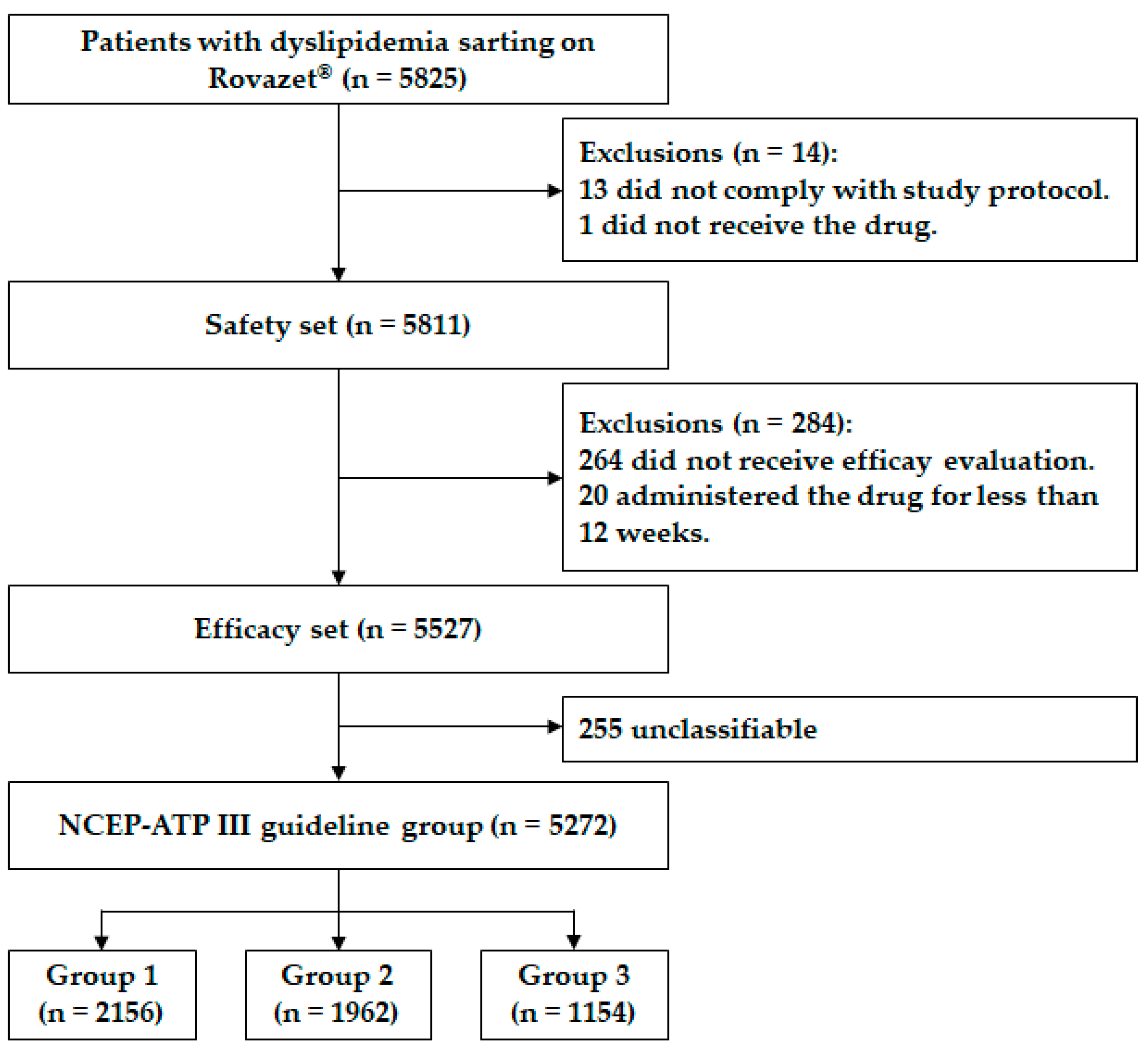
2.1. Study Protocol and Population
2.2. Clinical Data Collection
2.3. Lipid Profile
2.4. Grouping According to the NCEP-ATP III Guidelines
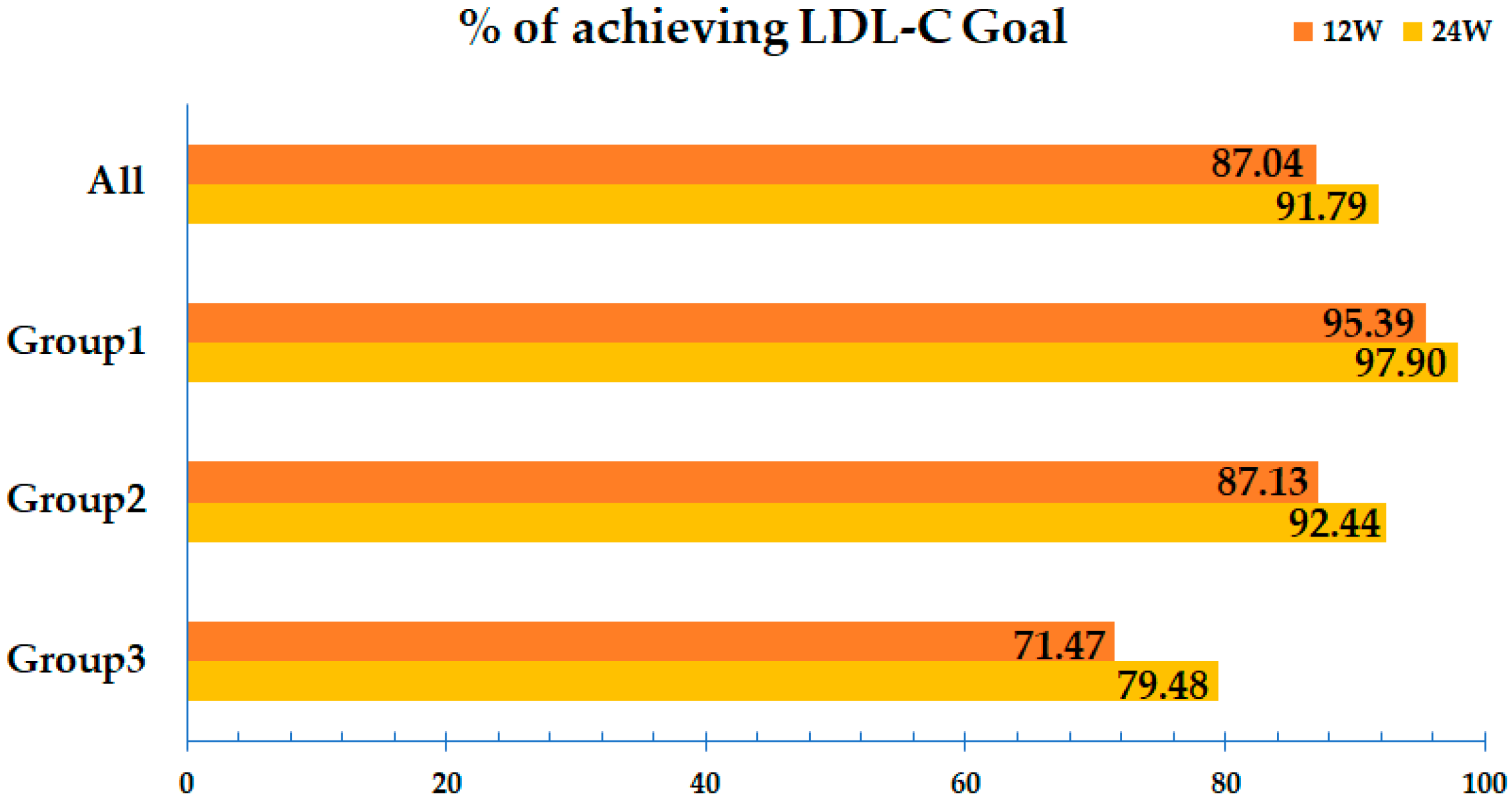
2.5. LDL-C Target
2.6. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report from the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef] [PubMed]
- Istvan, E.S.; Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 2001, 292, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Phan, B.A.; Dayspring, T.D.; Toth, P.P. Ezetimibe therapy: Mechanism of action and clinical update. Vasc. Health Risk Manag. 2012, 8, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Chilbert, M.R.; VanDuyn, D.; Salah, S.; Clark, C.M.; Ma, Q. Combination Therapy of Ezetimibe and Rosuvastatin for Dyslipidemia: Current Insights. Drug Des. Devel Ther. 2022, 16, 2177–2186. [Google Scholar] [CrossRef]
- Kim, B.K.; Hong, S.J.; Lee, Y.J.; Hong, S.J.; Yun, K.H.; Hong, B.K.; Heo, J.H.; Rha, S.W.; Cho, Y.H.; Lee, S.J.; et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): A randomised, open-label, non-inferiority trial. Lancet 2022, 400, 380–390. [Google Scholar] [CrossRef]
- Dadzie, S.K.; Tabowei, G.; Kaur, M.; Ahmed, S.; Thakur, A.; Khreis, K.; Bai, M.; Amin, A. A Comparison of Rosuvastatin Monotherapy and Rosuvastatin Plus Ezetimibe Combination Therapy in Patients with Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Cureus 2024, 16, e61526. [Google Scholar] [CrossRef]
- Lamb, Y.N. Rosuvastatin/Ezetimibe: A Review in Hypercholesterolemia. Am. J. Cardiovasc. Drugs 2020, 20, 381–392. [Google Scholar] [CrossRef]
- Hong, S.J.; Jeong, H.S.; Ahn, J.C.; Cha, D.H.; Won, K.H.; Kim, W.; Cho, S.K.; Kim, S.Y.; Yoo, B.S.; Sung, K.C.; et al. A Phase III, Multicenter, Randomized, Double-blind, Active Comparator Clinical Trial to Compare the Efficacy and Safety of Combination Therapy with Ezetimibe and Rosuvastatin Versus Rosuvastatin Monotherapy in Patients with Hypercholesterolemia: I-ROSETTE (Ildong Rosuvastatin & Ezetimibe for Hypercholesterolemia) Randomized Controlled Trial. Clin. Ther. 2018, 40, 226–241.e224. [Google Scholar] [CrossRef]
- Kim, W.; Yoon, Y.E.; Shin, S.H.; Bae, J.W.; Hong, B.K.; Hong, S.J.; Sung, K.C.; Han, S.H.; Kim, W.; Rhee, M.Y.; et al. Efficacy and Safety of Ezetimibe and Rosuvastatin Combination Therapy Versus Those of Rosuvastatin Monotherapy in Patients With Primary Hypercholesterolemia. Clin. Ther. 2018, 40, 993–1013. [Google Scholar] [CrossRef]
- Zambon, A.; Liberopoulos, E.; Dovizio, M.; Veronesi, C.; Degli Esposti, L.; Pérez de Isla, L. A real-world analysis of adherence, biochemical outcomes, and healthcare costs in patients treated with rosuvastatin/ezetimibe as single-pill combination vs. free combination in Italy. Eur. Heart J. Open 2024, 4, oeae074. [Google Scholar] [CrossRef]
- Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [CrossRef]
- Kim, K.J.; Kim, S.H.; Yoon, Y.W.; Rha, S.W.; Hong, S.J.; Kwak, C.H.; Kim, W.; Nam, C.W.; Rhee, M.Y.; Park, T.H.; et al. Effect of fixed-dose combinations of ezetimibe plus rosuvastatin in patients with primary hypercholesterolemia: MRS-ROZE (Multicenter Randomized Study of ROsuvastatin and eZEtimibe). Cardiovasc. Ther. 2016, 34, 371–382. [Google Scholar] [CrossRef]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.; Lee, Y.H.; Tsujita, K.; Gonzalez, J.A.; Kramer, C.M.; Kovarnik, T.; Kouvelos, G.N.; Suzuki, H.; Han, K.; Lee, C.J.; et al. Comparison of the Effects of Ezetimibe-Statin Combination Therapy on Major Adverse Cardiovascular Events in Patients with and without Diabetes: A Meta-Analysis. Endocrinol. Metab. 2018, 33, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Kang, S.H.; Jeong, S.W.; Yoon, C.H.; Youn, T.J.; Song, W.H.; Jeon, D.W.; Lim, S.W.; Lee, J.H.; Cho, S.W.; et al. Lipid-Lowering Efficacy of Combination Therapy with Moderate-Intensity Statin and Ezetimibe Versus High-Intensity Statin Monotherapy: A Randomized, Open-Label, Non-Inferiority Trial From Korea. J. Lipid Atheroscler. 2023, 12, 277–289. [Google Scholar] [CrossRef]
- Obońska, K.; Kasprzak, M.; Tymosiak, K.; Fabiszak, T.; Krintus, M.; Kubica, J. Low dose of ROSuvastatin in combination with EZEtimibe effectively and permanently reduce low density lipoprotein cholesterol concentration independently of timing of administration (ROSEZE): A randomized, crossover study—Preliminary results. Cardiol. J. 2021, 28, 58–66. [Google Scholar] [CrossRef]
- Perez de Isla, L.; Liberopoulos, E.; Dovizio, M.; Veronesi, C.; Degli Esposti, L.; Zambon, A. Differential Adherence to Free and Single-Pill Combination of Rosuvastatin/Ezetimibe: Findings from a Real-World Analysis in Italy. Adv. Ther. 2024, 41, 3407–3418. [Google Scholar] [CrossRef]
- Rodríguez-Saldaña, J.; Padilla-Padilla, F.; Cardona-Muñoz, E.G.; Romero-Antonio, Y.; Arguedas-Núñez, M.M.; Sander-Padilla, J.G.; Martínez-Muñoz, A.; Lugo-Sánchez, L.A.; Rodríguez-Vazquez, I.C.; González-Canudas, J. Real-World Evidence Evaluation on the Lipid Profile, Therapeutic Goals, and Safety of the Fixed-Dose Combination of Rosuvastatin/Ezetimibe (Trezete®) in Dyslipidemia Patients. Cardiol. Res. Pract. 2022, 2022, 9464733. [Google Scholar] [CrossRef]
- Samnaliev, M.; Khan, I.; Potukuchi, P.; Lee, K.; Garon, G.; Nicholls, C. Treatment adherence, persistence, and effectiveness of fixed dose combination versus free combination therapy of rosuvastatin-ezetimibe as a lipid-lowering therapy. Front. Cardiovasc. Med. 2025, 12, 1461416. [Google Scholar] [CrossRef]

| Characteristic | Value (n = 5527) |
|---|---|
| Age, years | 60.4 ± 11.6 |
| Female sex | 2599 (47.0) |
| Body weight, kg | 67.9 ± 13.1 |
| Height, cm | 163 ± 9 |
| Body mass index, kg/m2 | 25.4 ± 3.6 |
| Systolic blood pressure, mmHg | 128 ± 14 |
| Diastolic blood pressure, mmHg | 77 ± 10 |
| Medical history | |
| Hypertension | 2828 (51.2) |
| Diabetes mellitus | 1660 (30.0) |
| Coronary heart disease | 903 (20.1) |
| Heart failure | 254 (4.6) |
| Ischemic stroke | 293 (5.0) |
| Family history of cardiovascular disease or diabetes mellitus | 731 (13.2) |
| Current cigarette smoking | 1079 (19.5) |
| Prior anti-dyslipidemic medications | |
| Statin | 3246 (55.7) |
| Fibric acid | 70 (1.2) |
| Omega-3 fatty acid | 61 (1.0) |
| Ezetimibe | 7 (0.1) |
| Cholesterol Profile | Efficacy Set (n = 5527) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline (n = 5515) | At 12 Weeks (n = 4870) | % Change (n = 4861) | p | At 24 Weeks (n = 4941) | % Change (n = 4936) | p | |
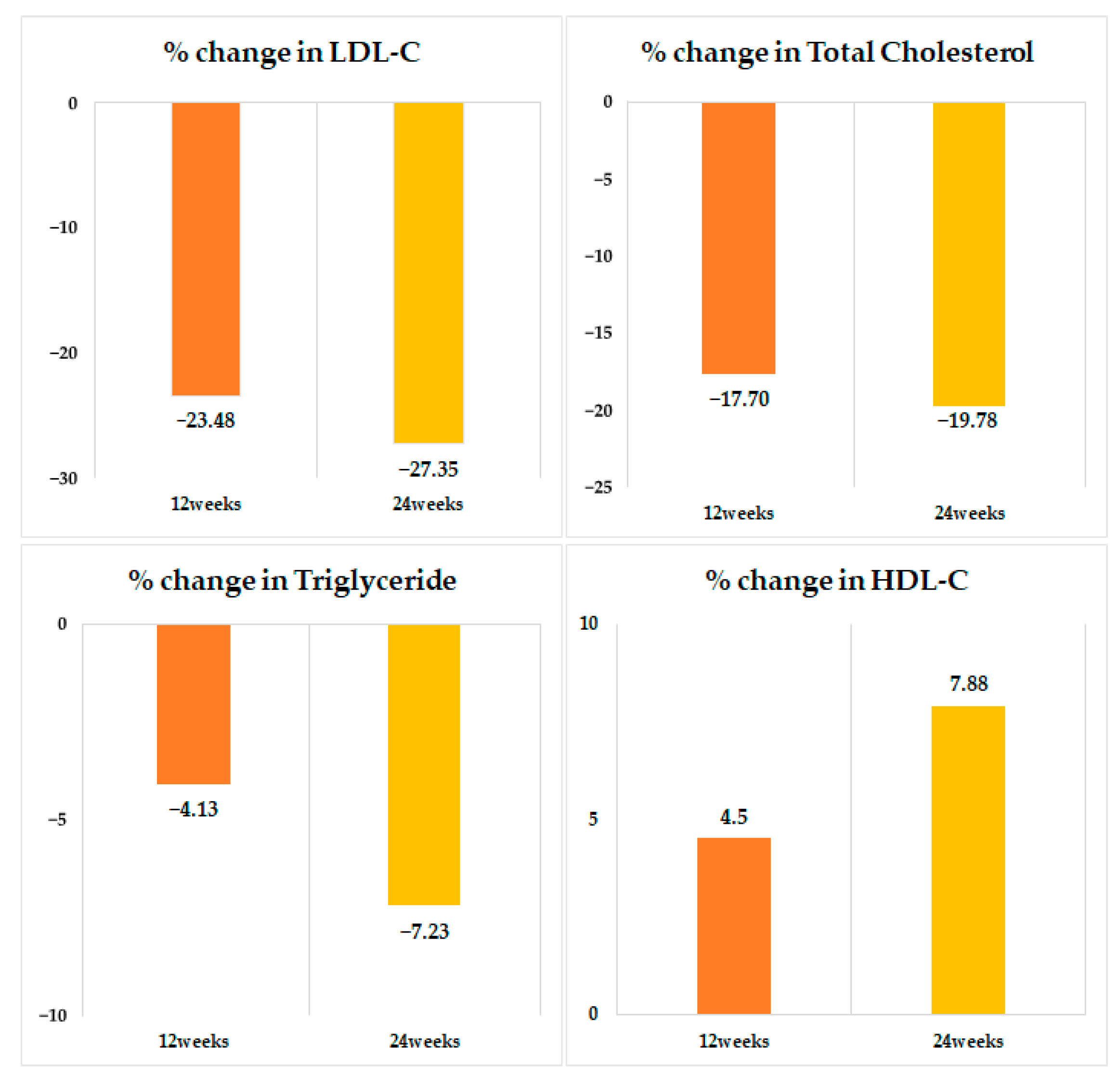
| TC, mg/dL | 0.0027 * | 0.0001 * | |||||
| Total population | 196 ± 59 | 156 ± 46 | −17.7 ± 21.9 | <0.0001 | 149 ± 39 | −19.7 ± 23.2 | <0.0001 |
| Group 1 (n = 2149) | 205 ± 58 | 161 ± 43 | −18.3 ± 21.9 | <0.0001 | 154 ± 37 | −20.6 ± 23.0 | <0.0001 |
| Group 2 (n = 1962) | 184 ± 56 | 149 ± 44 | −16.3 ± 21.5 | <0.0001 | 143 ± 38 | −18.0 ± 22.5 | <0.0001 |
| Group 3 (n = 1148) | 199 ± 62 | 157 ± 50 | −17.5 ± 22.5 | <0.0001 | 148 ± 44 | −20.5 ± 24.6 | <0.0001 |
| LDL-C, mg/dL | 0.0037 * | 0.0112 * | |||||
| Total population | 117 ± 51 | 81.1 ± 38.2 | −23.4 ± 87.8 | <0.0001 | 74.5 ± 33.3 | −27.3 ± 56.4 | <0.0001 |
| Group 1 (n = 2154) | 123 ± 52 | 82.9 ± 36.6 | −26.5 ± 34.7 | <0.0001 | 76.3 ± 31.7 | −28.9 ± 36.4 | <0.0001 |
| Group 2 (n = 1959) | 110 ± 48 | 78.8 ± 39.1 | −19.2 ± 140 | <0.0001 | 72.6 ± 33.5 | −24.4 ± 79.5 | <0.0001 |
| Group 3 (n = 1153) | 116 ± 50 | 81.1 ± 39.3 | −24.1 ± 36.6 | <0.0001 | 73.9 ± 35.9 | −28.2 ± 41.1 | <0.0001 |
| TG, mg/dL | <0.0001 * | <0.0001 * | |||||
| Total population | 176 ± 110 | 152 ± 100 | −13.0 ± −92.0 | <0.0001 | 142 ± 82 | −7.2 ± 53.7 | <0.0001 |
| Group 1 (n = 2156) | 153 ± 86 | 138 ± 75 | −0.81 ± 53.4 | <0.0001 | 132 ± 77 | −3.22 ± 52.0 | <0.0001 |
| Group 2 (n = 1962) | 190 ± 119 | 162 ± 125 | −4.79 ± 59.3 | <0.0001 | 150 ± 84 | −8.58 ± 58.7 | <0.0001 |
| Group 3 (n = 1153) | 192 ± 127 | 159 ± 95 | −7.34 ± 44.8 | <0.0001 | 149 ± 84 | −10.1 ± 49.3 | <0.0001 |
| HDL-C, mg/dL | <0.0001 * | <0.0001 * | |||||
| Total population | 50.8 ± 15.5 | 51.5 ± 15.5 | 4.50 ± 29.9 | <0.0001 | 52.2 ± 15.9 | 7.88 ± 50.0 | <0.0001 |
| Group 1 (n = 2156) | 58.5 ± 16.0 | 56.7 ± 13.7 | −0.59 ± 20.9 | <0.0001 | 57.0 ± 14.2 | 0.99 ± 45.9 | 0.0158 |
| Group 2 (n = 1962) | 45.4 ± 12.7 | 48.1 ± 17.3 | 8.31 ± 36.9 | <0.0001 | 49.1 ± 18.1 | 13.3 ± 61.2 | <0.0001 |
| Group 3 (n = 1150) | 47.1 ± 13.6 | 48.7 ± 13.5 | 6.39 ± 30.0 | <0.0001 | 49.5 ± 13.2 | 9.17 ± 36.1 | <0.0001 |
| Safety Set (n = 5811) | |||
|---|---|---|---|
| n (%) | 95% CI | Frequency | |
| Adverse event | 419 (7.21) | 6.56–7.91 | 558 |
| Adverse drug reaction | 163 (2.81) | 2.40–3.26 | 193 |
| Serious adverse event | 35 (0.60) | 0.42–0.84 | 38 |
| Serious adverse drug reaction | 4 (0.07) | 0.02–0.18 | 4 |
| Terminology | Safety Set (n = 5811) | |
|---|---|---|
| n (%) | Frequency | |
| Adverse event | ||
| Dizziness | 31 (0.53) | 31 |
| Hypertriglyceridemia | 28 (0.48) | 28 |
| Myalgia | 27 (0.46) | 29 |
| Abnormal hepatic function | 23 (0.40) | 23 |
| Chest pain | 21 (0.36) | 21 |
| Headache | 20 (0.34) | 21 |
| Abdominal pain | 14 (0.24) | 14 |
| Dyspepsia | 14 (0.24) | 14 |
| Conspitation | 12 (0.21) | 12 |
| Gastroesophageal reflux disease | 12 (0.21) | 12 |
| Total | 202 (3.48) | 205 |
| Adverse drug reaction | ||
| Hypertriglyceridemia | 27 (0.46) | 27 |
| Abnormal hepatic function | 20 (0.34) | 20 |
| Myalgia | 20 (0.34) | 20 |
| Dizziness | 10 (0.17) | 10 |
| Hyperlipidemia | 7 (0.12) | 7 |
| Hypercholesterolemia | 7 (0.12) | 7 |
| Gastroesophageal reflux disease | 6 (0.10) | 6 |
| Headache | 4 (0.07) | 4 |
| Chest pain | 4 (0.07) | 4 |
| Insomnia | 4 (0.07) | 4 |
| Total | 109 (1.88) | 109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-L.; Joh, H.S.; Kim, S.-H.; Kim, M.-A. Real-World Effectiveness of Rosuvastatin–Ezetimibe Single Pill (Rovazet®) in Korean Dyslipidemia Patients. J. Clin. Med. 2025, 14, 5480. https://doi.org/10.3390/jcm14155480
Kim H-L, Joh HS, Kim S-H, Kim M-A. Real-World Effectiveness of Rosuvastatin–Ezetimibe Single Pill (Rovazet®) in Korean Dyslipidemia Patients. Journal of Clinical Medicine. 2025; 14(15):5480. https://doi.org/10.3390/jcm14155480
Chicago/Turabian StyleKim, Hack-Lyoung, Hyun Sung Joh, Sang-Hyun Kim, and Myung-A Kim. 2025. "Real-World Effectiveness of Rosuvastatin–Ezetimibe Single Pill (Rovazet®) in Korean Dyslipidemia Patients" Journal of Clinical Medicine 14, no. 15: 5480. https://doi.org/10.3390/jcm14155480
APA StyleKim, H.-L., Joh, H. S., Kim, S.-H., & Kim, M.-A. (2025). Real-World Effectiveness of Rosuvastatin–Ezetimibe Single Pill (Rovazet®) in Korean Dyslipidemia Patients. Journal of Clinical Medicine, 14(15), 5480. https://doi.org/10.3390/jcm14155480






