Technological Advances in Pre-Operative Planning
Abstract
1. Introduction
2. Foundations of Pre-Operative Planning
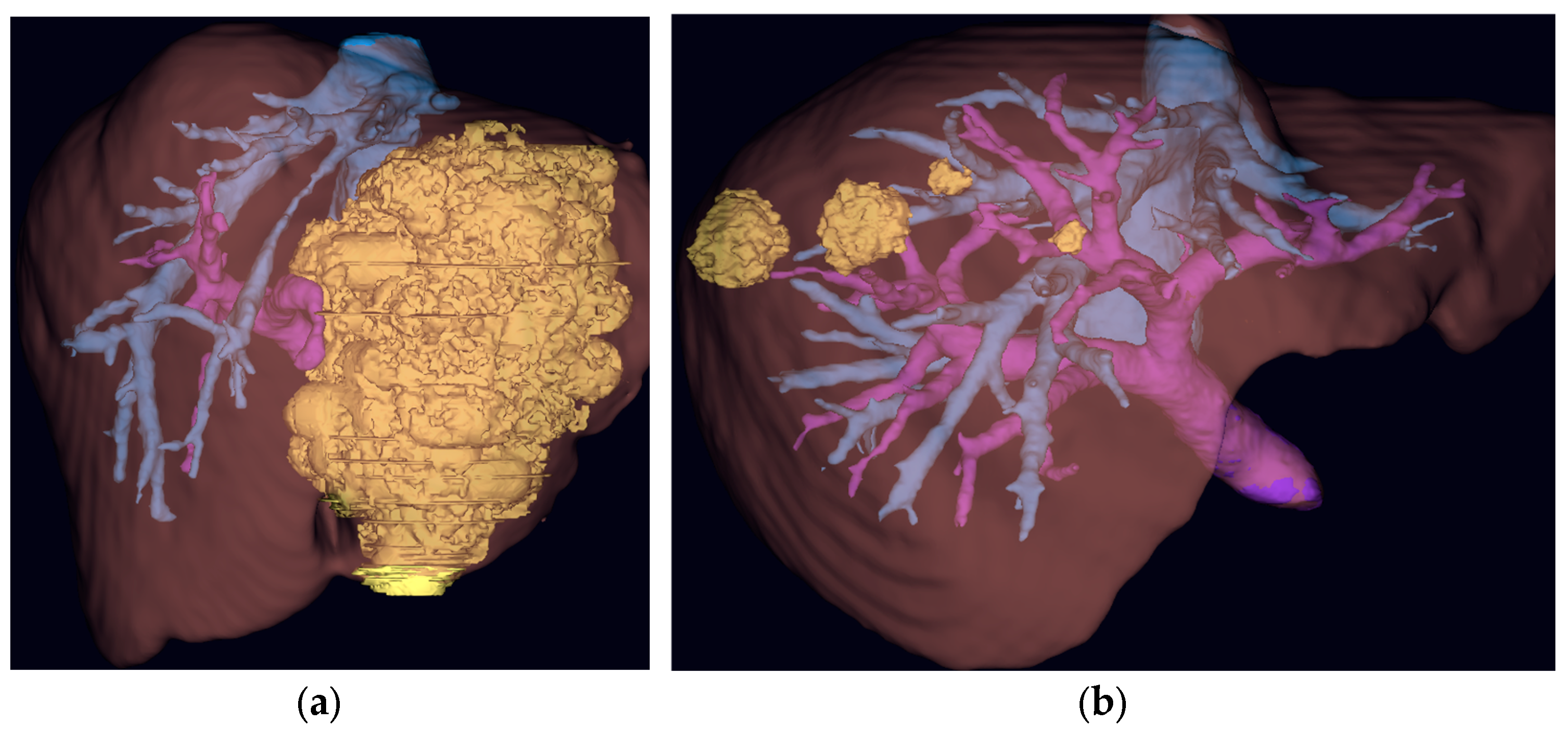
3. Generative Artificial Intelligence for Pre-Operative Planning
3.1. Predictive Analytics for Risk Stratification
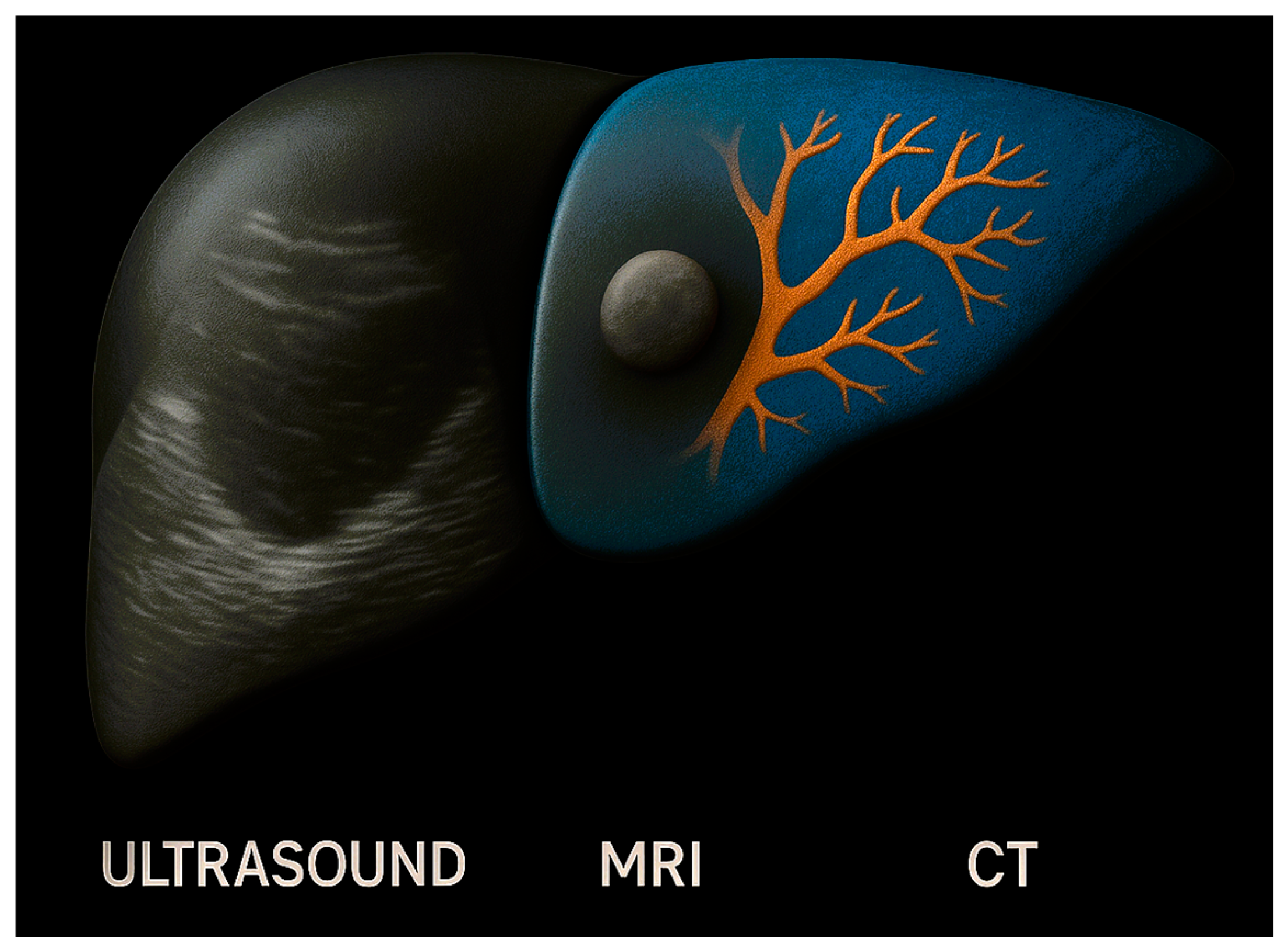
3.2. AI-Driven Imaging
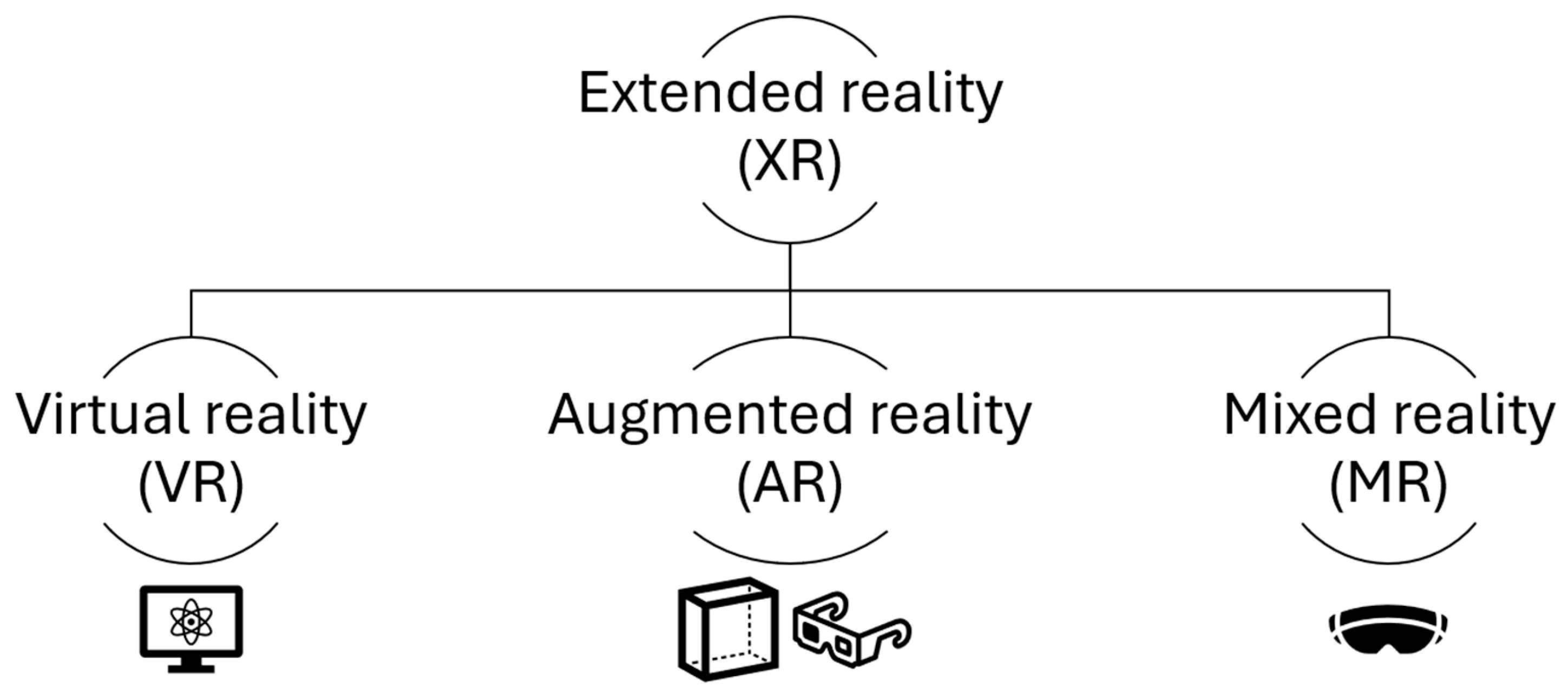
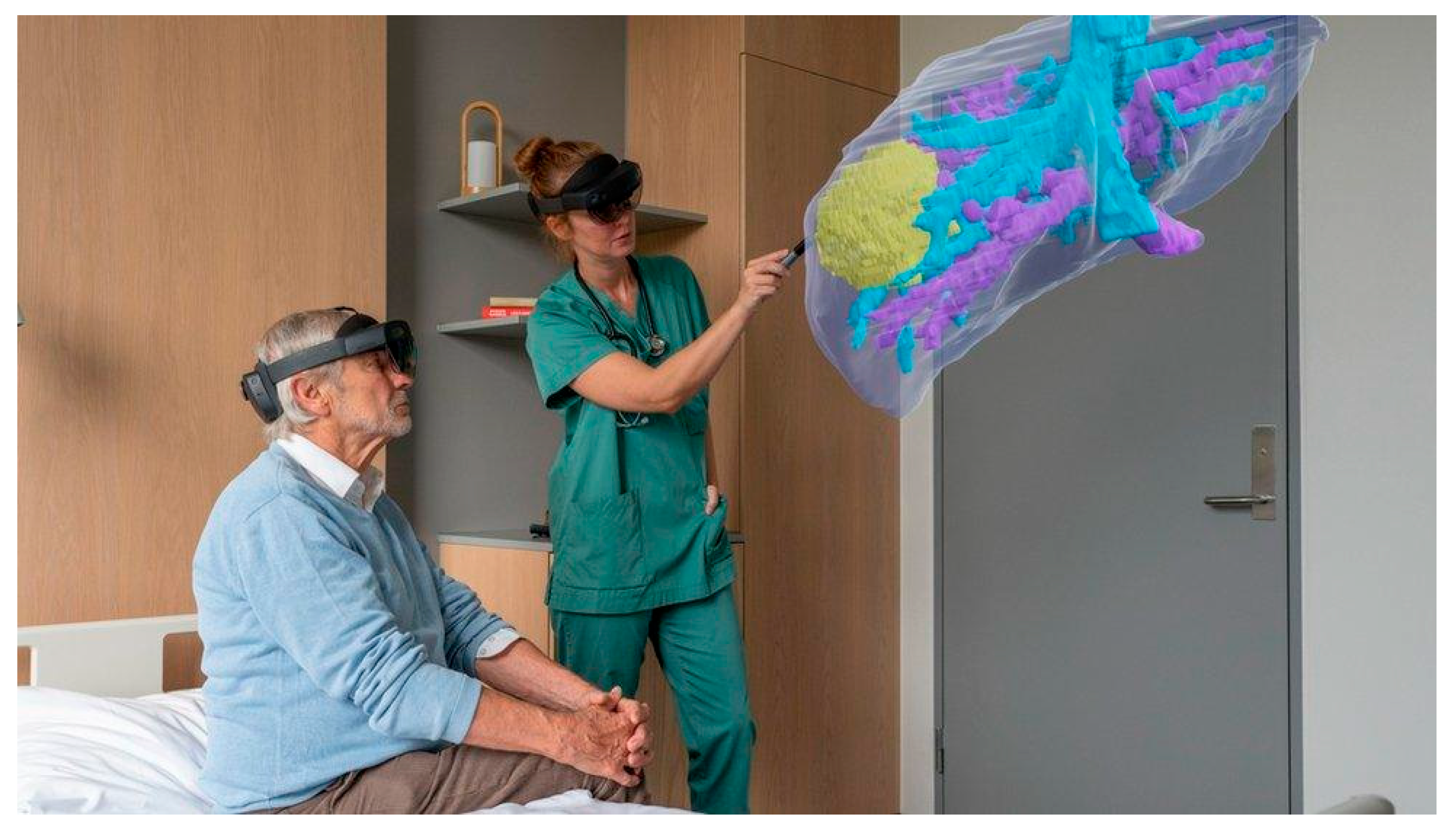
4. Extended Reality and Navigation Systems
4.1. Immersive Technologies
4.2. Surgical Navigation Systems
| Ref. | n | Intervention/Surgery Performed | Results | Significant Findings |
|---|---|---|---|---|
| [33] | ||||
| 85 | Augmented reality navigation system/laparoscopic anatomical hepatectomy for primary liver cancer | Length of stay: Intervention = 7 days Control = 10 days p = 0.003 | Decreased length of stay and estimated blood loss in the augmented reality group | |
| Estimated blood loss: Intervention = 200 mL Control = 300 mL p = 0.002 | ||||
| [34] | 45 | Mixed reality navigation combined with intra-operative ultrasound/laparoscopic anatomical hepatectomy for primary liver cancer | Estimated blood loss: Intervention = 103 mL Control = 259 mL p < 0.001 Complication rates: Intervention = 1 Control = 7 p = 0.021 | Decreased estimated blood loss, complication rates and operative time in the mixed reality group |
| Operative time: Intervention = 135 min Control = 199 min p < 0.001 | ||||
| [35] | 7 | Augmented reality navigation for pancreaticoduodenectomy | Estimated blood loss: Intervention = 901 mL Control = 825 mL p > 0.05 | No significant differences |
| Operative time: Intervention = 412 min Control = 425 min p > 0.05 | ||||
| [36] | 27 | Augmented reality navigation for laparoscopic cholecystectomy | Estimated blood loss: Intervention = 0 mL Control = 0 mL p > 0.05 | No significant differences |
| Operative time: Intervention = 74 min Control = 58 min p > 0.05 |
5. Surgical Education, Training and Patient Involvement
6. Current Challenges and Limitations
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| CRLM | Colorectal Liver Metastasis |
| CALI | Chemotherapy-Associated Liver Injury |
| GenAI | Generative Artificial Intelligence |
| HCC | Hepatocellular Carcinoma |
| XR | Extended Reality |
| PHLF | Post-hepatectomy liver failure |
| HPB | Hepato-pancreato-biliary |
| FLR | Future Liver Remnant |
| AUC | Area Under Curve |
| CT | Computed Tomography |
| MRI | Magnetic Resonance Imaging |
| MRCP | Magnetic Resonance Cholangiopancreatography |
| US | Ultrasound |
References
- International Surgical Outcomes Study, g. Global patient outcomes after elective surgery: Prospective cohort study in 27 low-, middle- and high-income countries. Br. J. Anaesth. 2016, 117, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Nepogodiev, D.; Martin, J.; Biccard, B.; Makupe, A.; Bhangu, A.; Nepogodiev, D.; Martin, J.; Biccard, B.; Makupe, A.; Ademuyiwa, A.; et al. Global burden of postoperative death. Lancet 2019, 393, 401. [Google Scholar] [CrossRef]
- Egeland, C.; Rostved, A.A.; Schultz, N.A.; Pommergaard, H.C.; Daugaard, T.R.; Thofner, L.B.; Rasmussen, A.; Hillingso, J.G. Morbidity and mortality after liver surgery for colorectal liver metastases: A cohort study in a high-volume fast-track programme. BMC Surg. 2021, 21, 312. [Google Scholar] [CrossRef] [PubMed]
- Topal, H.; Aerts, R.; Laenen, A.; Collignon, A.; Jaekers, J.; Geers, J.; Topal, B. Survival After Minimally Invasive vs Open Surgery for Pancreatic Adenocarcinoma. JAMA Netw. Open 2022, 5, e2248147. [Google Scholar] [CrossRef] [PubMed]
- Feussner, H.; Park, A. Surgery 4.0: The natural culmination of the industrial revolution? Innov. Surg. Sci. 2017, 2, 105–108. [Google Scholar] [CrossRef]
- Raza, M.M.; Venkatesh, K.P.; Diao, J.A.; Kvedar, J.C. Defining digital surgery for the future. NPJ Digit. Med. 2022, 5, 155. [Google Scholar] [CrossRef]
- Gilg, S.; Sandstrom, P.; Rizell, M.; Lindell, G.; Ardnor, B.; Stromberg, C.; Isaksson, B. The impact of post-hepatectomy liver failure on mortality: A population-based study. Scand. J. Gastroenterol. 2018, 53, 1335–1339. [Google Scholar] [CrossRef]
- Primavesi, F.; Maglione, M.; Cipriani, F.; Denecke, T.; Oberkofler, C.E.; Starlinger, P.; Dasari, B.V.M.; Heil, J.; Sgarbura, O.; Soreide, K.; et al. E-AHPBA-ESSO-ESSR Innsbruck consensus guidelines for preoperative liver function assessment before hepatectomy. Br. J. Surg. 2023, 110, 1331–1347. [Google Scholar] [CrossRef]
- Xu, Y.; Quan, R.; Xu, W.; Huang, Y.; Chen, X.; Liu, F. Advances in Medical Image Segmentation: A Comprehensive Review of Traditional, Deep Learning and Hybrid Approaches. Bioengineering 2024, 11, 1034. [Google Scholar] [CrossRef]
- Gorgec, B.; Hansen, I.S.; Kemmerich, G.; Syversveen, T.; Abu Hilal, M.; Belt, E.J.T.; Bosscha, K.; Burgmans, M.C.; Cappendijk, V.C.; D’Hondt, M.; et al. MRI in addition to CT in patients scheduled for local therapy of colorectal liver metastases (CAMINO): An international, multicentre, prospective, diagnostic accuracy trial. Lancet Oncol. 2024, 25, 137–146. [Google Scholar] [CrossRef]
- Aguiar, J.A.; Riaz, A.; Thornburg, B. Biliary Anatomy. Semin. Intervent. Radiol. 2021, 38, 251–254. [Google Scholar] [CrossRef]
- Strasberg, S.M.; Hertl, M.; Soper, N.J. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J. Am. Coll. Surg. 1995, 180, 101–125. [Google Scholar] [PubMed]
- Kalata, S.; Thumma, J.R.; Norton, E.C.; Dimick, J.B.; Sheetz, K.H. Comparative Safety of Robotic-Assisted vs Laparoscopic Cholecystectomy. JAMA Surg. 2023, 158, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.C.; Bell, G.; Fullarton, G.M. Incidence and nature of bile duct injuries following laparoscopic cholecystectomy: An audit of 5913 cases. West of Scotland Laparoscopic Cholecystectomy Audit Group. Br. J. Surg. 1996, 83, 1356–1360. [Google Scholar] [CrossRef] [PubMed]
- Broderick, R.C.; Lee, A.M.; Cheverie, J.N.; Zhao, B.; Blitzer, R.R.; Patel, R.J.; Soltero, S.; Sandler, B.J.; Jacobsen, G.R.; Doucet, J.J.; et al. Fluorescent cholangiography significantly improves patient outcomes for laparoscopic cholecystectomy. Surg. Endosc. 2021, 35, 5729–5739. [Google Scholar] [CrossRef]
- Kinami, S.; Maruyama, K.; Sannomiya, Y.; Saito, H.; Takamura, H. Benefits, problems, and optimal timing of administration of indocyanine green fluorescence cholangiography in laparoscopic cholecystectomy. BMJ Surg. Interv. Health Technol. 2025, 7, e000310. [Google Scholar] [CrossRef]
- Thirunavukarasu, A.J.; Ting, D.S.J.; Elangovan, K.; Gutierrez, L.; Tan, T.F.; Ting, D.S.W. Large language models in medicine. Nat. Med. 2023, 29, 1930–1940. [Google Scholar] [CrossRef]
- Morley, J.; DeVito, N.J.; Zhang, J. Generative AI for medical research. BMJ 2023, 382, 1551. [Google Scholar] [CrossRef]
- Khurana, D.; Koli, A.; Khatter, K.; Singh, S. Natural language processing: State of the art, current trends and challenges. Multimed. Tools Appl. 2023, 82, 3713–3744. [Google Scholar] [CrossRef]
- Kron, P.; Farid, S.; Ali, S.; Lodge, P. Artificial Intelligence: A Help or Hindrance to Scientific Writing? Ann. Surg. 2024, 280, 713–718. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J. A commentary of GPT-3 in MIT Technology Review 2021. Fundam. Res. 2021, 1, 831–833. [Google Scholar] [CrossRef]
- Vauthey, J.N.; Pawlik, T.M.; Ribero, D.; Wu, T.T.; Zorzi, D.; Hoff, P.M.; Xiong, H.Q.; Eng, C.; Lauwers, G.Y.; Mino-Kenudson, M.; et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J. Clin. Oncol. 2006, 24, 2065–2072. [Google Scholar] [CrossRef]
- Santol, J.; Kim, S.; Gregory, L.A.; Baumgartner, R.; Murtha-Lemekhova, A.; Birgin, E.; Gloor, S.; Braunwarth, E.; Ammann, M.; Starlinger, J.; et al. An APRI+ALBI-Based Multivariable Model as a Preoperative Predictor for Posthepatectomy Liver Failure. Ann. Surg. 2025, 281, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Mai, R.Y.; Lu, H.Z.; Bai, T.; Liang, R.; Lin, Y.; Ma, L.; Xiang, B.D.; Wu, G.B.; Li, L.Q.; Ye, J.Z. Artificial neural network model for preoperative prediction of severe liver failure after hemihepatectomy in patients with hepatocellular carcinoma. Surgery 2020, 168, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Kowal, M.; Smith, A.; Pandanaboyana, S.; Pathak, S. Editorial: Technological innovations and pancreatic cancer. Front. Oncol. 2024, 14, 1497367. [Google Scholar] [CrossRef]
- Vela Ulloa, J.; King Valenzuela, S.; Riquoir Altamirano, C.; Urrejola Schmied, G. Artificial intelligence-based decision-making: Can ChatGPT replace a multidisciplinary tumour board? Br. J. Surg. 2023, 110, 1543–1544. [Google Scholar] [CrossRef] [PubMed]
- Soga, K.; Ochiai, J.; Kassai, K.; Miyajima, T.; Itani, K.; Yagi, N.; Naito, Y. Development of a novel fusion imaging technique in the diagnosis of hepatobiliary-pancreatic lesions. J. Med. Imaging Radiat. Oncol. 2013, 57, 306–313. [Google Scholar] [CrossRef]
- Darzi, F.; Bocklitz, T. A Review of Medical Image Registration for Different Modalities. Bioengineering 2024, 11, 786. [Google Scholar] [CrossRef]
- Dakua, S.P. Performance divergence with data discrepancy: A review. Artif. Intell. Rev. 2013, 40, 429–455. [Google Scholar] [CrossRef]
- Teng, Z.; Li, L.; Xin, Z.; Xiang, D.; Huang, J.; Zhou, H.; Shi, F.; Zhu, W.; Cai, J.; Peng, T.; et al. A literature review of artificial intelligence (AI) for medical image segmentation: From AI and explainable AI to trustworthy AI. Quant. Imaging Med. Surg. 2024, 14, 9620–9652. [Google Scholar] [CrossRef]
- Fang, C.H.; Tao, H.S.; Yang, J.; Fang, Z.S.; Cai, W.; Liu, J.; Fan, Y.F. Impact of three-dimensional reconstruction technique in the operation planning of centrally located hepatocellular carcinoma. J. Am. Coll. Surg. 2015, 220, 28–37. [Google Scholar] [CrossRef]
- Andrews, C.; Southworth, M.K.; Silva, J.N.A.; Silva, J.R. Extended Reality in Medical Practice. Curr. Treat. Options Cardiovasc. Med. 2019, 21, 18. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, W.; Yang, J.; Xiang, N.; Zeng, N.; Hu, H.; Jia, F.; Fang, C. Augmented Reality Navigation for Stereoscopic Laparoscopic Anatomical Hepatectomy of Primary Liver Cancer: Preliminary Experience. Front. Oncol. 2021, 11, 663236. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Zhang, L.; Liu, H. Application effect of contrast-enhanced ultrasound combined with mixed reality technology in laparoscopic anatomical hepatectomy. Med. J. Chin. People’s Lib. Army 2023, 48, 1208. [Google Scholar] [CrossRef]
- Onda, S.; Okamoto, T.; Kanehira, M.; Suzuki, F.; Ito, R.; Fujioka, S.; Suzuki, N.; Hattori, A.; Yanaga, K. Identification of inferior pancreaticoduodenal artery during pancreaticoduodenectomy using augmented reality-based navigation system. J. Hepatobiliary Pancreat. Sci. 2014, 21, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, M.; Sugimoto, M.; Haruta, H.; Umezawa, A.; Kurokawa, Y. Intraoperative holography navigation using a mixed-reality wearable computer during laparoscopic cholecystectomy. Surgery 2022, 171, 1006–1013. [Google Scholar] [CrossRef]
- Vedula, S.S.; Ghazi, A.; Collins, J.W.; Pugh, C.; Stefanidis, D.; Meireles, O.; Hung, A.J.; Schwaitzberg, S.; Levy, J.S.; Sachdeva, A.K.; et al. Artificial Intelligence Methods and Artificial Intelligence-Enabled Metrics for Surgical Education: A Multidisciplinary Consensus. J. Am. Coll. Surg. 2022, 234, 1181–1192. [Google Scholar] [CrossRef]
- Winkler-Schwartz, A.; Bissonnette, V.; Mirchi, N.; Ponnudurai, N.; Yilmaz, R.; Ledwos, N.; Siyar, S.; Azarnoush, H.; Karlik, B.; Del Maestro, R.F. Artificial Intelligence in Medical Education: Best Practices Using Machine Learning to Assess Surgical Expertise in Virtual Reality Simulation. J. Surg. Educ. 2019, 76, 1681–1690. [Google Scholar] [CrossRef]
- Guerrero, D.T.; Asaad, M.; Rajesh, A.; Hassan, A.; Butler, C.E. Advancing Surgical Education: The Use of Artificial Intelligence in Surgical Training. Am. Surg. 2023, 89, 49–54. [Google Scholar] [CrossRef]
- Leon, S.; Lee, S.; Perez, J.E.; Hashimoto, D.A. Artificial intelligence and the education of future surgeons. Am. J. Surg. 2025, 246, 116257. [Google Scholar] [CrossRef]
- Skorka, P.; Kargul, M.; Seemannova, D.; Gajek, B.; Gutowski, P.; Kazimierczak, A.; Rynio, P. The Influence of Individualized Three-Dimensional Holographic Models on Patients’ Knowledge Qualified for Intervention in the Treatment of Peripheral Arterial Disease (PAD). J. Cardiovasc. Dev. Dis. 2023, 10, 464. [Google Scholar] [CrossRef]
- Clark, K.; Vendt, B.; Smith, K.; Freymann, J.; Kirby, J.; Koppel, P.; Moore, S.; Phillips, S.; Maffitt, D.; Pringle, M.; et al. The Cancer Imaging Archive (TCIA): Maintaining and operating a public information repository. J. Digit. Imaging 2013, 26, 1045–1057. [Google Scholar] [CrossRef]
- Kondylakis, H.; Kalokyri, V.; Sfakianakis, S.; Marias, K.; Tsiknakis, M.; Jimenez-Pastor, A.; Camacho-Ramos, E.; Blanquer, I.; Segrelles, J.D.; Lopez-Huguet, S.; et al. Data infrastructures for AI in medical imaging: A report on the experiences of five EU projects. Eur. Radiol. Exp. 2023, 7, 20. [Google Scholar] [CrossRef]
- McCulloch, P.; Altman, D.G.; Campbell, W.B.; Flum, D.R.; Glasziou, P.; Marshall, J.C.; Nicholl, J.; Balliol, C.; Aronson, J.K.; Barkun, J.S.; et al. No surgical innovation without evaluation: The IDEAL recommendations. Lancet 2009, 374, 1105–1112. [Google Scholar] [CrossRef]
- Shiferaw, K.B.; Roloff, M.; Balaur, I.; Welter, D.; Waltemath, D.; Zeleke, A.A. Guidelines and standard frameworks for artificial intelligence in medicine: A systematic review. JAMIA Open 2025, 8, ooae155. [Google Scholar] [CrossRef]
- Nathan, H.; Cameron, J.L.; Choti, M.A.; Schulick, R.D.; Pawlik, T.M. The volume-outcomes effect in hepato-pancreato-biliary surgery: Hospital versus surgeon contributions and specificity of the relationship. J. Am. Coll. Surg. 2009, 208, 528–538. [Google Scholar] [CrossRef]
- Birkmeyer, J.D.; Siewers, A.E.; Finlayson, E.V.; Stukel, T.A.; Lucas, F.L.; Batista, I.; Welch, H.G.; Wennberg, D.E. Hospital volume and surgical mortality in the United States. N. Engl. J. Med. 2002, 346, 1128–1137. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowal, M.R.; Ibrahim, M.; Mihaljević, A.L.; Kron, P.; Lodge, P. Technological Advances in Pre-Operative Planning. J. Clin. Med. 2025, 14, 5385. https://doi.org/10.3390/jcm14155385
Kowal MR, Ibrahim M, Mihaljević AL, Kron P, Lodge P. Technological Advances in Pre-Operative Planning. Journal of Clinical Medicine. 2025; 14(15):5385. https://doi.org/10.3390/jcm14155385
Chicago/Turabian StyleKowal, Mikolaj R., Mohammed Ibrahim, André L. Mihaljević, Philipp Kron, and Peter Lodge. 2025. "Technological Advances in Pre-Operative Planning" Journal of Clinical Medicine 14, no. 15: 5385. https://doi.org/10.3390/jcm14155385
APA StyleKowal, M. R., Ibrahim, M., Mihaljević, A. L., Kron, P., & Lodge, P. (2025). Technological Advances in Pre-Operative Planning. Journal of Clinical Medicine, 14(15), 5385. https://doi.org/10.3390/jcm14155385






