Evaluation of the Modified Early Warning Score (MEWS) in In-Hospital Cardiac Arrest in a Tertiary Healthcare Facility
Abstract
1. Introduction
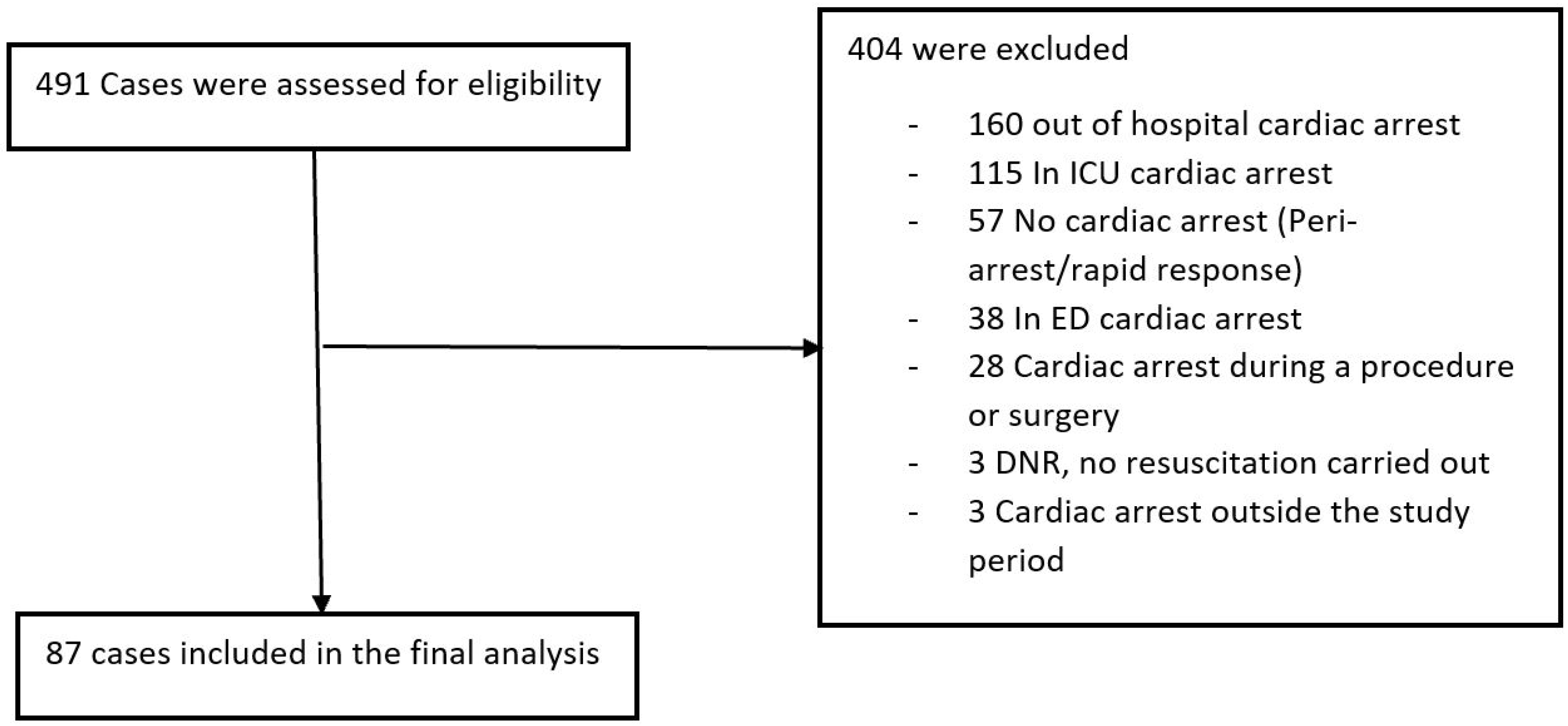
2. Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merchant, R.M.; Yang, L.; Becker, L.B.; Berg, R.A.; Nadkarni, V.; Nichol, G.; Carr, B.G.; Mitra, N.; Bradley, S.M.; Abella, B.S.; et al. Incidence of Treated Cardiac Arrest in Hospitalized Patients in the United States. Crit. Care Med. 2011, 39, 2401–2406. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.W.; Holmberg, M.J.; Berg, K.M.; Donnino, M.W.; Granfeldt, A. In-Hospital Cardiac Arrest: A Review. JAMA 2019, 321, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Hillman, K.; Chen, J.; Cretikos, M.; Bellomo, R.; Brown, D.; Doig, G.; Finfer, S.; Flabouris, A. Introduction of the Medical Emergency Team (Met) System: A Cluster-Randomised Controlled Trial. Lancet 2005, 365, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Kause, J.; Smith, G.; Prytherch, D.; Parr, M.; Flabouris, A.; Hillman, K. A Comparison of Antecedents to Cardiac Arrests, Deaths and Emergency Intensive Care Admissions in Australia and New Zealand, and the United Kingdom—The Academia study. Resuscitation 2004, 62, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Skrifvars, M.B.; Nurmi, J.; Ikola, K.; Saarinen, K.; Castren, M. Reduced Survival Following Resuscitation in Patients with Documented Clinically Abnormal Observations Prior to In-Hospital Cardiac Arrest. Resuscitation 2006, 70, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.A.; DeVita, M.A.; Bellomo, R. Rapid-Response Teams. N. Engl. J. Med. 2011, 365, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Kronick, S.L.; Kurz, M.C.; Lin, S.; Edelson, D.P.; Berg, R.A.; Billi, J.E.; Cabanas, J.G.; Cone, D.C.; Diercks, D.B.; Foster, J.J.; et al. Part 4: Systems of Care and Continuous Quality Improvement: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015, 132, S397–S413. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Physicians. National Early Warning Score (NEWS) 2: Standardising the Assessment of Acute-Illness Severity in the NHS; Updated Report of a Working Party; RCP: London, UK, 2017. [Google Scholar]
- Corfield, A.R.; Lees, F.; Zealley, I.; Houston, G.; Dickie, S.; Ward, K.; McGuffie, C. Utility of a Single Early Warning Score in Patients with Sepsis in the Emergency Department. Emerg. Med. J. 2014, 31, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, D.K. What Is the Proper Way to Apply the Multiple Comparison Test? Korean J. Anesthesiol. 2018, 71, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Spangfors, M.; Molt, M.; Samuelson, K. In-Hospital Cardiac Arrest and Preceding National Early Warning Score (NEWS): A Retrospective Case-Control Study. Clin. Med. 2020, 20, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Churpek, M.M.; Yuen, T.C.; Huber, M.T.; Park, S.Y.; Hall, J.B.; Edelson, D.P. Predicting Cardiac Arrest on the Wards: A Nested Case-Control Study. Chest 2012, 141, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Nallamothu, B.K.; Chan, P.S.; American Heart Association National Registry of Cardiopulmonary Resuscitation (NRCPR) Investigators. Body Mass Index and Survival after In-Hospital Cardiac Arrest. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Han, K.D.; Roh, S.Y.; Jeong, J.H.; Choi, Y.Y.; Min, K.; Shim, J.; Choi, J.I.; Kim, Y.H. Being Underweight Is Associated with Increased Risk of Sudden Cardiac Death in People with Diabetes Mellitus. J. Clin. Med. 2023, 12, 1045. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.Y.; Shin, Y.J.; Lee, J.M.; Huh, J.W.; Koh, Y.; Lim, C.M.; Hong, S.B. Modified Early Warning Score Changes Prior to Cardiac Arrest in General Wards. PLoS ONE 2015, 10, e0130523. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, I.; Oyadomari, S.; Maedomari, S.; Toma, R.; Igei, C.; Kobata, S.; Koyama, J.; Tomori, R.; Kawamitsu, N.; Yamamoto, Y.; et al. Use of a Modified Early Warning Score System to Reduce the Rate of In-Hospital Cardiac Arrest. J. Intensive Care 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Mullany, D.V.; Ziegenfuss, M.; Goleby, M.A.; Ward, H.E. Improved Hospital Mortality with a Low Met Dose: The Importance of a Modified Early Warning Score and Communication Tool. Anaesth. Intensive Care 2016, 44, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Heller, A.R.; Mees, S.T.; Lauterwald, B.; Reeps, C.; Koch, T.; Weitz, J. Detection of Deteriorating Patients on Surgical Wards Outside the Icu by an Automated Mews-Based Early Warning System with Paging Functionality. Ann. Surg. 2020, 271, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Hogan, H.; Hutchings, A.; Wulff, J.; Carver, C.; Holdsworth, E.; Nolan, J.; Welch, J.; Harrison, D.; Black, N. Type of Track and Trigger System and Incidence of in-Hospital Cardiac Arrest: An Observational Registry-Based Study. BMC Health Serv. Res. 2020, 20, 885. [Google Scholar] [CrossRef] [PubMed]
- Shaver, J. The State of Telehealth before and after the COVID-19 Pandemic. Prim. Care 2022, 49, 517–530. [Google Scholar] [CrossRef] [PubMed]

| Physiological Parameter | Score | ||||||
|---|---|---|---|---|---|---|---|
| 3 | 2 | 1 | 0 | 1 | 2 | 3 | |
| Respiratory rate | ≤8 | 9–11 | 12–20 | 21–24 | ≥25 | ||
| SpO2 Scale 1 (%) | ≤91 | 92–93 | 94–95 | ≥96 | |||
| SpO2 Scale 1 (%) (History of LTOT) | ≤83 | 84–85 | 86–87 | 88–92 ≥ 93 on air | 93–94 on oxygen | 95–96 on oxygen | ≥97 on oxygen |
| Air or oxygen | Oxygen | Air | |||||
| Systolic Blood Pressure (mmHg) | ≤90 | 91–100 | 101–110 | 111–219 | ≥220 | ||
| Pulse (BPM) | ≤40 | 41–50 | 51–90 | 91–100 | 111–130 | ≥131 | |
| Consciousness | Alert | CVPU | |||||
| Temperature | ≤35.0 | 35.1–36.0 | 36.1–38.0 | 38.1–39.0 | ≥39.1 | ||
| n (%) | Average Age (Years) | p-Value | Average 12 h MEWS | p-Value | Average 6 h MEWS | p-Value | Average 1 h MEWS | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Total cases | 87 | 69 ± 15 | 3.95 ± 2.4 | 4.65 ± 2.6 | 5.98 ± 3.5 | ||||
| Sex | |||||||||
| Male | 52 (59.8) | 68.6 ± 15.2 | 0.62 | 3.9 ± 2.4 | 0.81 | 4.46 ± 2.6 | 0.42 | 5.67 ± 3.7 | 0.41 |
| Female | 35 (40.2) | 70.3 ± 15.0 | 4.03 ± 2.5 | 4.46 ± 2.6 | 6.5 ± 3.2 | ||||
| Race | |||||||||
| Black | 17 (19.5) | 61.5 ± 15.7 | 0.016 | 3.0 ± 2.6 | 0.09 | 4.31 ± 2.5 | 0.56 | 6.33 ± 4.2 | 0.70 |
| White | 70 (80.5) | 71.2 ± 14.3 | 4.17 ± 2.3 | 4.73 ± 2.6 | 5.88 ± 3.4 |
| n (%) | Average 12 h MEWS | p-Value | Average 6 h MEWS | p-Value | Average 1 h MEWS | p-Value | |
|---|---|---|---|---|---|---|---|
| Total cases | 87 | 3.95 ± 2.4 | 4.65 ± 2.6 | 5.98 ± 3.5 | |||
| Weight class (BMI) | |||||||
| Underweight | 7 (8) | 5.33 ± 3.5 | 0.03 | 6.86 ± 2.1 | 0.066 | 10.4 ± 2.7 | 0.028 |
| Healthy weight | 33 (37.9) | 3.1 ± 2.2 | 4.87 ± 3.1 | 5.33 ± 3.4 | |||
| Overweight | 16 (18.4) | 5.0 ± 2.3 | 4.0 ± 2.1 | 5.55 ± 2.7 | |||
| Obese | 31 (35.6) | 4.0 ± 2.2 | 4.19 ± 2.1 | 5.75 ± 3.8 | |||
| COVID-19 | |||||||
| Yes | 15 (17.2) | 4.08 ± 2.5 | 0.84 | 5.14 ± 4.1 | 0.44 | 6.50 ± 2.9 | 0.61 |
| No | 72 (82.8) | 3.93 ± 2.4 | 4.55 ± 2.2 | 5.86 ± 3.7 | |||
| Hypertension | |||||||
| Yes | 47 (54) | 3.8 ± 2.5 | 0.53 | 4.47 ± 2.5 | 0.5 | 5.81 ± 3.5 | 0.67 |
| No | 40 (46) | 4.14 ± 2.3 | 4.86 ± 2.7 | 6.23 ± 3.7 | |||
| Diabetes | |||||||
| Yes | 24 (27.6) | 4.32 ± 2.5 | 0.40 | 5.25 ± 2.5 | 0.18 | 6.33 ± 3.4 | 0.65 |
| No | 63 (72.4) | 3.81 ± 2.4 | 4.39 ± 2.6 | 5.84 ± 3.6 | |||
| CKD | |||||||
| Yes | 9 (10.3) | 3.0 ± 1.9 | 0.21 | 5.67 ± 1.9 | 0.21 | 4.33 ± 2.7 | 0.23 |
| No | 78 (89.7) | 4.07 ± 2.4 | 4.52 ± 2.7 | 6.19 ± 3.6 | |||
| Rhythm | |||||||
| Shockable | 16 (18.4) | 3.5 ± 1.9 | 0.22 | 3.93 ± 2.8 | 0.38 | 4.67 ± 3.1 | 0.56 |
| Non-shockable | 56 (64.4) | 4.29 ± 2.4 | 4.94 ± 2.5 | 6.00 ± 3.2 | |||
| Undocumented | 15 917.2) | 3.15 ± 2.7 | 4.33 ± 2.7 | 6.64 ± 4.8 | |||
| Outcome | |||||||
| Death during same admission | 69 (79.3) | 4.03 ± 2.4 | 0.56 | 4.84 ± 2.6 | 0.23 | 6.42 ± 3.5 | 0.11 |
| Survived and discharged | 18 (20.7) | 3.65 ± 2.5 | 4.0 ± 2.5 | 4.62 ± 3.4 |
| (I) Time HrP | (J) Time HrP | Mean Difference in MEWS (I − J) | Standard Error | p-Value | 95% CI of Mean Difference |
|---|---|---|---|---|---|
| 12 | 6 | −0.70 | 0.440 | 0.341 | −1.76–0.36 |
| 1 | −2.03 | 0.494 | <0.001 | −3.22 to −0.84 | |
| 6 | 12 | 0.70 | 0.440 | 0.341 | −0.36–1.76 |
| 1 | −1.33 | 0.495 | 0.023 | −2.53 to −0.14 | |
| 1 | 12 | 2.03 | 0.494 | <0.001 | 0.84–3.22 |
| 6 | 1.33 | 0.495 | 0.023 | 0.14–2.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogbebor, O.; Niranjan, S.; Saini, V.; Ramanujam, D.; DiSilvio, B.; Cheema, T. Evaluation of the Modified Early Warning Score (MEWS) in In-Hospital Cardiac Arrest in a Tertiary Healthcare Facility. J. Clin. Med. 2025, 14, 5384. https://doi.org/10.3390/jcm14155384
Ogbebor O, Niranjan S, Saini V, Ramanujam D, DiSilvio B, Cheema T. Evaluation of the Modified Early Warning Score (MEWS) in In-Hospital Cardiac Arrest in a Tertiary Healthcare Facility. Journal of Clinical Medicine. 2025; 14(15):5384. https://doi.org/10.3390/jcm14155384
Chicago/Turabian StyleOgbebor, Osakpolor, Sitara Niranjan, Vikram Saini, Deeksha Ramanujam, Briana DiSilvio, and Tariq Cheema. 2025. "Evaluation of the Modified Early Warning Score (MEWS) in In-Hospital Cardiac Arrest in a Tertiary Healthcare Facility" Journal of Clinical Medicine 14, no. 15: 5384. https://doi.org/10.3390/jcm14155384
APA StyleOgbebor, O., Niranjan, S., Saini, V., Ramanujam, D., DiSilvio, B., & Cheema, T. (2025). Evaluation of the Modified Early Warning Score (MEWS) in In-Hospital Cardiac Arrest in a Tertiary Healthcare Facility. Journal of Clinical Medicine, 14(15), 5384. https://doi.org/10.3390/jcm14155384







