Alternative Arterial Access in Veno-Arterial ECMO: The Role of the Axillary Artery
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Axillary Artery Anatomy and Surgical Access
3.1.1. Surgical Technique for Axillary Artery Cannulation in V-A ECMO
3.1.2. Percutaneous Technique for Axillary Artery Cannulation in V-A ECMO
3.2. Overview of ECMO Cannulation Strategy
3.2.1. Central Cannulation in V-A ECMO
3.2.2. Peripheral V-A ECMO. Femoral Cannulation, North–South Syndrome, and the Emerging Role of Axillary Access
3.3. Axillary Artery Cannulation: An Evolving Strategy in Veno-Arterial ECMO
3.3.1. Clinical Evidence Supporting the Efficacy of Axillary Artery Cannulation
3.3.2. Femoro-Axillary vs. Femoro-Femoral Cannulation in V-A ECMO: A Comparative Insight into Hemodynamic and Clinical Outcomes
3.3.3. Cerebral Perfusion and Neurologic Outcomes: Comparing Axillary, Femoral and Central Cannulation Strategy
3.3.4. Complications Associated with Axillary Artery Cannulation for V-A ECMO
- Upper extremity ischemia, due to arterial spasm, thrombus formation or inadequate distal perfusion [35].
- Bleeding and hematoma formation, especially in anticoagulated patients or when surgical hemostasis is challenging due to anatomical constraints [50].
- Nerve injury, including brachial plexus trauma, may occur due to local hematoma or during surgical dissection [12].
- Iatrogenic pneumothorax: though uncommon, remains a procedural risk, particularly with percutaneous attempts in the infraclavicular region or in patients with emphysematous lungs, morbid obesity or altered thoracic anatomy [57].
- Limb hyper perfusion, compartment syndrome, and tissue necrosis [17].
- Cannulation performed in emergency settings, often under suboptimal conditions, may be associated with higher complications rates at the time of decannulation due to inadequate positioning, lack of vessel control or unrecognized arterial injury [58].
3.4. Indications for Axillary Cannulation in V-A ECMO
- Anatomic indications: Axillary access is primarily indicated in individuals with significant peripheral arterial disease (PAD), morbid obesity or when preservation of upper body perfusion and cerebral oxygenation is of paramount concern. In patients with severe PAD, the iliofemoral arteries may be heavily calcified or stenotic, increasing the technical complexity and clinical risks of femoral cannulation, including limb ischemia, inadequate systemic flow, and embolic complications [59]. Similarly, in obese patients, where femoral vessels are often difficult to access and prone to infection or hemorrhagic complications due to deep subcutaneous layers, the axillary artery offers a more superficial and surgically manageable target [60].
- Physiological and Hemodynamic indications: a particularly compelling advantage of axillary cannulation in ECMO lies in its ability to deliver true antegrade systemic perfusion, thereby optimizing oxygen delivery to the cerebral and coronary circulations. This feature is especially relevant in preventing North–South syndrome (NSS) [29].
- Clinical and organizational indications: the anatomical location and stability of the axillary cannulation site offer practical advantages in the context of long-term ECMO support. Compared to femoral access, axillary cannulation is better suited for patient mobilization and active rehabilitation, which are increasingly recognized as key components of care in prolonged extracorporeal support. This further reinforces its role in advanced ECMO management, particularly in patients with expected delayed recovery or as a bridge to transplant or durable mechanical circulatory support [61].
3.5. Contraindications, Pre-Procedural Imaging, and Limitations of Axillary Artery Cannulation
3.6. Risk of Decannulation Related Complications in V-A ECMO: Axillary vs. Femoral Artery Access
3.7. ECMELLA Configuration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ECMO | Extracorporeal membrane oxygenation |
| LV | Left ventricle |
| FF | Femoro-femoral |
| FAx | Femoro-axillary |
| V-A | Veno-arterial |
| NSS | North–South syndrome |
| LVEF | Left ventricular ejection fraction |
| PAD | Peripheral artery disease |
| BMI | Body mass index |
References
- Lafç, G.; Budak, A.B.; Yener, A.Ü.; Cicek, O.F. Use of extracorporeal membrane oxygenation in adults. Heart Lung Circ. 2014, 23, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Telukuntla, K.S.; Estep, J.D. Acute Mechanical Circulatory Support for Cardiogenic Shock. Methodist Debakey Cardiovasc. J. 2020, 16, 27–35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline. Contemporary Management of Cardiogenic Shock: A Scientific Statement from the American Heart Association. Circulation 2017, 136, e232–e268. [Google Scholar] [CrossRef] [PubMed]
- Ferrel, M.N.; Raza, S.S.; Tang, P.; Haft, J.; Ala, A.A.E. Cannulation strategies for extracorporeal membrane oxygenation. Indian J. Thorac. Cardiovasc. Surg. 2023, 39 (Suppl. S1), 91–100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Burrell, A.J.C.; Ihle, J.F.; Pellegrino, V.A.; Sheldrake, J.; Nixon, P.T. Cannulation technique: Femoro-femoral. J. Thorac. Dis. 2018, 10 (Suppl. S5), S616–S623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bonicolini, E.; Martucci, G.; Simons, J.; Raffa, G.M.; Spina, C.; Lo Coco, V.; Arcadipane, A.; Pilato, M.; Lorusso, R. Limb ischemia in peripheral veno-arterial extracorporeal membrane oxygenation: A narrative review of incidence, prevention, monitoring, and treatment. Crit. Care 2019, 23, 266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Falk, L.; Sallisalmi, M.; Lindholm, J.A.; Lindfors, M.; Frenckner, B.; Broomé, M.; Broman, L.M. Differential hypoxemia during venoarterial extracorporeal membrane oxygenation. Perfusion 2019, 34 (Suppl. S1), 22–29. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Safdar, A.; Chahal, D. Axillary Approach for Venoarterial Extracorporeal Membrane Oxygenation Cannulation. Cureus 2020, 12, e7788. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, K.; Lee, H.; Choi, I.J.; Jeong, W.; Kim, H.T.; Wei, Q.; Lee, J.H. Topography and Anatomical Variations of the Axillary Artery. Biomed. Res. Int. 2021, 2021, 6393780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takahashi, G.; Saiki, Y. Vascular Access for Axillary Artery, Femoral Artery and Femoral Vein. Kyobu Geka 2024, 77, 754–760. [Google Scholar] [PubMed]
- Standring, S. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 42nd ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Ohira, S.; Malekan, R.; Goldberg, J.B.; Lansman, S.L.; Spielvogel, D.; Kai, M.; Collaborators. Axillary artery cannulation for veno-arterial extracorporeal membrane oxygenation support in cardiogenic shock. JTCVS Tech. 2020, 5, 62–71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hillebrand, J.; Konerding, M.A.; Koch, M.; Kaufmann, T.; Steinseifer, U.; Moritz, A.; Dzemali, O. Anatomic and flow dynamic considerations for safe right axillary artery cannulation. J. Thorac. Cardiovasc. Surg. 2013, 146, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Mordhorst, A.; Yan, T.D.; Hoskins, N.; Gagnon, J.; Kazemi, K. Percutaneous proximal axillary artery versus femoral artery access for endovascular interventions. J. Vasc. Surg. 2022, 76, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Gregory, V.; Isath, A.; Grunfeld, M.; Kanwal, A.; Levine, A.; Kai, M.; Ohira, S. CARC8: Right versus Left Axillary Artery Access in Impella 5.5 Insertion. ASAIO J. 2023, 69 (Suppl. S2), 43. [Google Scholar] [CrossRef]
- Lorenz, V.; Muzzi, L.; Neri, E. How to perform a direct axillary artery cannulation. Multimed. Man. Cardiothorac. Surg. 2022, 2022. [Google Scholar] [CrossRef] [PubMed]
- Chamogeorgakis, T.; Lima, B.; Shafii, A.E.; Nagpal, D.; Pokersnik, J.A.; Navia, J.L.; Mason, D.; Gonzalez-Stawinski, G.V. Outcomes of axillary artery side graft cannulation for extracorporeal membrane oxygenation. J. Thorac. Cardiovasc. Surg. 2013, 145, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Sabik, J.F.; Lytle, B.W.; McCarthy, P.M.; Cosgrove, D.M. Axillary artery: An alternative site of arterial cannulation for patients with extensive aortic and peripheral vascular disease. J. Thorac. Cardiovasc. Surg. 1995, 109, 885–890; discussion 890–891. [Google Scholar] [CrossRef] [PubMed]
- Cakici, M.; Ozcinar, E.; Baran, C.; Bermede, A.O.; Sarıcaoglu, M.C.; Inan, M.B.; Durdu, M.S.; Aral, A.; Sirlak, M.; Akar, A.R. A retrospective cohort analysis of percutaneous versus side-graft perfusion techniques for veno-arterial extracorporeal membrane oxygenation in patients with refractory cardiogenic shock. Perfusion 2017, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Gawda, R.; Czarnik, T. Ultrasound-guided axillary artery cannulation in the infraclavicular area: A step-by-step approach. J. Vasc. Access 2025, 1, 11297298251334890. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, G.; Cao, Y.; Li, C.; Shi, B.; Zhao, M.; Lin, G.; Chang, X.; Ma, X.; Li, Q.; et al. Feasibility of Ultrasound-Guided Percutaneous Axillary Artery Cannulation for Veno-Arterial Extracorporeal Membrane Oxygenation and its Effect on the Recovery of Spontaneous Heartbeat in Patients with ECPR. Altern. Ther. Health Med. 2025, 31, 192–199. [Google Scholar] [PubMed]
- Camboni, D.; Philip, A.; Schmid, C.; Loforte, A. Double, triple and quadruple cannulation for veno-arterial extracorporeal membrane oxygenation support: Is there a limit? Ann. Cardiothorac. Surg. 2019, 8, 151–159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- MacLaren, G.; Butt, W.; Best, D.; Donath, S. Central extracorporeal membrane oxygenation for refractory pediatric septic shock. Pediatr. Crit. Care Med. 2011, 12, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Raffa, G.M.; Kowalewski, M.; Brodie, D.; Ogino, M.; Whitman, G.; Meani, P.; Pilato, M.; Arcadipane, A.; Delnoij, T.; Natour, E.; et al. Meta-Analysis of Peripheral or Central Extracorporeal Membrane Oxygenation in Postcardiotomy and Non-Postcardiotomy Shock. Ann. Thorac. Surg. 2019, 107, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Pooboni, S.K.; Gulla, K.M. Vascular access in ECMO. Indian J. Thorac. Cardiovasc. Surg. 2021, 37 (Suppl. S2), 221–231. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, A.; Forrest, P.; Loforte, A. Left ventricular decompression in veno-arterial extracorporeal membrane oxygenation. Ann. Cardiothorac. Surg. 2019, 8, 9–18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Falk, L.; Hultman, J.; Broman, L.M. Differential hypoxemia and the clinical significance of venous drainage position during extracorporeal membrane oxygenation. Perfusion 2023, 38, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Rozencwajg, S.; Wu, E.L.; Heinsar, S.; Stevens, M.; Chinchilla, J.; Fraser, J.F.; Pauls, J.P. A mock circulation loop to evaluate differential hypoxemia during peripheral venoarterial extracorporeal membrane oxygenation. Perfusion 2024, 39, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Hou, X. How to mange differential hypoxemia during veno-arterial extracorporeal membrane oxygenation. Perfusion 2024, 39, 443–444. [Google Scholar] [CrossRef] [PubMed]
- Matsuyoshi, T.; Shimizu, K.; Kaneko, H.; Kohsen, D.; Suzuki, H. Reconfiguration from veno-arterial to veno-arterio-venous extracorporeal membrane oxygenation for massive pulmonary embolism. J. Artif. Organs 2022, 25, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, S.W.; Kim, Y.U.; Kim, S.Y.; Kim, K.S.; Joo, S.J.; Lee, J.S. Application of veno-arterial-venous extracorporeal membrane oxygenation in differential hypoxia. Multidiscip. Respir. Med. 2014, 9, 55. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weber, C.; Deppe, A.C.; Sabashnikov, A.; Slottosch, I.; Kuhn, E.; Eghbalzadeh, K.; Scherner, M.; Choi, Y.H.; Madershahian, N.; Wahlers, T. Left ventricular thrombus formation in patients undergoing femoral veno-arterial extracorporeal membrane oxygenation. Perfusion 2018, 33, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Swartz, M.F.; Massey, H.T. Impella to unload the left ventricle during peripheral extracorporeal membrane oxygenation. ASAIO J. 2013, 59, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, R.; Shekar, K.; MacLaren, G.; Schmidt, M.; Pellegrino, V.; Meyns, B.; Haft, J.; Vercaemst, L.; Pappalardo, F.; Bermudez, C.; et al. ELSO Interim Guidelines for Venoarterial Extracorporeal Membrane Oxygenation in Adult Cardiac Patients. ASAIO J. 2021, 67, 827–844, Erratum in ASAIO J. 2022, 68, e133. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.; Baghdadi, K.; Popov, A.F.; Sandoval Boburg, R.; Risteski, P.; Schlensak, C.; Walter, T.; Berger, R.; Emrich, F. Right Axillary Artery Cannulation for Veno-Arterial Extracorporeal Membrane Oxygenation in Postcardiotomy Patients: A Single-Center Experience. Medicina 2023, 59, 2040. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jin, N.; Pang, X.; Song, S.; Zheng, J.; Liu, Z.; Gu, T.; Yu, Y. A comparative study of femoral artery and combined femoral and axillary artery cannulation in veno-arterial extracorporeal membrane oxygenation patients. Front. Cardiovasc. Med. 2024, 11, 1388577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pisani, A.; Braham, W.; Brega, C.; Lajmi, M.; Provenchere, S.; Danial, P.; Alkhoder, S.; Para, M.; Ghodbane, W.; Nataf, P. Right axillary artery cannulation for venoarterial extracorporeal membrane oxygenation: A retrospective single centre observational study. Eur. J. Cardiothorac. Surg. 2021, 59, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Hysi, I.; Fabre, O.; Renaut, C.; Guesnier, L. Extracorporeal membrane oxygenation with direct axillary artery perfusion. J. Card. Surg. 2014, 29, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Vale, J.D.; Kantor, E.; Papin, G.; Sonneville, R.; Braham, W.; Para, M.; Montravers, P.; Longrois, D.; Provenchère, S. Femoro-axillary versus femoro-femoral veno-arterial extracorporeal membrane oxygenation for refractory cardiogenic shock: A monocentric retrospective study. Perfusion 2025, 40, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Andrei, S.; Tran-Dinh, A.; Provenchere, S.; Lortat-Jacob, B.; Ghodbane, W.; Montravers, P.; Longrois, D. A quantified description of the interactions between the native cardiovascular system and femoro-femoral versus femoro-axillary extracorporeal life support using descending thoracic aorta velocity time integral. Artif. Organs 2019, 43, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, G.; Mariani, S.; Schaefer, A.K.; van Bussel, B.C.T.; Di Mauro, M.; Wiedemann, D.; Saeed, D.; Pozzi, M.; Botta, L.; Boeken, U.; et al. Neurologic complications in patients receiving aortic versus subclavian versus femoral arterial cannulation for post-cardiotomy extracorporeal life support: Results of the PELS observational multicenter study. Crit. Care 2024, 28, 265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nishikawa, M.; Willey, J.; Takayama, H.; Kaku, Y.; Ning, Y.; Kurlansky, P.A.; Brodie, D.; Masoumi, A.; Fried, J.; Takeda, K. Stroke patterns and cannulation strategy during veno-arterial extracorporeal membrane support. J. Artif. Organs 2022, 25, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Salna, M.; Ikegami, H.; Willey, J.Z.; Garan, A.R.; Cevasco, M.; Chan, C.; Takayama, H.; Colombo, P.C.; Naka, Y.; Takeda, K. Transcranial Doppler is an effective method in assessing cerebral blood flow patterns during peripheral venoarterial extracorporeal membrane oxygenation. J. Card Surg. 2019, 34, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Ohira, S.; Dhand, A.; Hirani, R.; Martinez, S.; Lanier, G.M.; Levine, A.; Pan, S.; Aggarwal-Gupta, C.; Gass, A.L.; Wolfe, K.; et al. Cannulation-related adverse events of peripheral veno-arterial extracorporeal membrane oxygenation support in heart transplantation: Axillary versus femoral artery cannulation. Clin. Transplant. 2023, 37, e14871. [Google Scholar] [CrossRef] [PubMed]
- Rastan, A.J.; Dege, A.; Mohr, M.; Doll, N.; Falk, V.; Walther, T.; Mohr, F.W. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J. Thorac. Cardiovasc. Surg. 2010, 139, 302–311.e1. [Google Scholar] [CrossRef] [PubMed]
- Navia, J.L.; Atik, F.A.; Beyer, E.A.; Ruda Vega, P. Extracorporeal membrane oxygenation with right axillary artery perfusion. Ann. Thorac. Surg. 2005, 79, 2163–2165. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.Q.; Cook, B.S.; Cauchi, M.P.; Foerst, J.R. A case series: Alternative access for refractory shock during cardiac arrest. Eur. Heart J. Case Rep. 2019, 3, ytz101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sibut-Pinote, V.; Reymond, P.; Cikirikcioglu, M.; Bendjelid, K.; Huber, C. Extracorporeal Membrane Oxygenation Cannulation Site Affects Coronary and Cerebral Perfusion When Combined with Intra-Aortic Balloon Pump. ASAIO J. 2025. [Google Scholar] [CrossRef] [PubMed]
- Feiger, B.; Kochar, A.; Gounley, J.; Bonadonna, D.; Daneshmand, M.; Randles, A. Determining the impacts of venoarterial extracorporeal membrane oxygenation on cerebral oxygenation using a one-dimensional blood flow simulator. J. Biomech. 2020, 104, 109707. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mittal, M.K.; Schears, G.J.; Wijdicks, E.F. Brachial plexus injury associated with extracorporeal membrane oxygenation. J. Clin. Neuromuscul. Dis. 2013, 15, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Joffre, J.; Preda, G.; Arrivé, L.; Maury, E. Fatal Aortic Dissection during Extracorporeal Membrane Oxygenation Axillary Cannulation Confirmed by Postmortem Computed Tomography Angiography. Am. J. Respir. Crit. Care Med. 2017, 195, 953–954. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Harada, K.; Funayama, H.; Kario, K. Massive Upper-extremity Edema after Transaxillary Venoarterial-extracorporeal Membrane Oxygenation. Intern. Med. 2023, 62, 1695–1696. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Omer, S.; Rajagopal, K. Commentary: When preserving life, do not neglect the limb: The role of axillary artery cannulation in venoarterial extracorporeal membrane oxygenation. JTCVS Tech. 2020, 5, 72–73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Capuano, F.; Danesi, T.H.; Roscitano, A.; Sinatra, R. How to ensure a good flow to the arm during direct axillary artery cannulation. Eur. J. Cardiothorac. Surg. 2011, 40, 520–521. [Google Scholar] [CrossRef] [PubMed]
- Moazami, N.; Moon, M.R.; Lawton, J.S.; Bailey, M.; Damiano, R., Jr. Axillary artery cannulation for extracorporeal membrane oxygenator support in adults: An approach to minimize complications. J. Thorac. Cardiovasc. Surg. 2003, 126, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.; Ahmad Ael, S.; Marinos, S.; Moritz, A.; Zierer, A. Simple and controlled method to avoid hyperperfusion of the right arm following axillary artery cannulation for extracorporeal membrane oxygenator support. Thorac. Cardiovasc. Surg. 2013, 61, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Flumignan, R.L.; Trevisani, V.F.; Lopes, R.D.; Baptista-Silva, J.C.; Flumignan, C.D.; Nakano, L.C. Ultrasound guidance for arterial (other than femoral) catheterisation in adults. Cochrane Database Syst. Rev. 2021, 10, CD013585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hadano, H.; Kamio, T.; Fukaguchi, K.; Sato, M.; Tsunano, Y.; Koyama, H. Analysis of adverse events related to extracorporeal membrane oxygenation from a nationwide database of patient-safety accidents in Japan. J. Artif. Organs 2024, 27, 15–22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alnahhal, K.I.; Majumdar, M.; Irshad, A.; Kapur, N.; Kumar, S.; Salehi, P. Peripheral artery disease and extracorporeal membrane oxygenation: Examining a high-risk cohort over time. Vascular 2024, 32, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, N.H.; O’Malley, T.J.; Abai, B.; Salvatore, D.M.; DiMuzio, P.J.; Hirose, H. Complications of Peripheral Cannulation Site in Obese Patients on Adult Extracorporeal Membrane Oxygenation. ASAIO J. 2021, 67, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.; Garan, A.R.; Brodie, D. Awake and fully mobile patients on cardiac extracorporeal life support. Ann. Cardiothorac. Surg. 2019, 8, 44–53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seto, A.H.; Estep, J.D.; Tayal, R.; Tsai, S.; Messenger, J.C.; Alraies, M.C.; Schneider, D.B.; Klein, A.J.; Duwayri, Y.; McCabe, J.M.; et al. SCAI Position Statement on Best Practices for Percutaneous Axillary Arterial Access and Training. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100041. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fleck, T.; Ehrlich, M.; Czerny, M.; Hutschala, D.; Tschernko, E.; Mares, P.; Wolner, E.; Grabenwoger, M. Cannulation of the axillary artery: The decision between direct cannulation and cannulation via side graft. Thorac. Cardiovasc. Surg. 2005, 53, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Talwar, A.; Wiadji, E.; Mathur, M.N. Experience with the Axillary Artery as an Arterial Cannulation Site in Patients with Acute Type A Aortic Dissection. Heart Lung Circ. 2019, 28, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Schachner, T.; Nagiller, J.; Zimmer, A.; Laufer, G.; Bonatti, J. Technical problems and complications of axillary artery cannulation. Eur. J. Cardiothorac. Surg. 2005, 27, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Mandel, J.L.; Yost, C.C.; Rosen, J.L.; Prochno, K.W.; Round, K.J.; Komlo, C.M.; Guy, T.S. An alternate approach: Percutaneous axillary cannulation for minimally invasive cardiac surgery. J. Card. Surg. 2022, 37, 5622–5625. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.; Kostibas, M.P. Echocardiographic and Point-of-Care Ultrasonography (POCUS) Guidance in the Management of the ECMO Patient. J. Clin. Med. 2024, 13, 2630. [Google Scholar] [CrossRef]
- Shetty, H.; Patil, V.; Mobin, N.; Gowda, M.H.N.; Puttamallappa, V.S.; Vamadevaiah, R.M.; Kunjappagounder, P. Study of course and termination of brachial artery by dissection and computed tomography angiography methods with clinical importance. Anat. Cell Biol. 2022, 55, 284–293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arnett, D.M.; Lee, J.C.; Harms, M.A.; Kearney, K.E.; Ramos, M.; Smith, B.M.; Anderson, E.C.; Tayal, R.; McCabe, J.M. Caliber and fitness of the axillary artery as a conduit for large-bore cardiovascular procedures. Catheter. Cardiovasc. Interv. 2018, 91, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.; Liu, C.; Xu, Z.; Chen, Y. Impact factors of POCUS-guided cannulation for peripheral venoarterial extracorporeal membrane oxygenation: One single-center retrospective clinical analysis. Medicine 2022, 101, e29489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, J.; Tse, J.R.; Chan, F.; Fleischmann, D. CT Angiography of Venoarterial Extracorporeal Membrane Oxygenation. Radiographics 2022, 42, 23–37, Erratum in Radiographics 2022, 42, E104. [Google Scholar] [CrossRef] [PubMed]
- Aboul Nour, H.; Poyiadji, N.; Mohamed, G.; Alsrouji, O.K.; Ramadan, A.R.; Griffith, B.; Marin, H.; Chebl, A.B. Challenges of acute phase neuroimaging in VA-ECMO, pitfalls and alternative imaging options. Interv. Neuroradiol. 2021, 27, 434–439. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.; Cho, Y.H.; Sung, K.; Park, T.K.; Lee, G.Y.; Lee, J.M.; Song, Y.B.; Hahn, J.Y.; Choi, J.H.; Choi, S.H.; et al. Impact of Cannula Size on Clinical Outcomes in Peripheral Venoarterial Extracorporeal Membrane Oxygenation. ASAIO J. 2019, 65, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Kobsa, S.; Kaku, Y.; Takayama, H. Commentary: Axillary or femoral cannulation-Which is the lesser of 2 evils? JTCVS Tech. 2020, 5, 74–75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nunes-Carvalho, J.; Silva, E.; Spath, P.; Araújo-Andrade, L.; Troisi, N.; Neves, J.R. Efficacy, safety, and complications of manta vascular closure device in VA-ECMO decannulation: A systematic review and meta-analysis. J. Vasc. Access 2025, 11297298251325391. [Google Scholar] [CrossRef] [PubMed]
- Au, S.Y.; Chan, K.S.; Fong, K.M.; Wong, H.R.; Fong, Y.H.; Chui, S.F.; Chan, K.T.; Lee, K.M.; Ng, W.G.; So, S.O.; et al. Comparing the outcomes of bedside percutaneous VA-ECMO decannulation by ProGlide and Manta in a high-ECMO-volume center in Hong Kong. Artif. Organs 2022, 46, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Guo, K.; Liu, Y.; Wei, W.; Zhao, K.; Huang, H.; Yao, Z. Perclose ProGlide closure devices vs. surgical removal for veno-arterial extracorporeal membrane oxygenation decannulation: A meta-analysis. Front. Cardiovasc. Med. 2025, 12, 1482305. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bisdas, T.; Beutel, G.; Warnecke, G.; Hoeper, M.M.; Kuehn, C.; Haverich, A.; Teebken, O.E. Vascular complications in patients undergoing femoral cannulation for extracorporeal membrane oxygenation support. Ann. Thorac. Surg. 2011, 92, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Lamb, K.M.; Hirose, H.; Cavarocchi, N.C. ECMO therapy: The role of surgical decannulation and closure techniques after femoral artery cannulation. J. Card. Surg. 2013, 28, 151–154. [Google Scholar] [CrossRef]
- Shah, A.; Robinson, J.; Leibowitz, J.; Singireddy, S.; Levy, L.; Ghoreishi, M.; Toursavadkohi, S.; Grazioli, A.; Rabin, J.; Kaczorowski, D.; et al. Vascular Closure Device vs Open Decannulation for Femoral Venoarterial Extracorporeal Membrane Oxygenation. Ann. Thorac. Surg. 2025, 120, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.; Desai, M.; Ryan, L.P.; Clevenger, L.; Speir, A.M.; Singh, R. Preclosure technique versus arterial cutdown after percutaneous cannulation for venoarterial extracorporeal membrane oxygenation. JTCVS Tech. 2021, 10, 322–330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hart, J.P.; Davies, M.G. Vascular Complications in Extracorporeal Membrane Oxygenation—A Narrative Review. J. Clin. Med. 2024, 13, 5170. [Google Scholar] [CrossRef] [PubMed]
- Tellioglu, T.M.; Iner, H.; Karaagac, E.; Yalcin, M.C.; Gurbuz, M.; Besir, Y.; Gokalp, O.; Yilik, L. Different Paths, Same Goals: A Comparative Study on the Safety of Femoral vs. Axillary Arterial Cannulation in VA ECMO. J. Clin. Med. 2025, 14, 4613. [Google Scholar] [CrossRef]
- Meani, P.; Lorusso, R.; Pappalardo, F. ECPella: Concept, Physiology and Clinical Applications. J. Cardiothorac. Vasc. Anesth. 2022, 36, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Isath, A.; Ohira, S.; Levine, A.; Lanier, G.M.; Pan, S.; Aggarwal-Gupta, C.; Mason, I.; Gregory, V.; Spielvogel, D.; Gass, A.L.; et al. Evolution of concomitant use of veno-arterial extracorporeal membrane oxygenation support with Impella in cardiogenic shock: From percutaneous femoral Impella to axillary Impella 5.5. Artif. Organs 2023, 47, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, A.M.; Potapov, E.; Schibilsky, D.; Ruhparwar, A.; Tschöpe, C.; Spillmann, F.; Benk, C.; Schmack, B.; Schmitto, J.D.; Napp, L.C.; et al. First in man evaluation of a novel circulatory support device: Early experience with the Impella 5.5 after CE mark approval in Germany. J. Heart Lung Transplant. 2021, 40, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Eulert-Grehn, J.J.; Starck, C.; Kempfert, J.; Falk, V.; Potapov, E. ECMELLA 2.0: Single Arterial Access Technique for a Staged Approach in Cardiogenic Shock. Ann. Thorac. Surg. 2021, 111, e135–e137. [Google Scholar] [CrossRef] [PubMed]
- Bitargil, M.; Pham, S.; Haddad, O.; Sareyyupoglu, B. Single arterial access for Ecpella and jugular venous cannulation provides full mobility on a status 1 heart transplant recipient. ESC Heart Fail. 2022, 9, 2003–2006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tadokoro, N.; Tonai, K.; Kainuma, S.; Fukushima, S. Single-vessel access venoarterial extracorporeal membrane oxygenation and Impella technique for acute cardiogenic shock complicated by lung congestion. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 37, ivad201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernhardt, A.M.; Schrage, B.; Westermann, D.; Reichenspurner, H. Extracorporeal membrane oxygenation evolution: Left ventricular unloading strategies. JTCVS Open 2021, 8, 85–89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Potapov, E.; Loforte, A.; Pappalardo, F.; Morshuis, M.; Schibilsky, D.; Zimpfer, D.; Lewin, D.; Riebandt, J.; Von Aspern, K.; Stein, J.; et al. Impact of a surgical approach for implantation of durable left ventricular assist devices in patients on extracorporeal life support. J. Card. Surg. 2021, 36, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Bertoldi, L.F.; Pappalardo, F.; Lubos, E.; Grahn, H.; Rybczinski, M.; Barten, M.J.; Legros, T.; Bertoglio, L.; Schrage, B.; Westermann, D.; et al. Bridging INTERMACS 1 patients from VA-ECMO to LVAD via Impella 5.0: De-escalate and ambulate. J. Crit. Care 2020, 57, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, A.M.; Zipfel, S.; Reiter, B.; Hakmi, S.; Castro, L.; Söffker, G.; Kluge, S.; Lubos, E.; Rybczinski, M.; Grahn, H.; et al. Impella 5.0 therapy as a bridge-to-decision option for patients on extracorporeal life support with unclear neurological outcomes. Eur. J. Cardiothorac. Surg. 2019, 56, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Kruse, J.; Silaschi, M.; Velten, M.; Wittmann, M.; Alaj, E.; Ahmad, A.E.; Zimmer, S.; Borger, M.A.; Bakhtiary, F. Femoral or Axillary Cannulation for Extracorporeal Circulation during Minimally Invasive Heart Valve Surgery (FAMI): Protocol for a Multi-Center Prospective Randomized Trial. J. Clin. Med. 2023, 12, 5344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Badulak, J.; Abrams, D.; Luks, A.M.; Zakhary, B.; Conrad, S.A.; Bartlett, R.; MacLaren, G.; Vercaemst, L.; Lorusso, R.; Broman, L.M.; et al. Extracorporeal Life Support Organization (ELSO). Position paper on the physiology and nomenclature of dual circulation during venoarterial ECMO in adults. Intensive Care Med. 2024, 50, 1994–2004, Erratum in Intensive Care Med. 2025, 51, 661–662. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simons, J.; Doddema, A.R.; Körver, E.P.; di Mauro, M.; Agricola, S.; Smets, J.; Metz, R.; Mariani, S.; De Piero, M.E.; Matteucci, M.; et al. Novel cannulation strategy with a bidirectional cannula for distal limb perfusion during peripheral veno-arterial extracorporeal life support: A preliminary, single-centre study. Perfusion 2023, 38 (Suppl. S1), 44–53. [Google Scholar] [CrossRef] [PubMed]
- Arons, D.; Dave, S.; Shah, A.; Deatrick, K.B. Contralateral Lower Extremity Ischemia on Femoral Veno-Arterial Extracorporeal Membrane Oxygenation. ASAIO J. 2023, 69, e128–e130. [Google Scholar] [CrossRef] [PubMed]
- Lunz, D.; Philipp, A.; Müller, T.; Pfister, K.; Foltan, M.; Rupprecht, L.; Schmid, C.; Lubnow, M.; Graf, B.; Sinner, B. Ischemia-related vascular complications of percutaneously initiated venoarterial extracorporeal membrane oxygenation: Indication setting, risk factors, manifestation and outcome. J. Crit. Care 2019, 52, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Lamb, K.M.; Hirose, H. Vascular Complications in Extracoporeal Membrane Oxygenation. Crit. Care Clin. 2017, 33, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Li, T. Delayed retroperitoneal hemorrhage during extracorporeal membrane oxygenation in COVID-19 patients: A case report and literature review. World J. Clin. Cases 2021, 9, 5203–5210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maybauer, M.O.; El Banayosy, A.; Koerner, M.M.; Hooker, R.L.; Swant, L.V.; Mihu, M.R.; Harper, M.D. Mechanical cardiopulmonary resuscitation for venoarterial ECMO implantation in pulmonary embolism complicated by type B aortic dissection and retroperitoneal hemorrhage. J. Card. Surg. 2020, 35, 2821–2824. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, A.K.; Latus, M.; Riebandt, J.; Goliasch, G.; Bernardi, M.H.; Laufer, G.; Zimpfer, D.; Wiedemann, D. Bleeding and thrombotic events in post-cardiotomy extracorporeal life support. Eur. J. Cardiothorac. Surg. 2023, 63, ezad072. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuonqui, K.; Diaddigo, S.E.; LaValley, M.N.; Dagi, A.F.; Dugue, D.; Imahiyerobo, T.A.; Bogue, J.T. Management of Venoarterial Extracorporeal Membrane Oxygenation Cannulation-Associated Groin Wound Complications With Muscle Flaps at a High-Acuity Cardiac Referral Center. Ann. Plast. Surg. 2024, 93, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Lamb, K.M.; DiMuzio, P.J.; Johnson, A.; Batista, P.; Moudgill, N.; McCullough, M.; Eisenberg, J.A.; Hirose, H.; Cavarocchi, N.C. Arterial protocol including prophylactic distal perfusion catheter decreases limb ischemia complications in patients undergoing extracorporeal membrane oxygenation. J. Vasc. Surg. 2017, 65, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- Siems, C.; Valentine, R.J.; Wang, Q.; Duke, J.; Brunsvold, M.; Reed, A.B. Risk factors for lower extremity vascular complications in adult patients on veno-arterial extracorporeal membrane oxygenation. J. Vasc. Surg. 2023, 77, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Banks, C.A.; Blakeslee-Carter, J.; Nkie, V.; Spangler, E.L.; Still, S.A.; Eudailey, K.W.; McElwee, S.K.; Blood, M.S.; Novak, Z.; Beck, A.W. Occurrence, predictors, and management of late vascular complications following extracorporeal membrane oxygenation. J. Vasc. Surg. 2024, 80, 864–872.e1. [Google Scholar] [CrossRef] [PubMed]
- Hager, H.H.; Burns, B. Artery Cannulation (Archived). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Lu, S.Y.; Ortoleva, J.; Colon, K.; Mueller, A.; Laflam, A.; Shelton, K.; Dalia, A.A. Association Between Body Mass Index and Outcomes in Venoarterial Extracorporeal Membrane Oxygenation. Anesth. Analg. 2022, 134, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Memon, S.; Drosou, M.E.; Caroline, M.; Casanova, E.; Gnall, E.M. Feasibility and outcomes with subclavian vein access for crescent jugular dual lumen catheter for venovenous extracorporeal membrane oxygenation in COVID-19 related acute respiratory distress syndrome. Perfusion 2024, 39, 304–309. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Becker, T.K.; Bruno, J.; Jeng, E.I.; Phillips, J.; Smith, T.N.; Roberts, D.L.; Carr, C.T.; Cooper, M.A.; Back, M.R.; Anderson, R.D.; et al. Successful Extracorporeal Cardiopulmonary Resuscitation Despite Aortic Occlusion. ASAIO J. 2025, 71, e81–e83. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.; Elhakim, A.; Rusch, R.; Berndt, R.; Panholzer, B.; Lutter, G.; Frank, D. Trans-Brachial TAVI in a Patient with Aortic Isthmus Stenosis: A Case Report. J. Clin. Med. 2024, 13, 308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

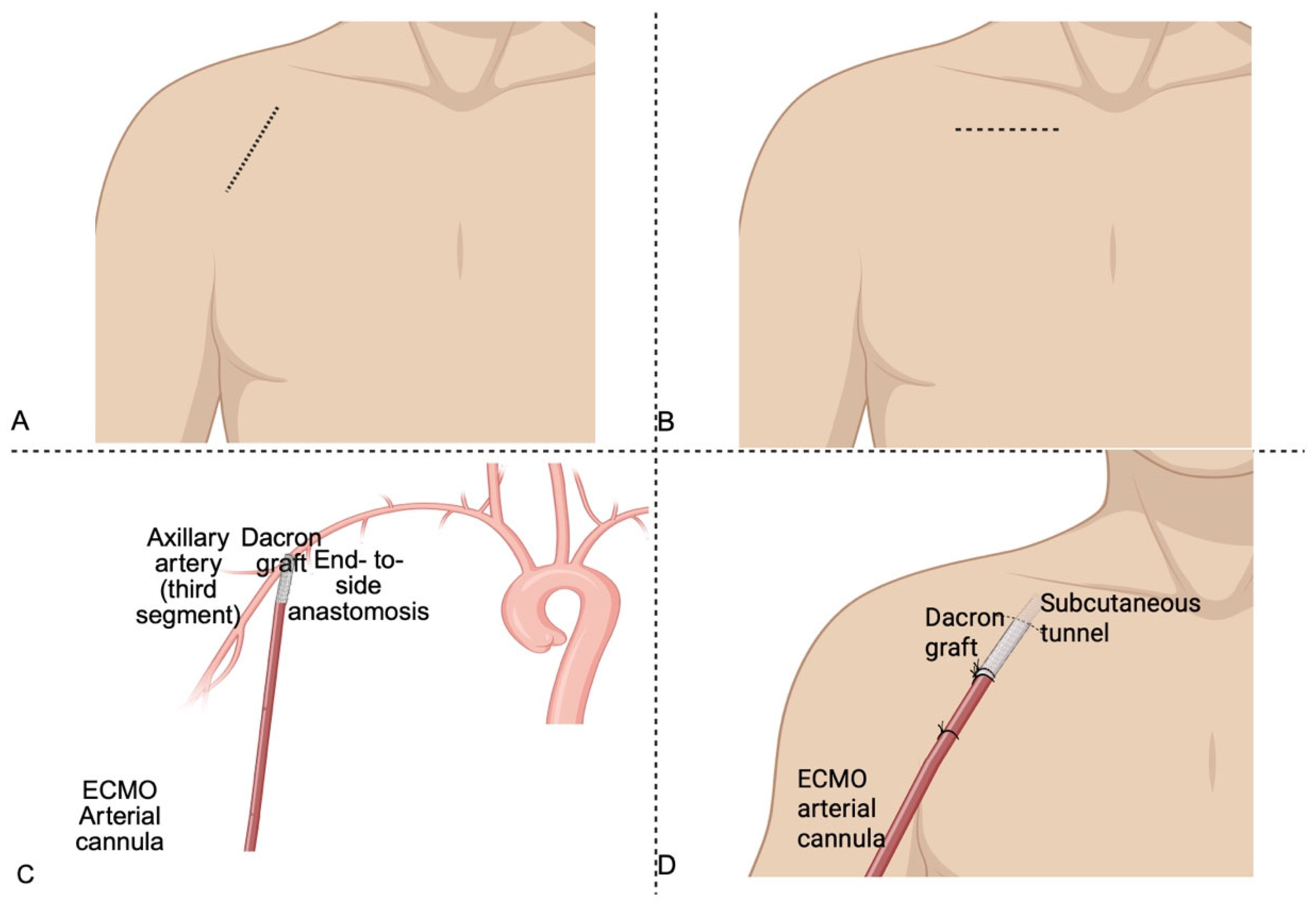

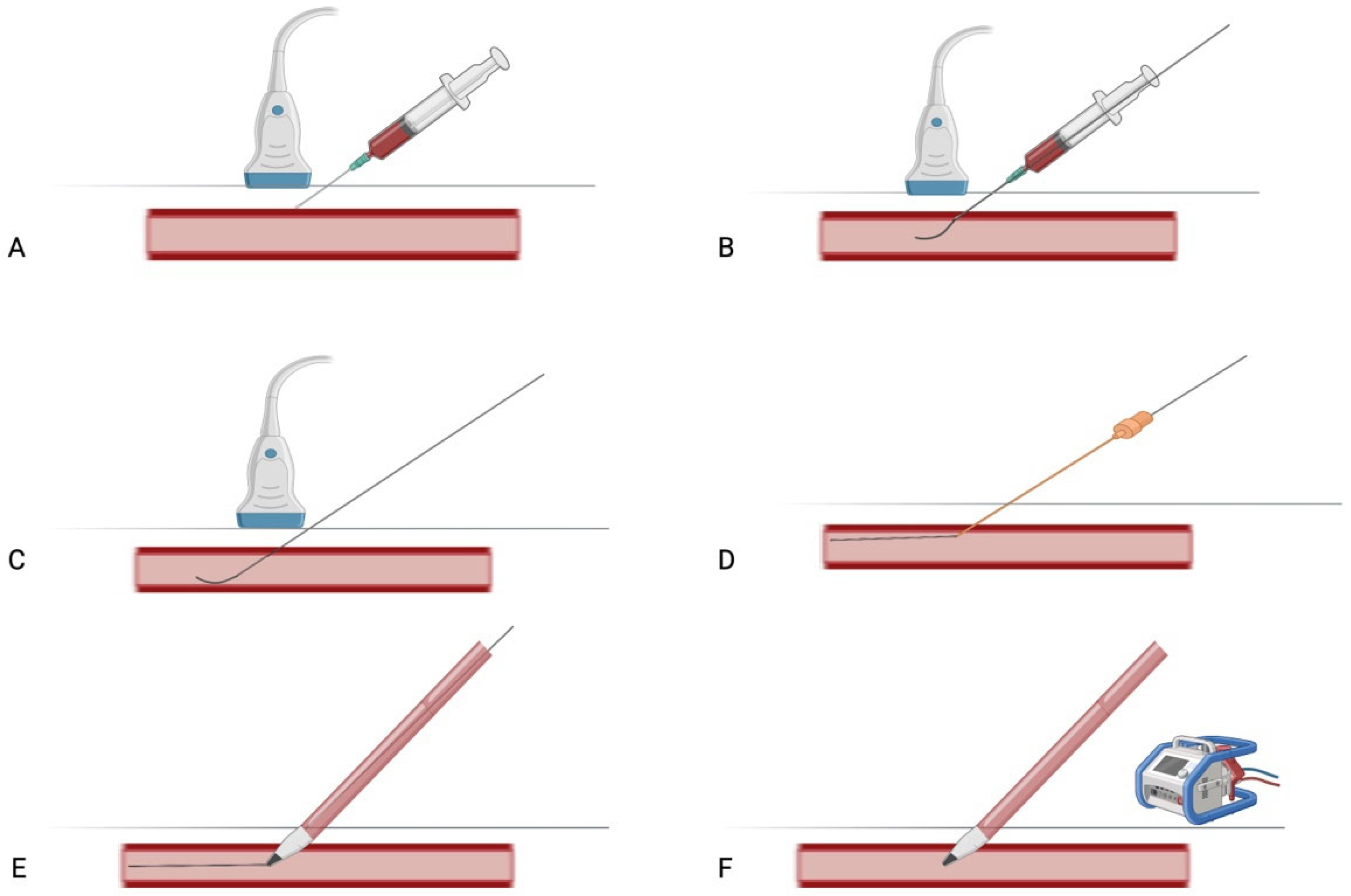
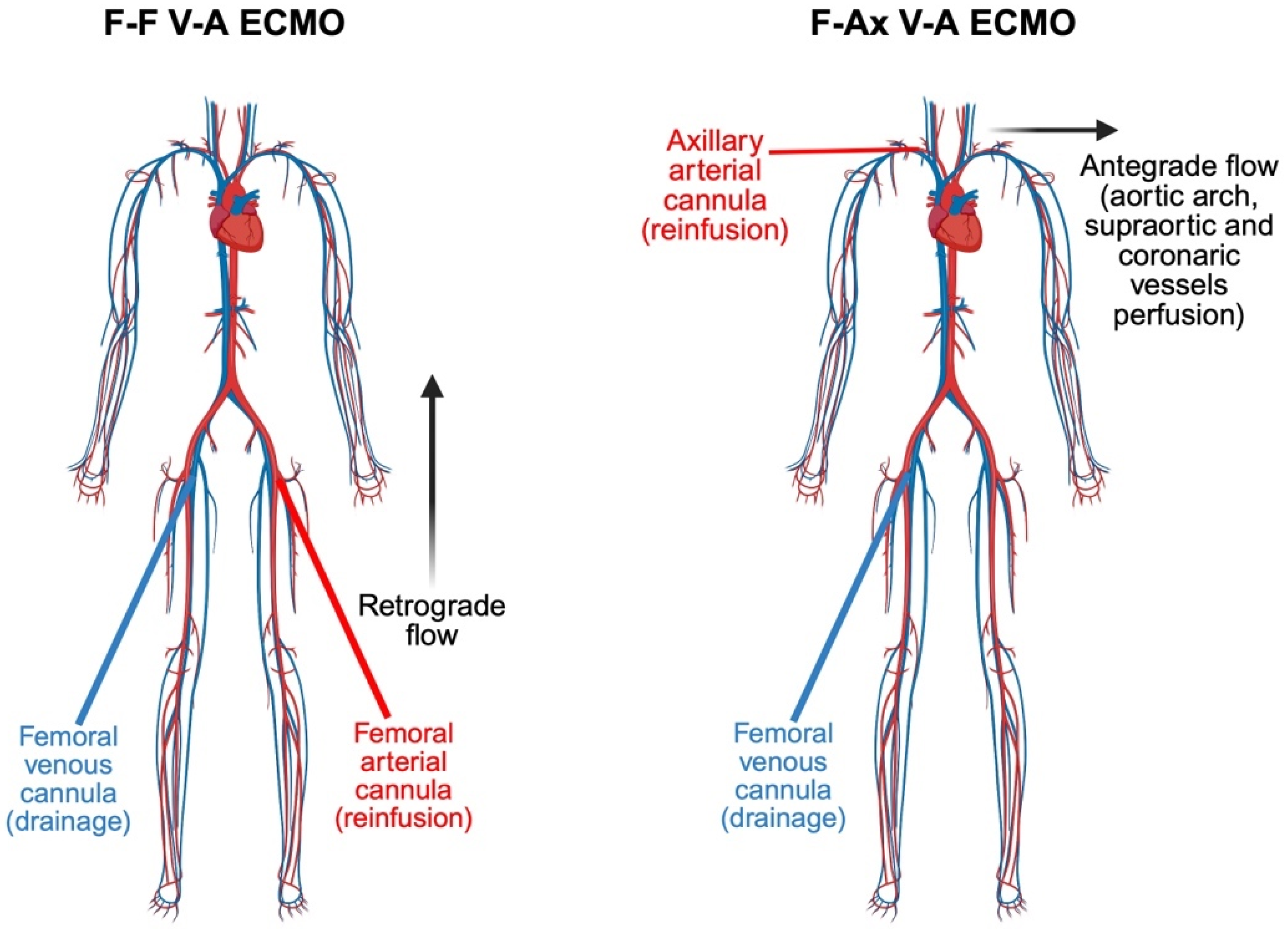
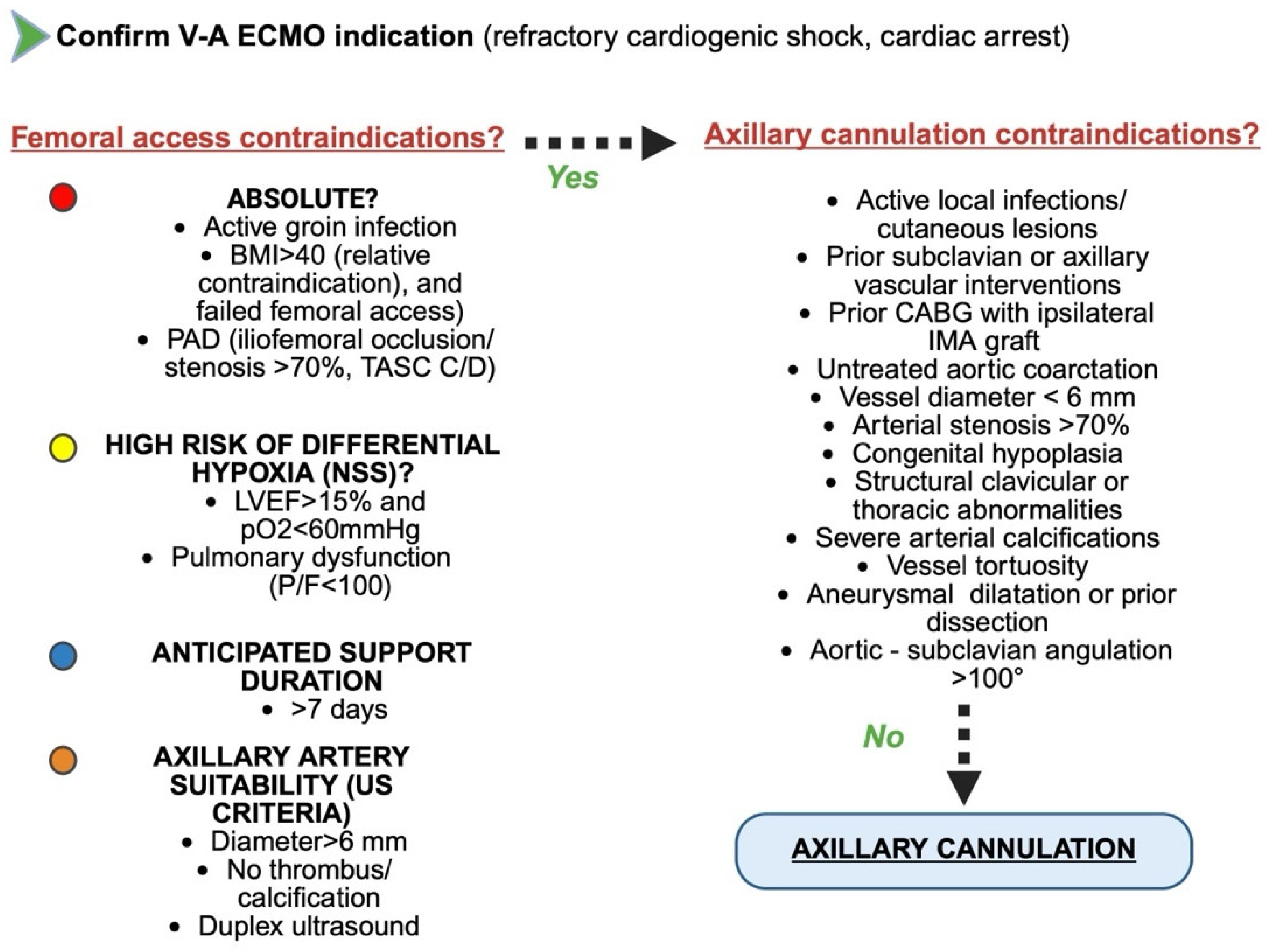
| Study | Design | Population | Key Findings | Complications |
|---|---|---|---|---|
| Ohira et al. (2020) [12] | Retrospective | Cardiogenic shock patients on V-A ECMO (n = 371) | Axillary access reduced limb ischemia, wound complications, and site conversion vs. femoral access, with equivalent survival and no increased bleeding or stroke | ↓ limb ischemia vs. femoral cohort |
| Chamogeorgakis et al. (2013) [17] | Retrospective | Adult V-A ECMO patients (n = 81 axillary side-graft, 166 femoral, 61 aortic) | Axillary artery cannulation using a side graft was associated with safe and effective extracorporeal support, facilitated patient mobilization, and significantly reduced lower limb ischemia compared to femoral access | Ipsilateral upper limb hyperperfusion syndrome (24.7%), graft site bleeding (17.3%). Femoral access was associated with higher rates of lower limb ischemia and fasciotomy |
| Cakici et al. (2017) [19] | Retrospective observational cohort | V-A ECMO (percutaneous vs. side graft) (n = 148) | Side-graft technique had fewer perfusion related complications and improved limb perfusion vs. percutaneous access | Acute limb ischemia (2.7% side-graft vs. 5.3% percutaneous), bleeding (12% side graft vs. 24.7% percutaneous), hyperperfusion syndrome (2.7% percutaneous vs. 30% side-graft). Survival outcomes were similar between groups |
| Liu et al. (2025) [21] | Prospective observational | ECPR patients with US-guided percutaneous axillary access (n = 7) | US-guided axillary access was feasible | US-guided percutaneous axillary cannulation is feasible |
| Radwan et al. (2023) [35] | Retrospective | Post-cardiotomy V-A ECMO via right axillary artery (n = 179) | FAx weaning success: 48.6%; in-hospital survival: 34.6%; 1-year survival: 74% (among weaned) | Subclavian bleeding (13.4%), upper limb ischemia (6.1%), stroke (10.6%), intracerebral hemorrhage (5%) |
| Jin et al. (2024) [36] | Retrospective | Post-cardiotomy V-A ECMO FF vs. Fax (n = 51) | FAx group: ↓ chronic renal failure (14.81% vs. 37.50%), ↑ platelets, ↓ creatinine vs. FF. Similar 30-day mortality | FAx reduced renal/metabolic complications |
| Pisani et al. (2021) [37] | Observational (n = 174) | V-A ECMO via right axillary artery (n = 174) | FAx feasible approach; 1-year survival: 72.7% (weaned patients) | Bleeding (4%), upper limb ischemia (1.1%), local infection (1.7%), brachial plexus injury (0.6%) |
| Hysi et al. (2013) [38] | Case series | ECMO requiring direct axillary cannulation (n = 16) | Axillary direct cannulation (no graft) provided reliable perfusion with low neurovascular complications | Minimal neurologic/vascular complications |
| Vale et al. (2024) [39] | Retrospective | Refractory cardiogenic shock: FAx vs. FF cannulation (n = 534) | FAx ↓ limb ischemia, local infections, bowel ischemia, pulmonary edema vs. FF. Similar 90-day mortality | ↑ Stroke in FAx group |
| Andrei et al. (2019) [40] | Prospective | FF vs. FAx ECLS configurations (n = 11) | FAx: ↑ LV ejection (↑ VTI in descending aorta with > ECMO flow). FF: ↓ VTI (LV outflow obstruction from retrograde flow) | Hemodynamic evidence of LV compromise with FF ECMO |
| Chiarini et al. (2024) [41] | Multicentric | Post-cardiotomy ECLS: aortic vs. axillary vs. femoral (n = 1897) | FAx: ↑ major neurologic events/seizures vs. aortic | Highest neurologic risk with axillary; |
| Nishikawa et al. (2021) [42] | Retrospective | V-A ECMO aortic vs. axillary vs. femoral (n = 414) | Stroke rates similar (6.2–6.5%); ischemic strokes (64%) across territories. | Uniform risk regardless of cannulation site |
| Salna et al. (2019) [43] | Prospective | Peripheral V-A ECMO: axillary vs. Femoral (n = 37) | FAx: ↑ MCA flow velocity, ↓ pulsatility index, ↑ cerebral perfusion stability. FF: ↑ pulsatility, suboptimal perfusion | Axillary optimizes cerebral hemodynamics |
| Ohira et al. (2022) [44] | Retrospective | Heart transplant recipient on V-A ECMO FAx vs. FF (n = 80) | FAx ↓ cannulation related wound infections vs. FF. Survival, stroke, bleeding, limb ischemia equivalent | Site-specific infection advantage with FAx |
| Rastan et al. (2010) [45] | Retrospective | Post cardiotomy cardiogenic shock patients on V-A ECMO. (n = 517) 60.8% central cannulation 39.2% peripheral cannulatio (30.3% Fax ECMO) | Peripheral cannulation, including Fax experienced suboptimal ECMO flow (80–90% of cardiac output) compared with central cannulation | Cerebrovascular events (17.4%), gastrointestinal bleeding (18.8%), renal failure requiring dialysis (65%). Comparable survival and complication rates between central and peripheral access |
| Study | Design | Population | Key Findings | Complications |
|---|---|---|---|---|
| Ahmed et al. (2020) [8] | Case report | Patient on V-A ECMO with cardiogenic shock with axillary access (n = 1) | Successful use of Axillary artery cannulation for V-A ECMO using a side graft approach. The technique allowed early ambulation and reduce the risk of lib ischemia. | No cannulation-related complications reported |
| Navia et al. (2005) [46] | Technical report | Not specified | Describes the utilization of right axillary artery cannulation for ECMO support, emphasizing the technique’s feasibility in preserving cerebral and upper body perfusion while minimizing limb ischemia | Not reported |
| Cui et al. (2019) [47] | Case series (n = 3) | Refractory cardiac arrest with percutaneous axillary access | The axillary artery provided a feasible and effective alternative access route for V-A ECMO initiation in the setting of cardiac arrest and refractory shock, especially when femoral access was contraindicated or technical challenging | No access-related complications |
| Sibut-Pinote et al. (2025) [48] | Perfusion simulation | Combined ECMO-IABP in low cardiac output | FAx ↑ coronary/cerebral perfusion vs. FF; ↓ renal perfusion | Site-dependent perfusion trade-offs (renal hypoperfusion with axillary) |
| Feiger et al. (2020) [49] | Computational model | Simulated V-A ECMO flows | Axillary/Central sites: adequate carotid perfusion at 1 L/min. Femoral: required > 4.9 L/min for equivalent perfusion | Potential implications for cerebral hypoperfusion or hyperperfusion depending on ECMO flow and cannulation strategy |
| Mittal et al. (2013) [50] | Case report (n = 2) | ECMO patients | Axillary cannulation linked to brachial plexus injury from hematoma-induced compression | Neurologic injury due to local hematoma |
| Joffre et al. (2017) [51] | Case report (n = 1) | ECMO via axillary cannulation | Fatal aortic dissection during cannulation | Catastrophic vascular injury |
| Saito et al. (2023) [52] | Case report (n = 1) | V-A ECMO via trans-axillary cannulation | Massive upper extremity edema from venous obstruction/inflammation | Massive upper extremity edema |
| Omer et al. (2020) [53] | Expert commentary | - | Highlights the limb-sparing and cerebral perfusion benefits of axillary cannulation. | - |
| Capuano et al. (2011) [54] | Technique description | - | Side-graft anastomosis (e.g., 8 mm8-mm Dacron) preserved antegrade limb flow, ↓ ischemia. Emphasizes the importance of graft orientation and anastomotic configuration to prevent upper limb hyperperfusion | Mitigated upper limb ischemia/compartment syndrome |
| Moazami et al. (2003) [55] | Technique description | - | Describes a surgical approach for axillary artery cannulation aimed at reducing access-related complication during ECMO support. Highlights the importance of graft tunneling and secure fixation to prevent limb ischemia | No significant complications reported; the technique was developed to minimize risk of bleeding and neurovascular injury. |
| Papadopoulos et al. (2012) [56] | Technique description | - | Side-graft anastomosis reduced hyperperfusion complications via balanced flow distribution | Optimized hemodynamic profile |
| Indication/Clinical Scenario | Rationale/Benefit |
|---|---|
| Severe femoral PAD | Avoids diseased femoral vessels; ensures reliable arterial inflow |
| Morbid obesity | Facilitates surgical access; reduces risk of infection and hemorrhagic complications |
| Risk of North–South syndrome | Provides antegrade perfusion to aortic arch; preserves cerebral and coronary perfusion |
| Requirement for preserved cerebral perfusion | Improves upper body oxygen delivery |
| Native cardiac output with recovering LV function | Promotes favorable mixing dynamics; limits excessive retrograde ECMO competition |
| Risk of increased afterload and impaired LV unloading | May offer modest hemodynamic benefits but does not eliminate the need for additional LV unloading strategies in patients with impaired ventricular function, as peripheral V-A ECMO intrinsically increases afterload |
| Anticipated long-term ECMO support | Better tolerated anatomically; allows improved patient management and access for vascular care |
| Early mobilization strategy | Provides stable cannula positioning; facilitates active physiotherapy and rehabilitation in selected cases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torre, D.E.; Pirri, C. Alternative Arterial Access in Veno-Arterial ECMO: The Role of the Axillary Artery. J. Clin. Med. 2025, 14, 5413. https://doi.org/10.3390/jcm14155413
Torre DE, Pirri C. Alternative Arterial Access in Veno-Arterial ECMO: The Role of the Axillary Artery. Journal of Clinical Medicine. 2025; 14(15):5413. https://doi.org/10.3390/jcm14155413
Chicago/Turabian StyleTorre, Debora Emanuela, and Carmelo Pirri. 2025. "Alternative Arterial Access in Veno-Arterial ECMO: The Role of the Axillary Artery" Journal of Clinical Medicine 14, no. 15: 5413. https://doi.org/10.3390/jcm14155413
APA StyleTorre, D. E., & Pirri, C. (2025). Alternative Arterial Access in Veno-Arterial ECMO: The Role of the Axillary Artery. Journal of Clinical Medicine, 14(15), 5413. https://doi.org/10.3390/jcm14155413








