A Novel Deep Learning Model for Predicting Colorectal Anastomotic Leakage: A Pioneer Multicenter Transatlantic Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population and Study Design
2.2. Criteria for Definition of Anastomosis Leak
2.3. Development of the Deep Learning Model
2.4. Model Performance and Statistical Analysis
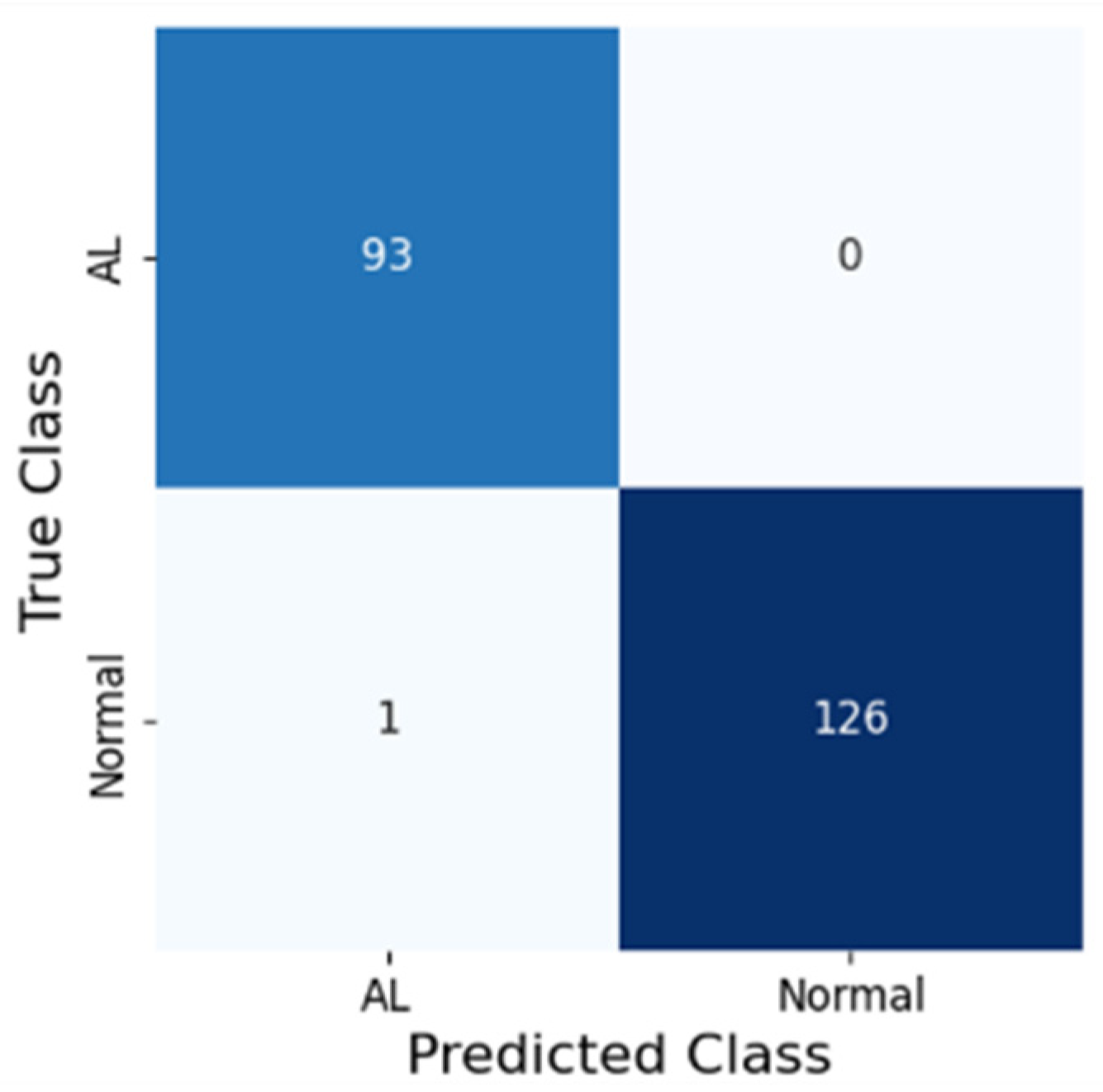
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hyman, N.; Manchester, T.L.; Osler, T.; Burns, B.; Cataldo, P.A. Anastomotic leaks after intestinal anastomosis: It’s later than you think. Ann. Surg. 2007, 245, 254–258. [Google Scholar] [CrossRef]
- Ha, G.W.; Kim, J.H.; Lee, M.R. Oncologic Impact of Anastomotic Leakage Following Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2017, 24, 3289–3299. [Google Scholar] [CrossRef]
- Ellis, C.T.; Maykel, J.A. Defining Anastomotic Leak and the Clinical Relevance of Leaks. Clin. Colon Rectal Surg. 2021, 34, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Choi, G.-S.; Oh, J.H.; Kim, N.K.; Park, J.S.; Kim, M.J.; Lee, K.Y.; Baik, S.H. Multicenter Analysis of Long-Term Oncologic Impact of Anastomotic Leakage After Laparoscopic Total Mesorectal Excision: The Korean Laparoscopic Colorectal Surgery Study Group. Medicine 2015, 94, e1202. [Google Scholar] [CrossRef]
- Midura, E.F.; Hanseman, D.; Davis, B.R.; Atkinson, S.J.; Abbott, D.E.; Shah, S.A.; Paquette, I.M. Risk factors and consequences of anastomotic leak after colectomy: A national analysis. Dis. Colon Rectum 2015, 58, 333–338. [Google Scholar] [CrossRef]
- Rahbari, N.N.; Weitz, J.; Hohenberger, W.; Heald, R.J.; Moran, B.; Ulrich, A.; Holm, T.; Wong, W.D.; Tiret, E.; Moriya, Y.; et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: A proposal by the International Study Group of Rectal Cancer. Surgery 2010, 147, 339–351. [Google Scholar] [CrossRef]
- Nijssen, D.J.; Wienholts, K.; Postma, M.J.; Tuynman, J.; Bemelman, W.A.; Laméris, W.; Hompes, R. The economic impact of anastomotic leakage after colorectal surgery: A systematic review. Tech. Coloproctol. 2024, 28, 55. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.Q.; Burns, E.M.; Jani, A.; Altman, S.; Young, J.D.; Cunningham, C.; Faiz, O.; Mortensen, N.J. The economic impact of anastomotic leakage after anterior resections in English NHS hospitals: Are we adequately remunerating them? Color. Dis. 2013, 15, e190–e198. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.; Tozer, P.; Paterson-Brown, S.; Garden, O.J. Colorectal Surgery: A Companion to Specialist Surgical Practice; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Litchinko, A.; Buchs, N.; Balaphas, A.; Toso, C.; Liot, E.; Meurette, G.; Ris, F.; Meyer, J. Score prediction of anastomotic leak in colorectal surgery: A systematic review. Surg. Endosc. 2024, 38, 1723–1730. [Google Scholar] [CrossRef]
- Stearns, A.T.; Liccardo, F.; Tan, K.; Sivrikoz, E.; Aziz, O.; Jenkins, J.T.; Kennedy, R.H. Physiological changes after colorectal surgery suggest that anastomotic leakage is an early event: A retrospective cohort study. Color. Dis. 2019, 21, 297–306. [Google Scholar] [CrossRef]
- Tsalikidis, C.; Mitsala, A.; Mentonis, V.I.; Romanidis, K.; Pappas-Gogos, G.; Tsaroucha, A.K.; Pitiakoudis, M. Predictive Factors for Anastomotic Leakage Following Colorectal Cancer Surgery: Where Are We and Where Are We Going? Curr. Oncol. 2023, 30, 3111–3137. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, M.M.; Fransvea, P.; Cariati, M.; Adams, N.J.; Bianchi, V.; Brisinda, G. Anastomotic leakage in colorectal cancer surgery. Surg. Oncol. 2022, 40, 101708. [Google Scholar] [CrossRef]
- Erb, L.; Hyman, N.H.; Osler, T. Abnormal vital signs are common after bowel resection and do not predict anastomotic leak. J. Am. Coll. Surg. 2014, 218, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.; Marland, J.R.K.; Murray, A.F.; Argyle, D.J.; Potter, M.A. Predictive and Diagnostic Biomarkers of Anastomotic Leakage: A Precision Medicine Approach for Colorectal Cancer Patients. J. Pers. Med. 2021, 11, 471. [Google Scholar] [CrossRef]
- Wit, A.; Daams, F. Considerations in case of suspected anastomotic leakage in the lower GI tract. Best Pract. Res. Clin. Gastroenterol. 2024, 70, 101925. [Google Scholar] [CrossRef]
- McDermott, F.D.; Heeney, A.; Kelly, M.E.; Steele, R.J.; Carlson, G.L.; Winter, D.C. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br. J. Surg. 2015, 102, 462–479. [Google Scholar] [CrossRef]
- Nicksa, G.A.; Dring, R.V.; Johnson, K.H.; Sardella, W.V.; Vignati, P.V.; Cohen, J.L. Anastomotic leaks: What is the best diagnostic imaging study? Dis. Colon Rectum 2007, 50, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Power, N.; Atri, M.; Ryan, S.; Haddad, R.; Smith, A.C.T. assessment of anastomotic bowel leak. Clin. Radiol. 2007, 62, 37–42. [Google Scholar] [CrossRef]
- Ikeda, T.; Kumashiro, R.; Taketani, K.; Ando, K.; Kimura, Y.; Saeki, H.; Oki, E.; Morita, M.; Akahoshi, T.; Hashizume, M.; et al. Endoscopic evaluation of clinical colorectal anastomotic leakage. J. Surg. Res. 2015, 193, 126–134. [Google Scholar] [CrossRef]
- Hirst, N.A.; Tiernan, J.P.; Millner, P.A.; Jayne, D.G. Systematic review of methods to predict and detect anastomotic leakage in colorectal surgery. Color. Dis. 2014, 16, 95–109. [Google Scholar] [CrossRef]
- Kryzauskas, M.; Bausys, A.; Dulskas, A.; Imbrasaite, U.; Danys, D.; Jotautas, V.; Stratilatovas, E.; Strupas, K.; Poskus, E.; Poskus, T. Comprehensive testing of colorectal anastomosis: Results of prospective observational cohort study. Surg. Endosc. 2022, 36, 6194–6204. [Google Scholar] [CrossRef]
- Arpaia, P.; Bracale, U.; Corcione, F.; De Benedetto, E.; Di Bernardo, A.; Di Capua, V.; Duraccio, L.; Peltrini, R.; Prevete, R. Assessment of blood perfusion quality in laparoscopic colorectal surgery by means of Machine Learning. Sci. Rep. 2022, 12, 14682. [Google Scholar] [CrossRef]
- Kryzauskas, M.; Bausys, A.; Jakubauskas, M.; Valciukiene, J.; Makunaite, G.; Jasiunas, E.; Bausys, R.; Poskus, E.; Strupas, K.; Poskus, T. Intraoperative testing of colorectal anastomosis and the incidence of anastomotic leak: A meta-analysis. Medicine 2020, 99, e23135. [Google Scholar] [CrossRef]
- Irani, J.L.; Hedrick, T.L.; Miller, T.E.; Lee, L.; Steinhagen, E.; Shogan, B.D.; Goldberg, J.E.; Feingold, D.L.; Lightner, A.L.; Paquette, I.M. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American Society of Colon and Rectal Surgeons and the Society of American Gastrointestinal and Endoscopic Surgeons. Surg. Endosc. 2023, 37, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Anteby, R.; Horesh, N.; Soffer, S.; Zager, Y.; Barash, Y.; Amiel, I.; Rosin, D.; Gutman, M.; Klang, E. Deep learning visual analysis in laparoscopic surgery: A systematic review and diagnostic test accuracy meta-analysis. Surg. Endosc. 2021, 35, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Graaf, F.W.; Lange, M.M.; Spakman, J.I.; van Grevenstein, W.M.U.; Lips, D.; Graaf, E.J.R.; Menon, A.G.; Lange, J.F. Comparison of Systematic Video Documentation With Narrative Operative Report in Colorectal Cancer Surgery. JAMA Surg. 2019, 154, 381–389. [Google Scholar] [CrossRef]
- Jalal, N.A.; Alshirbaji, T.A.; Docherty, P.D.; Arabian, H.; Laufer, B.; Krueger-Ziolek, S.; Neumuth, T.; Moeller, K. Laparoscopic Video Analysis Using Temporal, Attention, and Multi-Feature Fusion Based-Approaches. Sensors 2023, 23, 1958. [Google Scholar] [CrossRef]
- Quero, G.; Mascagni, P.; Kolbinger, F.R.; Fiorillo, C.; De Sio, D.; Longo, F.; Schena, C.A.; Laterza, V.; Rosa, F.; Menghi, R.; et al. Artificial Intelligence in Colorectal Cancer Surgery: Present and Future Perspectives. Cancers 2022, 14, 3803. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Park, H.M.; Baek, K.R.; Ahn, H.M.; Lee, I.Y.; Son, G.M. Artificial intelligence based real-time microcirculation analysis system for laparoscopic colorectal surgery. World J. Gastroenterol. 2020, 26, 6945–6962. [Google Scholar] [CrossRef]
- Kitaguchi, D.; Takeshita, N.; Matsuzaki, H.; Takano, H.; Owada, Y.; Enomoto, T.; Oda, T.; Miura, H.; Yamanashi, T.; Watanabe, M.; et al. Real-time automatic surgical phase recognition in laparoscopic sigmoidectomy using the convolutional neural network-based deep learning approach. Surg. Endosc. 2020, 34, 4924–4931. [Google Scholar] [CrossRef]
- Morris, M.X.; Rajesh, A.; Asaad, M.; Hassan, A.; Saadoun, R.; Butler, C.E. Deep Learning Applications in Surgery: Current Uses and Future Directions. Am. Surg. 2023, 89, 36–42. [Google Scholar] [CrossRef]
- Foppa, C.; Ng, S.C.; Montorsi, M.; Spinelli, A. Anastomotic leak in colorectal cancer patients: New insights and perspectives. Eur. J. Surg. Oncol. 2020, 46, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Morse, B.C.; Simpson, J.P.; Jones, Y.R.; Johnson, B.L.; Knott, B.M.; Kotrady, J.A. Determination of independent predictive factors for anastomotic leak: Analysis of 682 intestinal anastomoses. Am. J. Surg. 2013, 206, 950–955. [Google Scholar] [CrossRef]
- Daniel, V.T.; Alavi, K.; Davids, J.S.; Sturrock, P.R.; Harnsberger, C.R.; Steele, S.R.; Maykel, J.A. The utility of the delphi method in defining anastomotic leak following colorectal surgery. Am. J. Surg. 2020, 219, 75–79. [Google Scholar] [CrossRef]
- Tan, M.; Le, Q. EfficientNet: Rethinking Model Scaling for Convolutional Neural Networks. In Proceedings of the 36th International Conference on Machine Learning, Long Beach, CA, USA, 9–15 June 2019; pp. 6105–6114. [Google Scholar]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep Residual Learning for Image Recognition. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 770–778. [Google Scholar]
- Sandler, M.; Howard, A.; Zhu, M.; Zhmoginov, A.; Chen, L.-C. MobileNetV2: Inverted Residuals and Linear Bottlenecks. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018; pp. 4510–4520. [Google Scholar]
- Yung, H.C.; Daroch, A.K.; Parikh, R.; Mathur, D.V.; Kafexhiu, I.K.; Goodman, E. Diagnostic Modalities for Early Detection of Anastomotic Leak After Colorectal Surgery. J. Surg. Res. 2024, 301, 520–533. [Google Scholar] [CrossRef]
- Gielen, A.H.; Heuvelings, D.J.; Sylla, P.; van Loon, Y.-T.; Melenhorst, J.; Bouvy, N.D.; Kimman, M.L.; Breukink, S.O.; On behalf of the CoReAL collaborative. Impact of Anastomotic Leakage After Colorectal Cancer Surgery on Quality of Life: A Systematic Review. Dis. Colon Rectum. 2025, 68, 154–170. [Google Scholar] [CrossRef]
- Arezzo, A.; Filippini, C.; Morino, M. The REAL (REctal Anastomotic Leak) score for prediction of anastomotic leak after rectal cancer surgery. Tech. Coloproctol. 2021, 25, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Sammour, T.; Cohen, L.; Karunatillake, A.I.; Lewis, M.; Lawrence, M.J.; Hunter, A.; Moore, J.W.; Thomas, M.L. Validation of an online risk calculator for the prediction of anastomotic leak after colon cancer surgery and preliminary exploration of artificial intelligence-based analytics. Tech. Coloproctol. 2017, 21, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Takeaki, I. “Bon mariage” of artificial intelligence and intraoperative fluorescence imaging for safer surgery. Artif. Intell. Surg. 2023, 3, 163–165. [Google Scholar] [CrossRef]
- Mascarenhas, M.; Afonso, J.; Ribeiro, T.; Andrade, P.; Cardoso, H.; Macedo, G. The Promise of Artificial Intelligence in Digestive Healthcare and the Bioethics Challenges It Presents. Medicina 2023, 59, 790. [Google Scholar] [CrossRef]
- Mascarenhas:, M.; Mendes, F.; Martins, M.; Ribeiro, T.; Afonso, J.; Cardoso, P.; Ferreira, J.; Fonseca, J.; Macedo, G. Explainable AI in Digestive Healthcare and Gastrointestinal Endoscopy. J. Clin. Med. 2025, 14, 549. [Google Scholar] [CrossRef] [PubMed]

| Patients (n = 26) | |
|---|---|
| Age, years (SD) | 62.3 (11.5) |
| Sex, n (%) | |
| Female | 13 (50.0) |
| Male | 13 (50.0) |
| Surgery, n (%) | |
| Right hemicolectomy | 5 (19.2%) |
| Left hemicolectomy | 3 (11.5%) |
| Proctosigmoidectomy | 4 (15.4%) |
| Segmental sigmoid resection | 6 (23.1%) |
| Anterior rectal resection | 8 (30.8%) |
| Study Center, n (%) | |
| Instituto Português de Oncologia de Lisboa Francisco Gentil, Portugal | 12 (46.2) |
| Royal Liverpool University Hospital, United Kingdom | 6 (23.1) |
| Hospital das Clínicas de Ribeirão Preto, Brazil | 8 (30.8) |
| Surgical Indication | |
| Neoplasia, n (%) | 25 (95.2) |
| Diverticulitis, n (%) | 1 (4.8) |
| Robotic Surgery, n (%) | 6 (23.1) |
| Anastomotic Leak, n (%) | 6 (23.1) |
| Hyperparameter | Possible Values | ||
|---|---|---|---|
| Learning Rate | 1 × 10−7 | 1 × 10−6 | 1 × 10−5 |
| Batch Size | 16 | 32 | 64 |
| Dropout | 0.3 | 0.4 | 0.5 |
| Accuracy (%) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUROC (%) |
|---|---|---|---|---|---|
| 99.5 | 99.2 | 100.0 | 100.0 | 98.9 | 99.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mascarenhas, M.; Mendes, F.; Fonseca, F.; Carvalho, E.; Santos, A.; Cavadas, D.; Barbosa, G.; Pinto da Costa, A.; Martins, M.; Bunaiyan, A.; et al. A Novel Deep Learning Model for Predicting Colorectal Anastomotic Leakage: A Pioneer Multicenter Transatlantic Study. J. Clin. Med. 2025, 14, 5462. https://doi.org/10.3390/jcm14155462
Mascarenhas M, Mendes F, Fonseca F, Carvalho E, Santos A, Cavadas D, Barbosa G, Pinto da Costa A, Martins M, Bunaiyan A, et al. A Novel Deep Learning Model for Predicting Colorectal Anastomotic Leakage: A Pioneer Multicenter Transatlantic Study. Journal of Clinical Medicine. 2025; 14(15):5462. https://doi.org/10.3390/jcm14155462
Chicago/Turabian StyleMascarenhas, Miguel, Francisco Mendes, Filipa Fonseca, Eduardo Carvalho, Andre Santos, Daniela Cavadas, Guilherme Barbosa, Antonio Pinto da Costa, Miguel Martins, Abdullah Bunaiyan, and et al. 2025. "A Novel Deep Learning Model for Predicting Colorectal Anastomotic Leakage: A Pioneer Multicenter Transatlantic Study" Journal of Clinical Medicine 14, no. 15: 5462. https://doi.org/10.3390/jcm14155462
APA StyleMascarenhas, M., Mendes, F., Fonseca, F., Carvalho, E., Santos, A., Cavadas, D., Barbosa, G., Pinto da Costa, A., Martins, M., Bunaiyan, A., Vasconcelos, M., Feitosa, M. R., Willoughby, S., Ahmed, S., Javed, M. A., Ramião, N., Macedo, G., & Limbert, M. (2025). A Novel Deep Learning Model for Predicting Colorectal Anastomotic Leakage: A Pioneer Multicenter Transatlantic Study. Journal of Clinical Medicine, 14(15), 5462. https://doi.org/10.3390/jcm14155462








