Comparison of Zeiss MEL90 and Alcon WaveLight EX500 Excimer Lasers in FDA Premarket Approval Trials for the Treatment of Myopia, Hyperopia, and Mixed Astigmatism
Abstract
1. Introduction
2. Materials and Methods
2.1. FDA Approval Timeline
2.2. Laser Specifications
2.3. PMA Cohorts
2.4. Outcome Measures
2.5. Selection of Published Studies
2.6. Statistical Analysis
3. Results
3.1. Patient Demographics
3.2. Efficacy
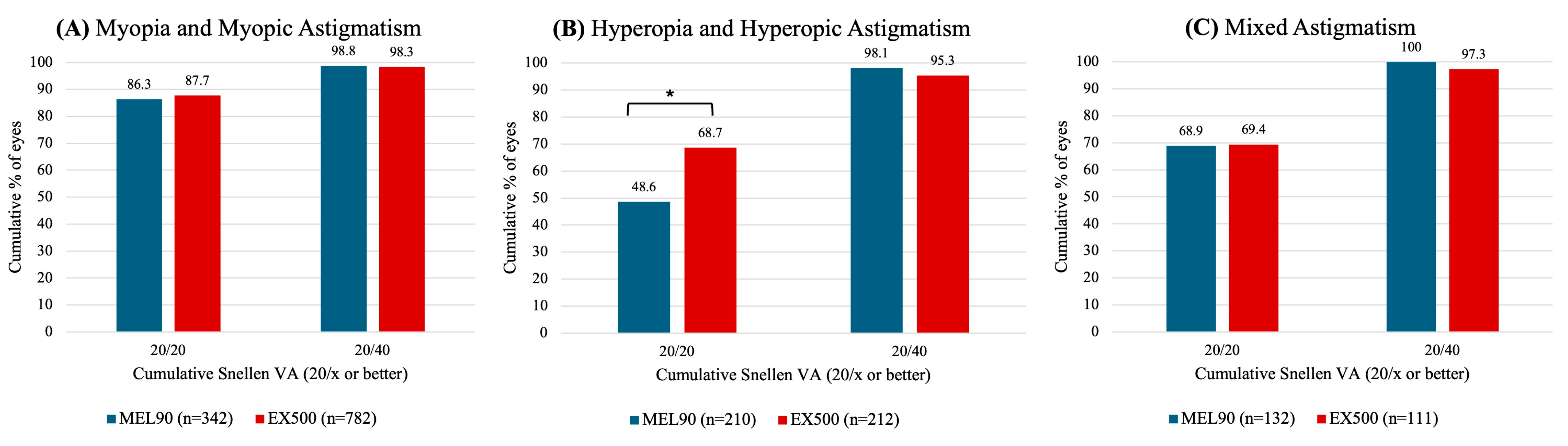
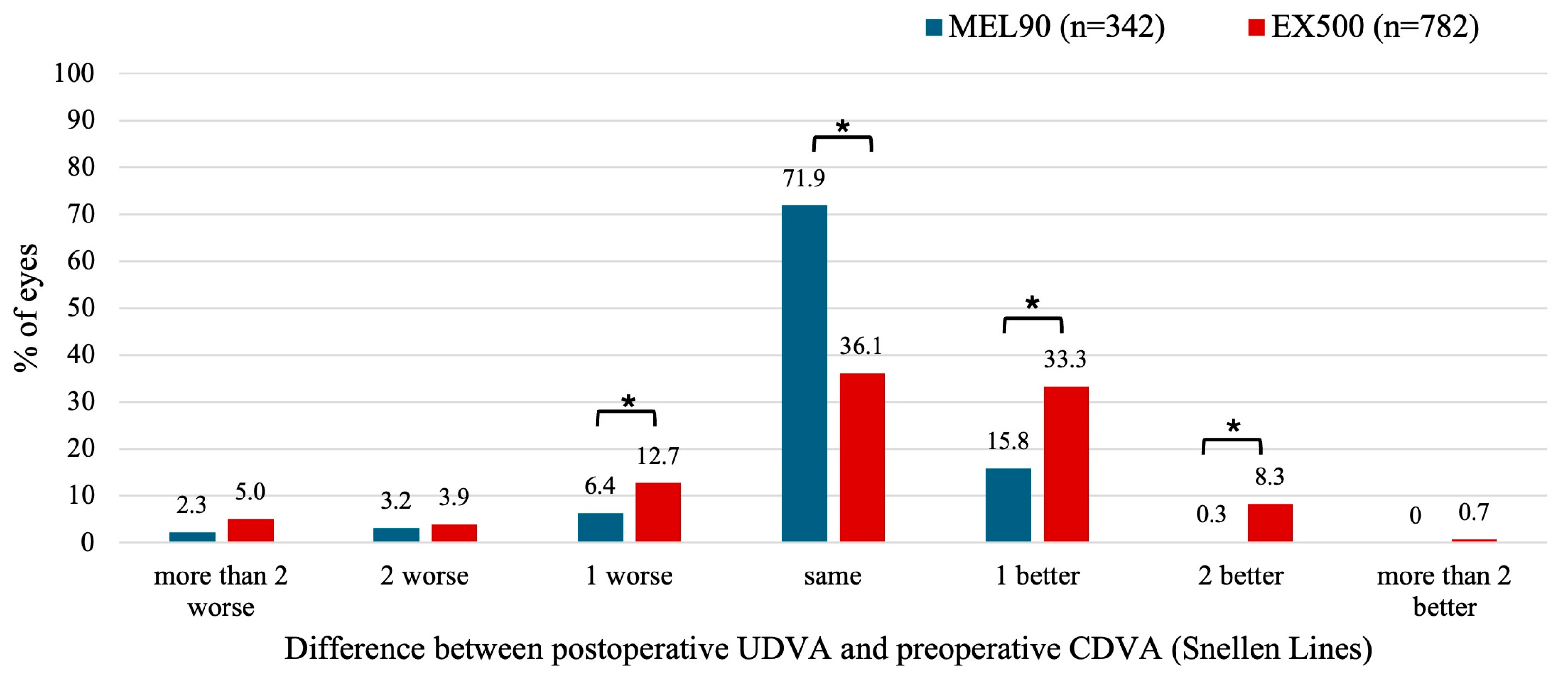
3.2.1. Myopia with and Without Astigmatism
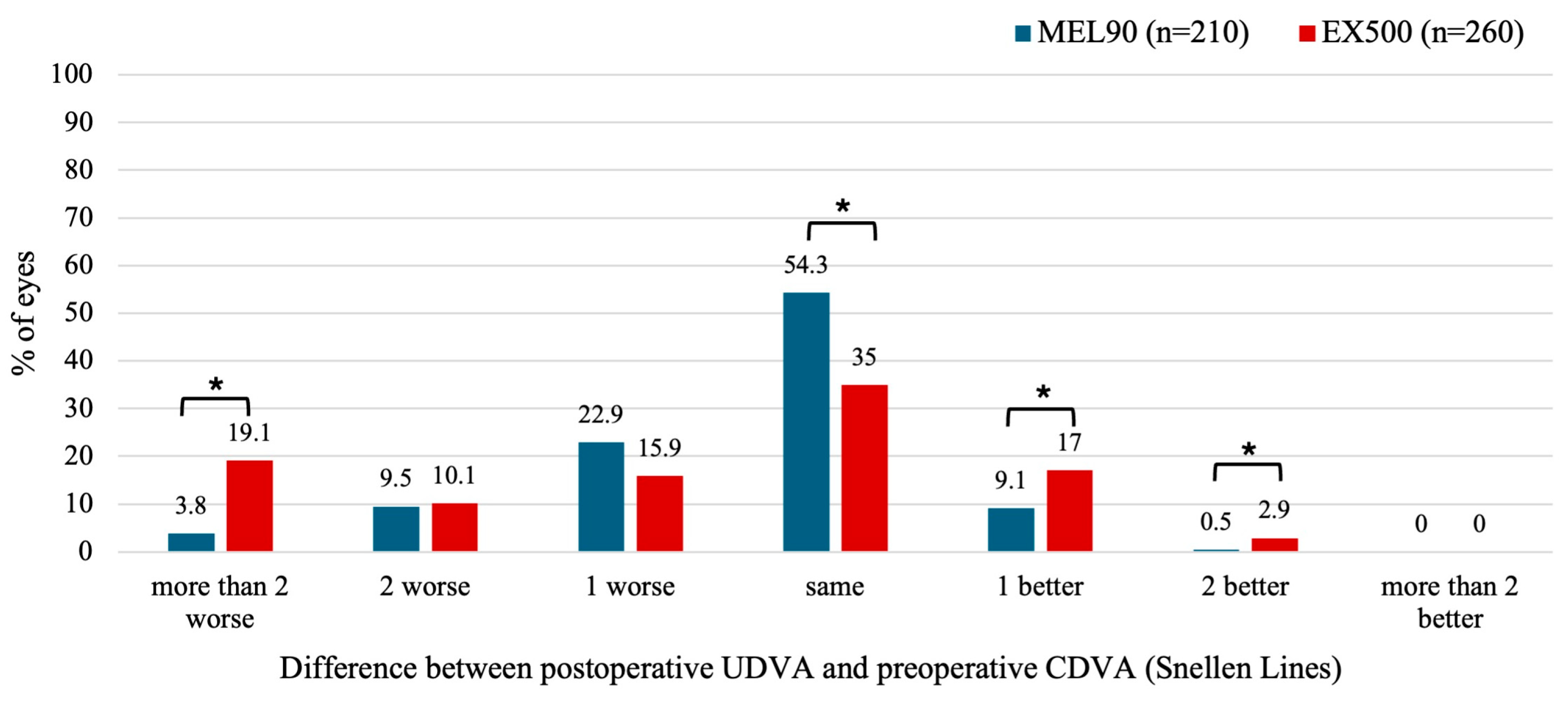
3.2.2. Hyperopia with and Without Astigmatism
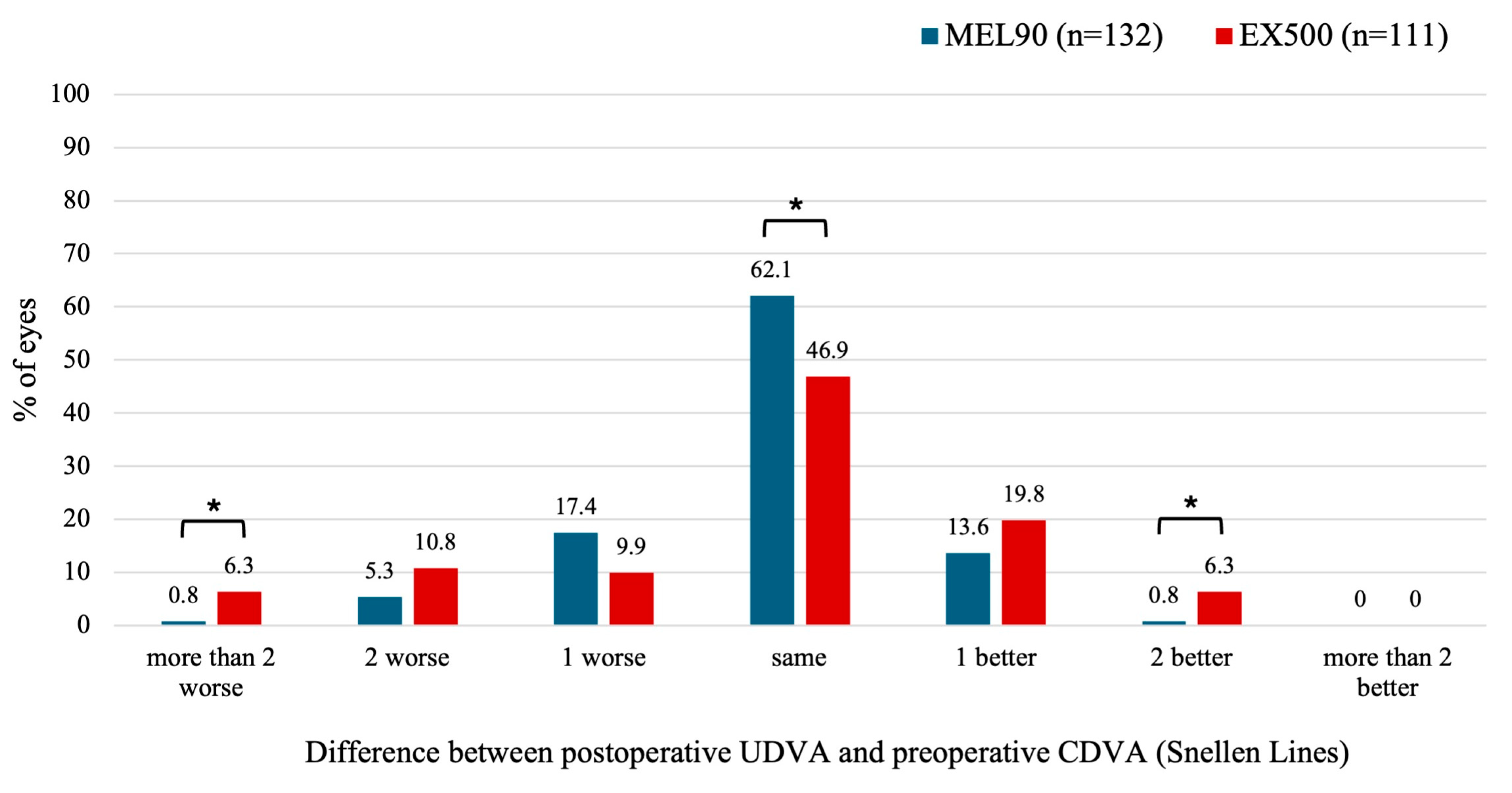
3.2.3. Mixed Astigmatism
3.3. Safety
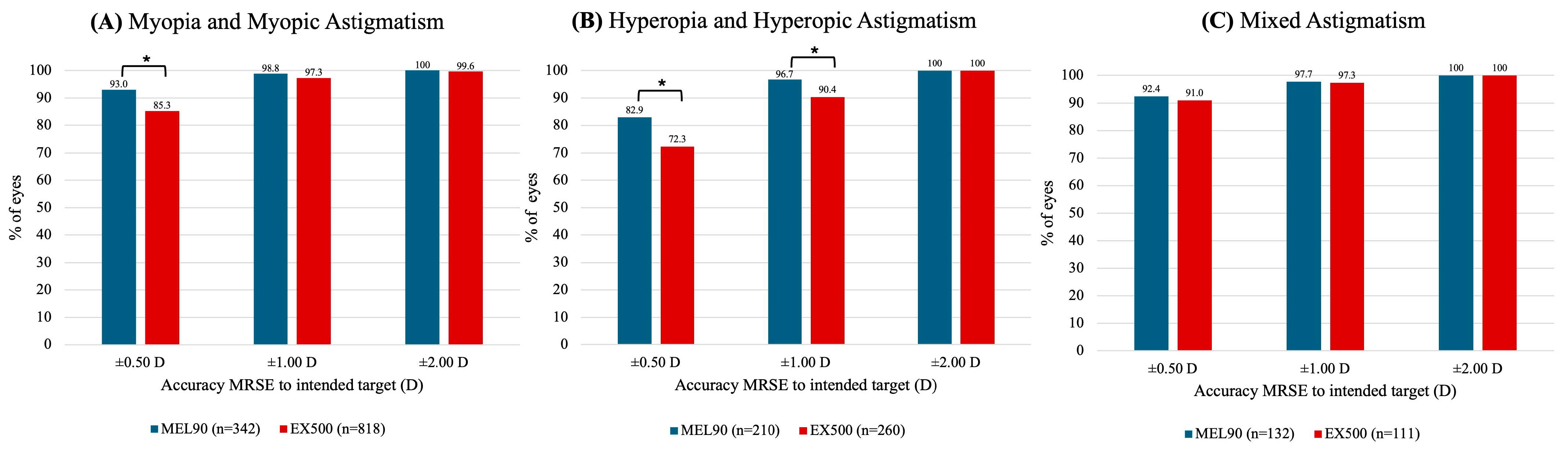
3.4. Accuracy
3.5. Astigmatic Correction
3.6. Patient-Reported Outcomes
3.7. Retreatment
3.8. Complications
3.9. Review of Current Literature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LASIK | Laser assisted in situ keratomileusis |
| FDA | Food and Drug Administration |
| PMA | Premarket approval |
| SSED | Summary of Safety and Effectiveness Data |
| MRSE | Manifest refraction spherical equivalence |
| CDVA | Corrected distance visual acuity |
| UDVA | Uncorrected distance visual acuity |
| ArF | Argon Fluoride |
| CYL | Cylinder |
| D | Diopter |
| VA | Visual Acuity |
| PROWL | Patient reported outcomes with LASIK |
| FBS | Foreign body sensation |
| DLK | Diffuse lamellar keratitis |
| ANSI | American National Standards Institute |
References
- Shehata, A.M. Femtosecond Lasik Versus SMILE for Management of High Myopic Astigmatism: Six Months Outcome. Med. J. Cairo Univ. 2023, 91, 849–856. [Google Scholar] [CrossRef]
- Hashemi, H.; Fotouhi, A.; Yekta, A.; Pakzad, R.; Ostadimoghaddam, H.; Khabazkhoob, M. Global and regional estimates of prevalence of refractive errors: Systematic review and meta-analysis. J. Curr. Ophthalmol. 2018, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- FDA, Center for Devices and Radiological Health. List of FDA-Approved Lasers for LASIK. FDA. Published Online 3 November 2018. Available online: https://www.fda.gov/medical-devices/lasik/list-fda-approved-lasers-lasik (accessed on 19 June 2025).
- Pidro, A.; Biscevic, A.; Pjano, M.; Mravicic, I.; Bejdic, N.; Bohac, M. Excimer Lasers in Refractive Surgery. Acta Inform. Medica 2019, 27, 278–283. [Google Scholar] [CrossRef]
- United States Food and Drug Administration. Summary of Safety and Effectiveness Data (P060004/S006). Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf6/P060004S006B.pdf (accessed on 3 May 2025).
- United States Food and Drug Administration. Summary of Safety and Effectiveness Data (P020050). Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf2/P020050.pdf (accessed on 3 May 2025).
- United States Food and Drug Administration. Summary of Safety and Effectiveness Data (P030008). Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf3/P030008B.pdf (accessed on 3 May 2025).
- United States Food and Drug Administration. Summary of Safety and Effectiveness Data (P030008/S004). Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf3/P030008S004B.pdf (accessed on 3 May 2025).
- United States Food and Drug Administration. Summary of Safety and Effectiveness Data (P020050/S004). Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf2/P020050S004B.pdf (accessed on 3 May 2025).
- United States Food and Drug Administration. Summary of Safety and Effectiveness Data (P020050/S012). Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf2/p020050s012b.pdf (accessed on 3 May 2025).
- United States Food and Drug Administration. Summary of Safety and Effectiveness Data (P020050/S023). Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf2/P020050S023B.pdf (accessed on 3 May 2025).
- Shehata, M.D. Visumax Femtosecod and MEL90 Excimer Laser Outcome in Mild, Moderate and High Myopic Astigmatism: Six Months Follow-up. Med. J. Cairo Univ. 2023, 91, 409–416. [Google Scholar] [CrossRef]
- Vaswani, S. Short-term LASIK outcomes for myopia with and without astigmatism using the MEL 90 excimer laser and VisuMax femtosecond laser. Optom Pract. 2021, 22, 1. [Google Scholar]
- Brar, S.; Rathod, D.P.; Roopashree, C.R.; Ganesh, S. One-Year Visual and Refractive Outcomes following LASIK for Myopia and Myopic Astigmatism with MEL 90 versus Schwind Amaris 750S Excimer Laser: A Comparative Study. J Ophthalmol. 2021, 2021, 9929181. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Carp, G.I.; Lewis, T.A.; Archer, T.J.; Gobbe, M. Outcomes for Myopic LASIK With the MEL 90 Excimer Laser. J. Refract. Surg. 2015, 31, 316–321. [Google Scholar] [CrossRef]
- Rowen, S.L.; Tooma, T.; Trieu, N.; Hall, B. Retrospective Study Comparing Topography-Guided and Wavefront-Optimized LASIK Procedures in a Single Center. Clin. Ophthalmol. 2024, 18, 1615–1622. [Google Scholar] [CrossRef]
- Agarwal, S.; Thornell, E.; Hodge, C.; Sutton, G.; Hughes, P. Visual Outcomes and Higher Order Aberrations Following LASIK on Eyes with Low Myopia and Astigmatism. Open Ophthalmol. J. 2018, 12, 84–93. [Google Scholar] [CrossRef]
- Niparugs, M.; Tananuvat, N.; Chaidaroon, W.; Tangmonkongvoragul, C.; Ausayakhun, S. Outcomes of LASIK for Myopia or Myopic Astigmatism Correction with the FS200 Femtosecond Laser and EX500 Excimer Laser Platform. Open Ophthalmol. J. 2018, 12, 63–71. [Google Scholar] [CrossRef]
- Sáles, C.S.; Manche, E.E. One-Year Outcomes from a Prospective, Randomized, Eye-to-Eye Comparison of Wavefront-Guided and Wavefront-Optimized LASIK in Myopes. Ophthalmology 2013, 120, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, D.Z.; Carp, G.I.; Archer, T.J.; Day, A.C.; Vida, R.S. Outcomes for Hyperopic LASIK With the MEL 90® Excimer Laser. J. Refract. Surg. 2018, 34, 799–808. [Google Scholar] [CrossRef]
- Moshirfar, M.; Megerdichian, A.; West, W.B.; Miller, C.M.; Sperry, R.A.; Neilsen, C.D.; Tingey, M.T.; Hoopes, P.C. Comparison of Visual Outcome After Hyperopic LASIK Using a Wavefront-Optimized Platform Versus Other Excimer Lasers in the Past Two Decades. Ophthalmol. Ther. 2021, 10, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Durrie, D.S.; Smith, R.T.; Waring, G.O.; Stahl, J.E.; Schwendeman, F.J. Comparing Conventional and Wavefront-optimized LASIK for the Treatment of Hyperopia. J. Refract. Surg. 2010, 26, 356–363. [Google Scholar] [CrossRef]
- Reinstein, D.Z.; Carp, G.I.; Archer, T.J.; Day, A.C.; Vida, R.S. Outcomes for Mixed Cylinder LASIK With the MEL 90® Excimer Laser. J. Refract. Surg. 2018, 34, 672–680. [Google Scholar] [CrossRef]
- Moshirfar, M.; Durnford, K.; Megerdichian, A.; Thomson, A.; Martheswaran, T.; West, W., Jr.; McCabe, S.; Ronquillo, Y.; Hoopes, P. Refractive Outcomes After LASIK for the Treatment of Mixed Astigmatism with the Allegretto WaveLight EX500. Ophthalmol. Ther. 2022, 11, 785–795. [Google Scholar] [CrossRef]
- Stonecipher, K.G.; Kezirian, G.M.; Stonecipher, K. LASIK for Mixed Astigmatism Using the ALLEGRETTO WAVE: 3- and 6-Month Results with the 200- and 400-Hz Platforms. J. Refract. Surg. 2010, 26, S819–S823. [Google Scholar] [CrossRef]
- Eydelman, M.B.; Drum, B.; Holladay, J.; Hilmantel, G.; Kezirian, G.; Durrie, D.; Stulting, R.D.; Sanders, D.; Wong, B. Standardized Analyses of Correction of Astigmatism by Laser Systems That Reshape the Cornea. J. Refract. Surg. 2006, 22, 81–95. [Google Scholar] [CrossRef]
- ANSI Z80.11-2012; The Accredited Committee Z80 for Ophthalmic Standards. American National Standards Institute (ANSI): Washington, DC, USA, 2012. Available online: https://webstore.ansi.org/preview-pages/VC%20(ASC%20Z80)/preview_ANSI+Z80.11-2012.pdf?srsltid=AfmBOorrBUkyFJOQ_eEDJRGiQJUBOZRW9eDBJC_hSBN8f-5aW2UqrDvv (accessed on 13 June 2025).
- Moshirfar, M.; Basharat, N.F.; Kelkar, N.; Bundogji, N.; Ronquillo, Y.C.; Hoopes, P.C. Visual Outcomes of Photorefractive Keratectomy Enhancement After Primary LASIK. J. Refract. Surg. 2022, 38, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Manche, E.E. Comparison of femtosecond and excimer laser platforms available for corneal refractive surgery. Curr. Opin. Ophthalmol. 2016, 27, 316–322. [Google Scholar] [CrossRef]
- Biscevic, A.; Bohac, M.; Koncarevic, M.; Anticic, M.; Dekaris, I.; Patel, S. Vector analysis of astigmatism before and after LASIK: A comparison of two different platforms for treatment of high astigmatism. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Motwani, M.; Pei, R. Treatment of moderate-to-high hyperopia with the WaveLight Allegretto 400 and EX500 excimer laser systems. Clin. Ophthalmol. 2017, 11, 999–1007. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mrochen, M.; Donitzky, C.; Wüllner, C.; Löffler, J. Wavefront-optimized ablation profiles: Theoretical background. J. Cataract. Refract. Surg. 2004, 30, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, D.Z.; Waring, G.O. Graphic Reporting of Outcomes of Refractive Surgery. J. Refract. Surg. 2009, 25, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Alpins, N.A. A new method of analyzing vectors for changes in astigmatism. J. Cataract. Refract. Surg. 1993, 19, 524–533. [Google Scholar] [CrossRef]

| Zeiss | Alcon | ||||
|---|---|---|---|---|---|
| Excimer Laser | MEL80 | MEL90 | Allegretto Wave | Allegretto Wave Eye-Q | EX500 |
| Approval (year) | 2006 | 2024 | 2003 | 2007 | 2010 |
| Type | ArF | ArF | ArF | ArF | ArF |
| Wavelength (nm) | 193 | 193 | 193 | 193 | 193 |
| Frequency (Hz) | 250 | 500 | 200 | 400 | 500 |
| Eye Tracker (Hz) | 250 | 1050 | 200 | 400 | 1050 |
| Pulse Duration (ns) | 4–7 | 4–7 | 10 | 10 | 6 |
| Optical Zone (mm) | 6.0–6.5 | 6.0–7.0 | 4.5–8.0 | 4.5–8.0 | 6.0–6.5 |
| Ablation Zone (mm) | 9.2 a | 9.2 a | 9.0 | 9.0 | 9.0 |
| Peak Fluence (mJ/cm2) | >150 | >150 | 400 | 400 | 400 |
| MEL90 | EX500 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | Myopia ± Astigmatism | Hyperopia ± Astigmatism | Mixed Astigmatism | Myopia ± Astigmatism | Hyperopia ± Astigmatism | Mixed Astigmatism | p d | p e | p f |
| Eyes (n) | 358 | 221 | 135 | 901 | 290 | 162 | |||
| Sex, male/female (n) | 88/95 | 52/65 | 46/31 | 436 b/465 b | 142 b/148 b | 109 b/53 b | |||
| Age (years) a | 33.1 ± 7.5 (19 to 63) | 39.9 ± 11.5 (18 to 62) | 35.5 ± 9.1 (20 to 57) | 38.07 ± 9.7 (18 to 67) | 51.55 ± 8.8 (25 to 69) | 39.0 ± 9.4 (22 to 70) | <0.001 | <0.001 | 0.001 |
| Preoperative MRSE (D) a | −5.14 ± 3.26 | 2.48 ± 1.83 | −0.04 ± 1.49 | −4.46 ± 2.35 c | 2.27 ± 1.30 c | −0.21 ± 0.05 c | <0.001 | 0.156 | 0.192 |
| Preoperative CYL (D) a | −1.00 ± 1.05 (−4.00 to 0.00) | −0.99 ± 1.04 (−4.00 to 0.00) | −2.60 ± 0.99 (−4.00 to −0.75) | −0.85 ± 0.86 c (−5.00 to 0.00) | −0.66 ± 0.73 c (−4.50 to 0.00) | −2.40 ± 1.15 c (−6.00 to 0.00) | 0.017 | <0.001 | 0.110 |
| MEL90 | EX500 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter (n/N) (%) | Myopia ± Astigmatism | Hyperopia ± Astigmatism | Mixed Astigmatism | Myopia ± Astigmatism | Hyperopia ± Astigmatism | Mixed Astigmatism | p c | p d | p e |
| CDVA loss ≥ 2 lines | 1/342 (0.5%) | 0/210 (0%) | 0/133 (0%) | 6/818 (0.7%) | 4/260 (1.5%) | 1/111 (0.9%) | 0.131 | 0.455 | 0.681 |
| CDVA worse than 20/40 a | 0/342 (0%) | 0/210 (0%) | 0/133 (0%) | 0/818 (0%) | 1/260 (0.4%) | 0/111 (0%) | NA | NA | NA |
| CDVA worse than 20/25 a | 1/342 (0.5%) | 0/210 (0%) | 0/133 (0%) | 2/779 (0.3%) | 0/241 (0%) | 0/97 (0%) | 0.998 | NA | NA |
| Increased CYL > 2.0 D | 0/342 (0%) | 0/210 (0%) | 1/133 (0.8%) | 0/242 b (0%) | 0/79 b (0%) | – | NA | NA | NA |
| MEL90 | EX500 | |||||
|---|---|---|---|---|---|---|
| Complication n (%) | Myopia ± Astigmatism (n = 358) | Hyperopia ± Astigmatism (n = 221) | Mixed Astigmatism (n = 135) | Myopia ± Astigmatism (n = 876) | Hyperopia ± Astigmatism (n = 285) | Mixed Astigmatism (n = 161) |
| Interface debris a | 13 (3.6) | 1 (0.5) | 0 | 0 | 0 | 0 |
| Persistent FBS/pain | 0 | 1 (0.5) | 0 | 7 (0.8) | 5 (1.8) | 2 (1.2) |
| Epithelium in interface | 4 (1.1) | 4 (1.8) | 0 | 3 (0.3) | 7 (2.5) | 0 |
| Retinal detachment | 1 (0.3) | 0 | 0 | 0 | 1 (0.4) | 0 |
| DLK | 6 (1.7) | 0 | 0 | 0 | 0 | 0 |
| Corneal striae | 4 (1.1) | 0 | 0 | 0 | 0 | 0 |
| Persistent corneal edema | 1 (0.3) | 2 (1.5) | 2 (1.5) | 0 | 0 | 0 |
| Epithelial defect | 1 (0.3) | 0 | 0 | 16 (1.8) | 3 (1.0) | 0 |
| Flap dislocation | 1 (0.3) | 0 | 0 | 2 (0.2) | 0 | 0 |
| Flap tear/damage | 0 | 2 (0.9) a | 0 | 0 | 0 | 0 |
| Study (Year) | Country | Follow-Up (mo) | N | MRSE, Mean ± SD | CYL ≤0.5 D | MRSE ±0.5 D | MRSE ±1.0 D | UDVA ≥20/20 | UDVA ≥20/40 | Loss of ≥2 Lines of CDVA | Loss of 1 Line of CDVA | No Change of CDVA | Gain of 1 Line of CDVA | Gain of ≥2 Lines of CDVA | Safety Index | Efficacy Index | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preop (D) | Postop (D) | ||||||||||||||||
| MEL90 | |||||||||||||||||
| PMA trial (2024) [6] | US | 6 | 342 | −5.14 ± 3.26 | - | - | 93.0 | 98.8 | 86.3 | 98.8 | 0.5 | 3.2 | 71.9 | 22.2 | 2.4 | - | - |
| Shehata et al. (2023) a [13] | EGY | 6 | 150 | −4.89 ± 0.77 | −0.05 ± 0.05 | - | 98.7 | - | 54 | - | - | - | - | - | - | - | - |
| Vaswani et al. (2021) [14] | UK | 3 | 382 | - | - | 91 | 98 | 92 | - | 0 | - | - | - | - | - | - | |
| Brar et al. (2021) [15] | IND | 12 | 165 | −3.98 ± 1.90 | −0.23 ± 0.23 | 97 | 91 | 100 | 96 | 100 | 0 | 3 | 59 | 35 | 3 | 1.08 | 1.00 |
| Reinstein et al. (2015) [16] | UK | 3 | 286 | −3.83 ± 1.83 | −0.13 | 90 | 88 | 100 | 92 | 99 b | 0 | 6 | 59 | 31 | 4 | - | - |
| EX500 | |||||||||||||||||
| PMA trial (2003) [7] | US | 12 | 901 | −4.46 ± 2.35 | - | - | 85.1 | 97.7 | 87.4 | 99.0 | 0.5 | - | - | - | - | - | - |
| Rowen et al. (2024) [17] | US | 3 | 121 | −4.35 ± 2.33 | −0.01 ± 0.24 | 92 | 96 | 100 | 95 | 100 | 0 | 8 | 69 | 23 | 0 | 1.05 c | 0.98 d |
| Agarwal et al. (2018) [18] | AUS | 3 | 76 | −2.49 ± 1.00 | −0.09 ± 0.26 | - | 95 | - | 96.1 | 100 | 0 | 3 | 20 | 62 | 14 | 1.26 c | 1.12 d |
| Niparugs et al. (2018) [19] | THA | 12 | 254 | −5.15 ± 2.41 | −0.14 ± 0.30 | 91.3 | 98.5 | 89.0 | 98.7 | 0 | 14.0 | 58.8 | 27.2 | 0 | - | - | |
| Salés & Manche et al. (2013) [20] | US | 12 | 34 | −3.99 ± 1.71 | −0.33 ± 0.34 | 97 | 85 | 97 | 97 | 100 | 0 | 15 | 47 | 32 | 6 | - | - |
| Study (Year) | Country | Follow-Up (mo) | N | MRSE, Mean ± SD | CYL ≤0.5 D | MRSE ±0.5 D | MRSE ±1.0 D | UDVA ≥20/20 | UDVA ≥20/40 | Loss of ≥2 Lines of CDVA | Loss of 1 Line of CDVA | No Change of CDVA | Gain of 1 Line of CDVA | Gain of ≥2 Lines of CDVA | Safety Index | Efficacy Index | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preop (D) | Postop (D) | ||||||||||||||||
| MEL90 | |||||||||||||||||
| PMA trial (2024) [6] | US | 12 | 210 | 2.48 ± 1.83 | - | - | 82.9 | 96.7 | 48.6 | 98.1 | 0 | 9.1 | 77.1 | 12.9 | 1.6 | - | - |
| Reinstein et al. (2018) [21] | UK | 12 | 1383 | 2.77 ± 1.34 | −0.11 ± 0.55 | 75 | 73 | 93 | 75 | 99 | 0.6 | 17 | 64 | 19 | 0 | - | - |
| EX500 | |||||||||||||||||
| PMA trial (2003) [8] | US | 12 | 290 | 2.27 ± 1.30 | - | - | 65.3 | 90.8 | 67.5 | 98.8 | 1.5 | - | - | - | - | - | - |
| Moshirfar et al. (2021) [22] | US | 12 | 379 | 1.33 ± 1.10 | −0.46 ± 0.79 | 76 | 78 | 96 | 69 | 97 | 1.1 | 4.8 | 74 | 19 | 1.1 | 1.03 | 0.93 |
| Durrie et al. (2009) [23] | US | 6 | 26 | 1.33 ± 0.76 | 0.16 ± 0.27 | - | 96.2 | 100 | 84 | 92 | 0 | 3.8 | 73.1 | 19.2 | 3.8 | - | - |
| Study (Year) | Country | Follow-Up (mo) | N | MRSE, Mean ± SD | CYL ≤0.5 D | MRSE ±0.5 D | MRSE ±1.0 D | UDVA ≥20/20 | UDVA ≥20/40 | Loss of ≥2 Lines of CDVA | Loss of 1 Line of CDVA | No Change of CDVA | Gain of 1 Line of CDVA | Gain of ≥2 Lines of CDVA | Safety Index | Efficacy Index | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Preop (D) | Postop (D) | ||||||||||||||||
| MEL90 | |||||||||||||||||
| PMA trial (2024) [6] | US | 6 | 132 | −0.04 ± 1.49 | - | - | 92.4 | 97.7 | 68.9 | 100 | 0 | 3.8 | 75.2 | 19.6 | 1.6 | - | - |
| Reinstein et al. (2018) [24] | UK | 12 | 105 | −0.30 ± 0.90 | −0.21 ± 0.38 | 65 | 85 | 99 | 73 | 94 | 0 | 10 | 57 | 32 | 1 | - | - |
| EX500 | |||||||||||||||||
| PMA trial (2003) [9] | US | 6 | 162 | −0.98 ± 0.80 | - | 78.4 | 91.0 | 97.3 | 69.4 | 97.3 | 0.9 | - | - | - | - | - | - |
| Moshirfar et al. (2022) [25] | US | 12 | 179 | −0.61 ± 0.70 | −0.36 ± 0.57 | 80 | 88 | 100 | 74 | 100 | 0 | 3 | 72 | 25 | 0 | 1.02 a | 0.83 b |
| Stonecipher et al. (2010) c [26] | US | 6 | 111 | 0.78 ± 0.52 | - | 100 | 95 | - | 79 | 99 | 0 | 10 | 50 | 40 | 0 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandenburg, T.M.; Sitto, M.M.; Hoopes, P.C.; Moshirfar, M. Comparison of Zeiss MEL90 and Alcon WaveLight EX500 Excimer Lasers in FDA Premarket Approval Trials for the Treatment of Myopia, Hyperopia, and Mixed Astigmatism. J. Clin. Med. 2025, 14, 5403. https://doi.org/10.3390/jcm14155403
Brandenburg TM, Sitto MM, Hoopes PC, Moshirfar M. Comparison of Zeiss MEL90 and Alcon WaveLight EX500 Excimer Lasers in FDA Premarket Approval Trials for the Treatment of Myopia, Hyperopia, and Mixed Astigmatism. Journal of Clinical Medicine. 2025; 14(15):5403. https://doi.org/10.3390/jcm14155403
Chicago/Turabian StyleBrandenburg, Traeson M., Mina M. Sitto, Phillip C. Hoopes, and Majid Moshirfar. 2025. "Comparison of Zeiss MEL90 and Alcon WaveLight EX500 Excimer Lasers in FDA Premarket Approval Trials for the Treatment of Myopia, Hyperopia, and Mixed Astigmatism" Journal of Clinical Medicine 14, no. 15: 5403. https://doi.org/10.3390/jcm14155403
APA StyleBrandenburg, T. M., Sitto, M. M., Hoopes, P. C., & Moshirfar, M. (2025). Comparison of Zeiss MEL90 and Alcon WaveLight EX500 Excimer Lasers in FDA Premarket Approval Trials for the Treatment of Myopia, Hyperopia, and Mixed Astigmatism. Journal of Clinical Medicine, 14(15), 5403. https://doi.org/10.3390/jcm14155403






