Diagnostic Value of Point-of-Care Ultrasound for Sarcopenia in Geriatric Patients Hospitalized for Hip Fracture
Abstract
1. Introduction
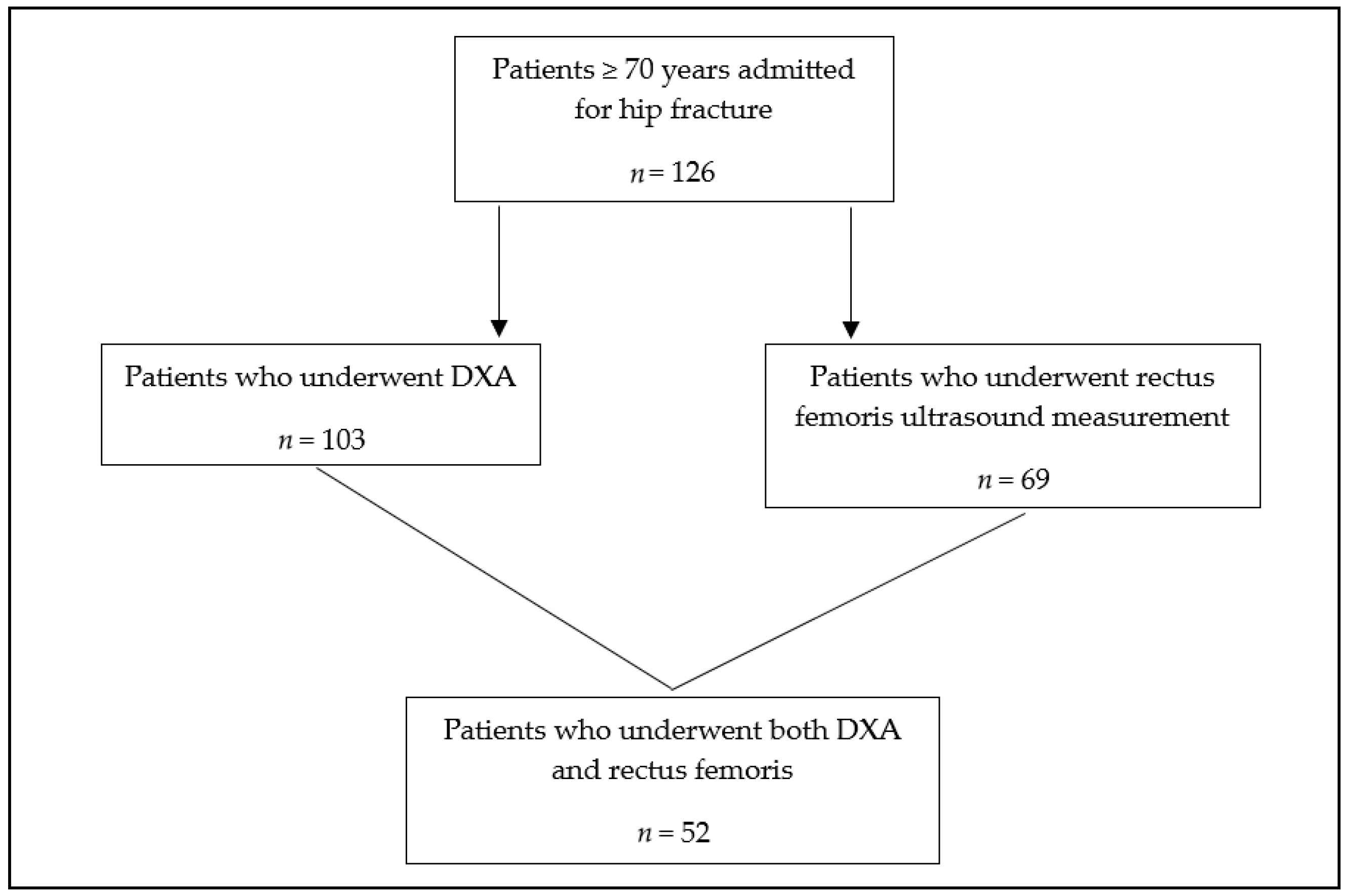
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Data Analysis
- Socio-demographic, clinical, and geriatric assessment data: age, gender, living situation prior to admission (home or nursing home), body mass index, comorbidities (hypertension, diabetes, heart failure, valvular disease, chronic kidney disease, chronic obstructive pulmonary disease, or history of cerebrovascular disease), polypharmacy (use of >5 medications), basic activities of daily living (ADL), instrumental activities of daily living (IADL), nutritional status (MNA-sf score), comorbidity (CIRS-G score), cognitive impairment, and depression.
- Surgical data: type of fracture (intra- or extracapsular), type of surgical procedure (intramedullary nail, hemiarthroplasty, total hip replacement, triple screw fixation, or plate and screws), delay between admission and surgery (hours), and surgery duration (minutes).
- Biological parameters at admission: hemoglobin, white blood cell count, CRP, albumin, and Vitamin D levels.
- Postoperative complications: the Clavien–Dindo classification was used within 72 h, and the scores were dichotomized into non-severe (grades 1 and 2) and severe (grades 3 to 5) complications [19,20]. Inpatient falls, delirium (detected by at least one positive CAM—Confusion Assessment Method—score), administration of red blood cell transfusions, pressure injuries, and length of stay were also recorded. Inpatient mortality rates were assessed, as well as at 1 month, 3 months, and 6 months post-discharge via electronic health records or contact with the patient’s General Practitioner.
- Sarcopenia assessment: Sarcopenia was defined according to the revised EWGSOP2 criteria [6]. Probable sarcopenia was considered if the maximal grip value of the dominant hand was inferior to 27 kg in men and 16 kg in women. Handgrip strength (HGS) was assessed before surgery when possible, or far from the time of surgery, by two trials of the dominant hand using a standard hand-held dynamometer (JAMAR; TEC; Clifton, NJ, USA), and the maximum value was retained. Sarcopenia was diagnosed as a low grip strength associated with a low muscle mass index. ASMI was measured by DXA, and a low muscle mass was determined by using the cut-off of 5.5 kg/m2 for women and 7.0 kg/m2 for men. Sarcopenia severity was assessed via the mobility component of the MNA-SF, due to the inability of patients to perform physical performance tests postoperatively. This component is considered as a valid to predict gait speed in old, hospitalized patients [21]. The EWGSOP2 categories were dichotomized into two groups: the non-sarcopenic control group including patients with no sarcopenia and probable sarcopenia and the sarcopenia group including confirmed sarcopenia and severe sarcopenia. Rectus femoris thickness was measured using a portable ultrasound device (Clarius PA HD3 Wireless, Belfast, Ireland), with the patient in a supine position, halfway between the anterior superior iliac spine and the superior border of the patella, on the non-fractured leg with the knee extended and relaxed, as according to the recommendations [22]. Measurements were performed by two trained ultrasound operators. Inter-operator reliability was analyzed prior to the study and showed an intraclass correlation coefficient (ICC) between 0.964 and 0.997 (n = 9).
2.4. Statistical Analysis
3. Results
3.1. Descriptive Analysis
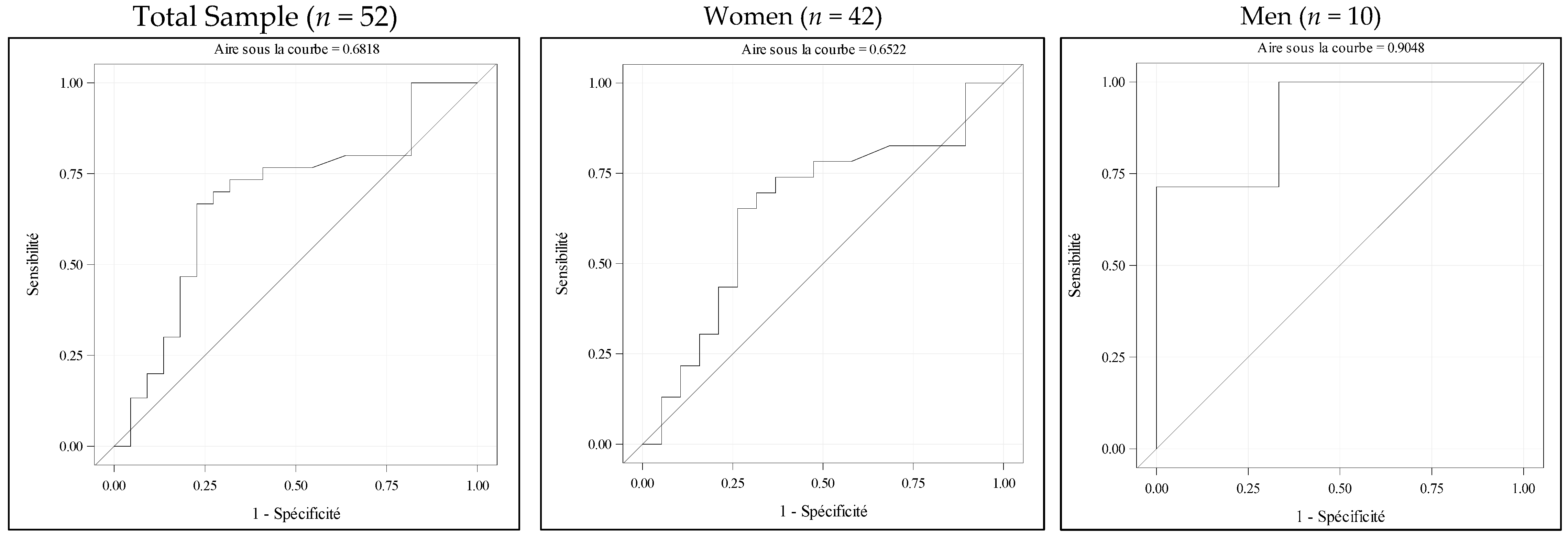
3.2. Analysis of the Association Between Rectus Femoris Thickness by POCUS and ASMI by DXA
3.3. Analysis of Factors Associated with Severe Complications and Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADL | Activities of Daily Living |
| ASMI | Appendicular Skeletal Muscle Mass Index |
| AUC | Area Under the Curve |
| AWGS | Asian Working Group for Sarcopenia |
| BIA | Bioelectrical Impedance Analysis |
| BMI | Body Mass Index |
| CAM | Confusion Assessment Method |
| CI | Confidence Interval |
| CIRS-G | Cumulative Illness Rating Scale-Geriatric version |
| COPD | Chronic Obstructive Pulmonary Disease |
| CRP | C-reactive Protein |
| CT | Computed Tomography |
| DXA | Dual-energy X-ray Absorptiometry |
| EWGSOP | European Working Group on Sarcopenia in Older People |
| HGS | Handgrip Strength |
| IADL | Instrumental Activities of Daily Living |
| ICC | Intraclass Correlation Coefficient |
| MNA-SF | Mini Nutritional Assessment-Short Form |
| MRI | Magnetic Resonance Imaging |
| OR | Odds Ratio |
| POCUS | Point-of-Care Ultrasound |
| RF | Rectus Femoris |
| ROC | Receiver Operating Characteristics |
| SPPB | Short Physical Performance Battery |
References
- Kim, J.W.; Kim, R.; Choi, H.; Lee, S.-J.; Bae, G.-U. Understanding of sarcopenia: From definition to therapeutic strategies. Arch. Pharmacal Res. 2021, 44, 876–889. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachex- Sarcopenia Muscle 2021, 13, 86–99. [Google Scholar] [CrossRef]
- Yuan, S.; Larsson, S.C. Epidemiology of sarcopenia: Prevalence, risk factors, and consequences. Metabolism 2023, 144, 155533. [Google Scholar] [CrossRef] [PubMed]
- Cervera-Díaz, M.d.C.; López-Gómez, J.J.; García-Virto, V.; Aguado-Hernández, H.J.; De Luis-Román, D.A. Prevalence of sarcopenia in patients older than 75 years admitted for hip fracture. Endocrinol. Diabetes Y Nutr. 2023, 70, 396–407. [Google Scholar] [CrossRef]
- Chiang, M.-H.; Kuo, Y.-J.; Chen, Y.-P. The Association Between Sarcopenia and Postoperative Outcomes Among Older Adults With Hip Fracture: A Systematic Review. J. Appl. Gerontol. 2021, 40, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- Morri, M.; Ambrosi, E.; Chiari, P.; Magli, A.O.; Gazineo, D.; Alessandro, F.D.; Forni, C. One-year mortality after hip fracture surgery and prognostic factors: A prospective cohort study. Sci. Rep. 2019, 9, 18718. [Google Scholar] [CrossRef]
- Espinosa, K.A.; Gélvez, A.G.; Torres, L.P.; García, M.F.; Peña, O.R. Pre-operative factors associated with increased mortality in elderly patients with a hip fracture: A cohort study in a developing country. Injury 2018, 49, 1162–1168. [Google Scholar] [CrossRef]
- Chang, W.; Lv, H.; Feng, C.; Yuwen, P.; Wei, N.; Chen, W.; Zhang, Y. Preventable risk factors of mortality after hip fracture surgery: Systematic review and meta-analysis. Int. J. Surg. 2018, 52, 320–328. [Google Scholar] [CrossRef]
- Malafarina, V.; Malafarina, C.; Ugarte, A.B.; Martinez, J.A.; Goñi, I.A.; Zulet, M.A. Factors Associated with Sarcopenia and 7-Year Mortality in Very Old Patients with Hip Fracture Admitted to Rehabilitation Units: A Pragmatic Study. Nutrients 2019, 11, 2243. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Minetto, M.A.; Busso, C.; Gamerro, G.; Lalli, P.; Massazza, G.; Invernizzi, M. Quantitative assessment of volumetric muscle loss: Dual-energy X-ray absorptiometry and ultrasonography. Curr. Opin. Pharmacol. 2021, 57, 148–156. [Google Scholar] [CrossRef]
- Nagae, M.; Umegaki, H.; Yoshiko, A.; Fujita, K. Muscle ultrasound and its application to point-of-care ultrasonography: A narrative review. Ann. Med. 2023, 55, 190–197. [Google Scholar] [CrossRef]
- Nijholt, W.; Scafoglieri, A.; Jager-Wittenaar, H.; Hobbelen, J.S.; van der Schans, C.P. The reliability and validity of ultrasound to quantify muscles in older adults: A systematic review. J. Cachex- Sarcopenia Muscle 2017, 8, 702–712. [Google Scholar] [CrossRef]
- Sanz-Paris, A.; González-Fernandez, M.; Río, L.E.H.-D.; Ferrer-Lahuerta, E.; Monge-Vazquez, A.; Losfablos-Callau, F.; Sanclemente-Hernández, T.; Sanz-Arque, A.; Arbones-Mainar, J.M. Muscle Thickness and Echogenicity Measured by Ultrasound Could Detect Local Sarcopenia and Malnutrition in Older Patients Hospitalized for Hip Fracture. Nutrients 2021, 13, 2401. [Google Scholar] [CrossRef]
- Díaz-Gómez, J.L.; Mayo, P.H.; Koenig, S.J.; Ingelfinger, J.R. Point-of-Care Ultrasonography. N. Engl. J. Med. 2021, 385, 1593–1602. [Google Scholar] [CrossRef]
- Hogenbirk, R.N.M.; Viddeleer, A.R.; Hentzen, J.E.K.R.; van der Plas, W.Y.; van der Schans, C.P.; de Bock, G.H.; Kruijff, S.; Klaase, J.M. Thickness of Biceps and Quadriceps Femoris Muscle Measured Using Point-of-Care Ultrasound as a Representation of Total Skeletal Muscle Mass. J. Clin. Med. 2022, 11, 6606. [Google Scholar] [CrossRef]
- Madden, K.M.; Feldman, B.; Arishenkoff, S.; Meneilly, G.S. A rapid point-of-care ultrasound marker for muscle mass and muscle strength in older adults. Age Ageing 2021, 50, 505–510. [Google Scholar] [CrossRef]
- Poenaru, D. Ultrasound Diagnostic: Rapid Detection of Second Metatarsal Stress Fracture, Case Report and Literature Study. Curr. Med. Imaging 2023, 20, e310823220566. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Yu, S.; Zhou, C.; Wang, D.; Tang, D.; Jiang, G.; Zhang, Y. Use of the modified Clavien-Dindo classification to determine the risk factors for early complications following radical gastrectomy and the effect of such complications on long-term prognosis. World Acad. Sci. J. 2021, 3, 59. [Google Scholar] [CrossRef]
- Song, X.; Ma, Y.; Shi, H.; Liu, Y. Application of Clavien–Dindo classfication-grade in evaluating overall efficacy of laparoscopic pancreaticoduodenectomy. Front. Surg. 2023, 10, 1043329. [Google Scholar] [CrossRef]
- De Breucker, S.; Mets, T.; Pepersack, T. Relating the MNA Mobility Single Item to Usual Walking Speed in Hospitalized and Community-Dwelling Geriatric Patients. J. Frailty Aging 2019, 8, 138–140. [Google Scholar] [CrossRef]
- Perkisas, S.; Baudry, S.; Bauer, J.; Beckwée, D.; De Cock, A.-M.; Hobbelen, H.; Jager-Wittenaar, H.; Kasiukiewicz, A.; Landi, F.; Marco, E.; et al. Application of ultrasound for muscle assessment in sarcopenia: Towards standardized measurements. Eur. Geriatr. Med. 2018, 9, 739–757. [Google Scholar] [CrossRef]
- Berger, J.; Bunout, D.; Barrera, G.; de la Maza, M.P.; Henriquez, S.; Leiva, L.; Hirsch, S. Rectus femoris (RF) ultrasound for the assessment of muscle mass in older people. Arch. Gerontol. Geriatr. 2015, 61, 33–38. [Google Scholar] [CrossRef]
- Nies, I.; Ackermans, L.; Poeze, M.; Blokhuis, T.; Bosch, J.A.T. The Diagnostic Value of Ultrasound of the Rectus Femoris for the diagnosis of Sarcopenia in adults: A systematic review. Injury 2022, 53 (Suppl. 3), S23–S29. [Google Scholar] [CrossRef]
- Sri-On, J.; Rueanthip, S.; Vanichkulbodee, A.; Paksopis, T.; Chetanasilpin, C. The Validity of Ultrasonographic Measurements of the Rectus Femoris Muscle in Older Adults with Sarcopenia in Thai Population. Clin. Interv. Aging 2022, 17, 1249–1259. [Google Scholar] [CrossRef]
- Rustani, K.; Kundisova, L.; Capecchi, P.L.; Nante, N.; Bicchi, M. Ultrasound measurement of rectus femoris muscle thickness as a quick screening test for sarcopenia assessment. Arch. Gerontol. Geriatr. 2019, 83, 151–154. [Google Scholar] [CrossRef]
- Pedauyé-Rueda, B.; García-Fernández, P.; Maicas-Pérez, L.; Maté-Muñoz, J.L.; Hernández-Lougedo, J. Different Diagnostic Criteria for Determining the Prevalence of Sarcopenia in Older Adults: A Systematic Review. J. Clin. Med. 2024, 13, 2520. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, J.; Lee, Y.; Yoo, J.; Choi, Y.; Yoon, B.; Ha, Y.; Koo, K. Prevalence of sarcopenia and mortality rate in older adults with hip fracture. J. Am. Geriatr. Soc. 2022, 70, 2379–2385. [Google Scholar] [CrossRef]
- Saito, H.; Fujimoto, Y.; Matsue, Y.; Yoshioka, K.; Maekawa, E.; Kamiya, K.; Toki, M.; Iwata, K.; Saito, K.; Murata, A.; et al. Ultrasound-measured Quadriceps Muscle Thickness and Mortality in Older Patients With Heart Failure. Can. J. Cardiol. 2024, 40, 2555–2564. [Google Scholar] [CrossRef]
- Sabatino, A.; Kooman, J.P.; Di Motta, T.; Cantarelli, C.; Gregorini, M.; Bianchi, S.; Regolisti, G.; Fiaccadori, E. Quadriceps muscle thickness assessed by ultrasound is independently associated with mortality in hemodialysis patients. Eur. J. Clin. Nutr. 2022, 76, 1719–1726. [Google Scholar] [CrossRef]
- Nagae, M.; Umegaki, H.; Yoshiko, A.; Fujita, K.; Komiya, H.; Watanabe, K.; Yamada, Y.; Kuzuya, M. Echo intensity is more useful in predicting hospital-associated complications than conventional sarcopenia-related parameters in acute hospitalized older patients. Exp. Gerontol. 2021, 150, 111397. [Google Scholar] [CrossRef]
- Benton, E.; Liteplo, A.S.; Shokoohi, H.; Loesche, M.A.; Yacoub, S.; Thatphet, P.; Wongtangman, T.; Liu, S.W. A pilot study examining the use of ultrasound to measure sarcopenia, frailty and fall in older patients. Am. J. Emerg. Med. 2021, 46, 310–316. [Google Scholar] [CrossRef]
- Sabatino, A.; Maggiore, U.; Regolisti, G.; Rossi, G.M.; Di Mario, F.; Gentile, M.; Farina, M.T.; Fiaccadori, E. Ultrasound for Non-invasive Assessment and Monitoring of Quadriceps Muscle Thickness in Critically Ill Patients With Acute Kidney Injury. Front. Nutr. 2021, 8, 622823. [Google Scholar] [CrossRef] [PubMed]
- Le Neindre, A.; Fossat, G. Intérêt de l’échographie thoracique et musculaire en kinésithérapie de réanimation. Med. Intensiv. Reanim. 2017, 26, 425–434. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Fernández-De-Las-Peñas, C.; Varol, U.; Ortega-Santiago, R.; Gallego-Sendarrubias, G.M.; Arias-Buría, J.L. L’imagerie par ultrasons comme outil de biofeedback visuel en rééducation: Une revue systématique actualisée», Revue internationale de recherche environnementale et de santé publique. Int. J. Environ. Res. Public Health 2021, 18, 7554. [Google Scholar] [CrossRef]
| Variable | n | n (%) Mean ± SD Median [IQR] |
|---|---|---|
| Socio-demographic, clinical, and geriatric data | ||
| Age (years) | 126 | 85.3 ± 6.6 |
| Sex (Men) | 126 | 35 (27.8) |
| Place of residence (living at home) | 126 | 77 (61.1) |
| BMI (kg/m2) | 126 | 23.0 [20.3–26.0] |
| Comorbidities | 126 | |
| Hypertension | 86 (68.3) | |
| Diabetes | 25 (19.9) | |
| Heart failure | 46 (36.5) | |
| Moderate to severe valvular disease | 19 (15.1) | |
| Chronic kidney disease | 42 (33.3) | |
| COPD | 21 (16.7) | |
| History of cerebrovascular disease | 23 (18.3) | |
| ADL (/24) | 126 | 8 [7–15] |
| IADL (/8) | 126 | 2 [0–5] |
| MNA-SF (/14) | 126 | 7.8 ± 2.7 |
| CIRS-G (/56) | 126 | 16.4 ± 6.2 |
| Cognitive impairment | 126 | 55 (43.7) |
| Depression | 126 | 36 (28.6) |
| Polypharmacy (>5 medications) | 126 | 85 (67.5) |
| Surgical characteristics | ||
| Type of hip fracture | 126 | |
| Intracapsular femoral neck fracture | 65 (51.6) | |
| Extracapsular intertrochanteric fracture | 54 (42.8) | |
| Extracapsular subtrochanteric fracture | 7 (5.6) | |
| Type of surgery | 126 | |
| Intramedullary nail | 61 (48.4) | |
| Hemiarthroplasty | 52 (41.3) | |
| Total hip replacement | 7 (5.6) | |
| Triple screw fixation | 5 (3.9) | |
| Dynamic hip screw | 1 (0.8) | |
| Time from admission to surgery (hours) | 126 | 27 [15–50] |
| Duration of surgery (minutes) | 126 | 84 [60–107] |
| Laboratory characteristics | ||
| Hemoglobin (g/dL) | 126 | 12.2 ± 2.0 |
| White blood cell count (×103/mm3) | 126 | 10.7 [8.0–13.8] |
| C-reactive protein (mg/L) | 126 | 11.0 [2.0–46.7] |
| Albumin (g/L) | 126 | 36.0 [32.0–38.0] |
| 25 hydroxy-Vitamin D (ng/mL) | 126 | 26.0 [15.3–33.0] |
| Postoperative outcomes | ||
| Severe complications (Clavien–Dindo score > 2) | 126 | 9 (7.3) |
| Fall(s) | 126 | 11 (8.7) |
| Delirium | 126 | 24 (19.0) |
| Red blood cell transfusion | 126 | 29 (23.0) |
| Pressure injury (injuries) | 126 | 12 (9.5) |
| Length of hospital stay (days) | 126 | 18 [16–23] |
| Mortality | ||
| In-hospital | 6 (4.8) | |
| At 1 month | 8 (6.3) | |
| At 3 months | 20 (16.0) | |
| At 6 months | 23 (19.5) | |
| Variable | n | n (%) Mean ± SD Median [IQR] |
|---|---|---|
| Handgrip strength (kg) | 123 | 12 [10–20] |
| Women | 88 | 12 [8–16] |
| Men | 35 | 23 [14–25] |
| ASMI (kg/m2) | 103 | 5.5 [5.0–6.4] |
| Women | 79 | 5.4 [4.9–6.2] |
| Men | 24 | 6.3 [5.4–6.9] |
| Sarcopenia according to EWGSOP2 | 100 | |
| No sarcopenia | 27 (27) | |
| Probable sarcopenia | 30 (30) | |
| Confirmed sarcopenia | 28 (28) | |
| Severe sarcopenia | 15 (15) | |
| Sarcopenia (binary variable) | 100 | 43 (43) |
| Women | 76 | 27 (35.5) |
| Men | 24 | 16 (66.7) |
| Rectus femoris muscle thickness (mm) | 69 | 9.4 ± 3.2 |
| Women | 51 | 9.2 ± 3.1 |
| Men | 18 | 10.2 ± 3.4 |
| Variable | OR | CI 95% | p-Value |
|---|---|---|---|
| ASMI | 0.80 | [0.34–1.85] | 0.598 |
| Rectus femoris muscle thickness | 1.11 | [0.83–1.48] | 0.483 |
| Presence of sarcopenia (EWGSOP2) | 0.80 | [0.40–1.56] | 0.508 |
| Mortality | In-Hospital | 1 Month | 3 Months | 6 Months | ||||
|---|---|---|---|---|---|---|---|---|
| N (%) | 6 (4.8%) | 8 (6.3%) | 20 (16%) | 23 (19.5%) | ||||
| OR [95%] | p | OR [95%] | p | OR [95%] | p | OR [95%] | p | |
| ASMI | 0.07 [0.004–1.10] | 0.058 | 0.66 [0.23–1.89] | 0.436 | 0.84 [0.48–1.47] | 0.540 | 1.14 [0.65–2.02] | 0.641 |
| RF thickness | 0.78 [0.46–1.33] | 0.361 | 0.86 [0.58–1.29] | 0.471 | 0.81 [0.65–1.01] | 0.062 | 0.82 [0.64–1.06] | 0.126 |
| Presence of sarcopenia (EWGSOP2) | NA | NA | NA | NA | 0.36 [0.09–1.38] | 0.135 | 0.44 [0.11–1.76] | 0.248 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondo, L.; Louis, C.; Saboul, H.; Beernaert, L.; De Breucker, S. Diagnostic Value of Point-of-Care Ultrasound for Sarcopenia in Geriatric Patients Hospitalized for Hip Fracture. J. Clin. Med. 2025, 14, 5424. https://doi.org/10.3390/jcm14155424
Mondo L, Louis C, Saboul H, Beernaert L, De Breucker S. Diagnostic Value of Point-of-Care Ultrasound for Sarcopenia in Geriatric Patients Hospitalized for Hip Fracture. Journal of Clinical Medicine. 2025; 14(15):5424. https://doi.org/10.3390/jcm14155424
Chicago/Turabian StyleMondo, Laure, Chloé Louis, Hinda Saboul, Laetitia Beernaert, and Sandra De Breucker. 2025. "Diagnostic Value of Point-of-Care Ultrasound for Sarcopenia in Geriatric Patients Hospitalized for Hip Fracture" Journal of Clinical Medicine 14, no. 15: 5424. https://doi.org/10.3390/jcm14155424
APA StyleMondo, L., Louis, C., Saboul, H., Beernaert, L., & De Breucker, S. (2025). Diagnostic Value of Point-of-Care Ultrasound for Sarcopenia in Geriatric Patients Hospitalized for Hip Fracture. Journal of Clinical Medicine, 14(15), 5424. https://doi.org/10.3390/jcm14155424







