Ruptured Posterior Inferior Cerebellar Artery Aneurysms: Integrating Microsurgical Expertise, Endovascular Challenges, and AI-Driven Risk Assessment
Abstract
1. Introduction
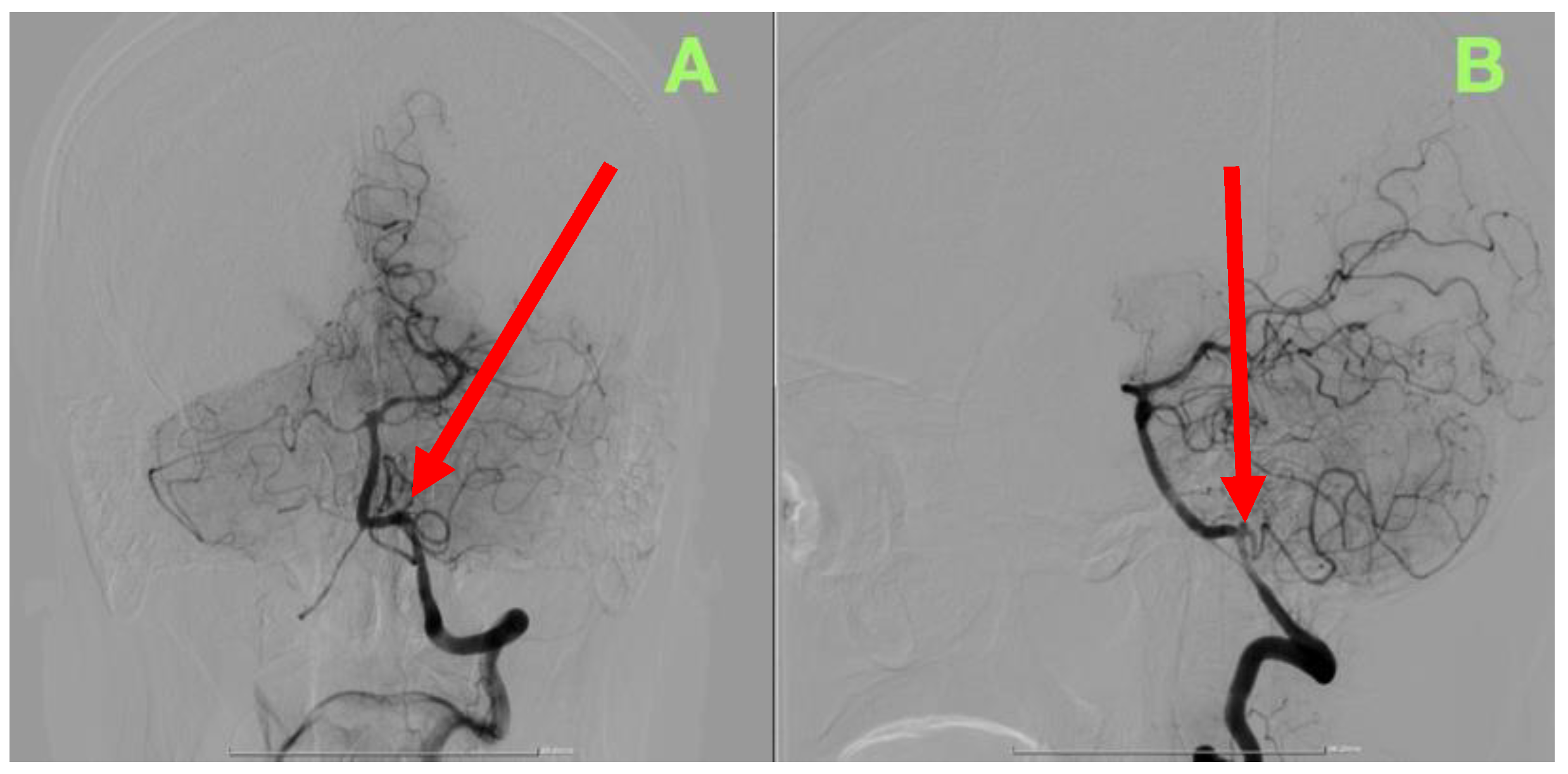
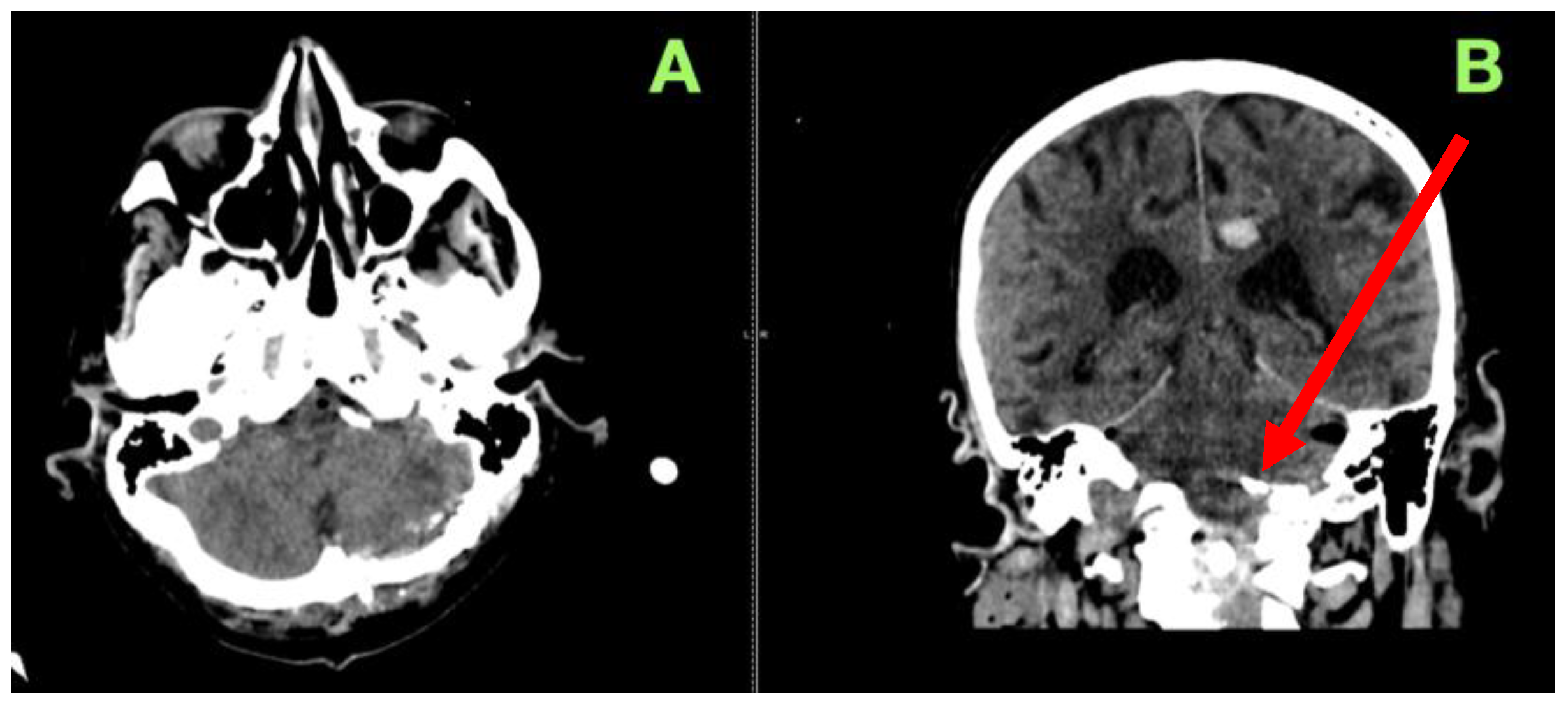
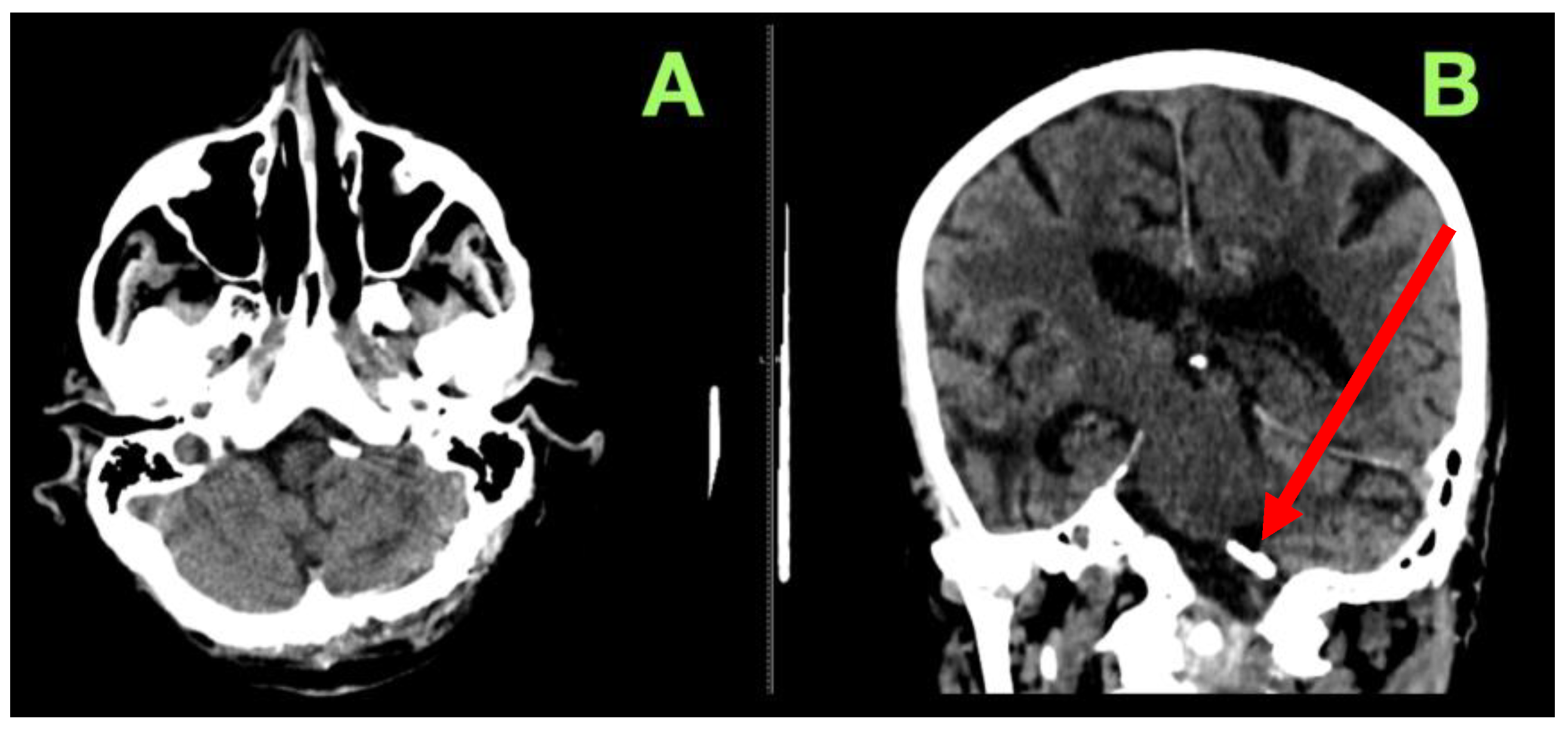
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miao, H.-L.; Zhang, D.-Y.; Wang, T.; Jiao, X.-T.; Jiao, L.-Q. Clinical Importance of the Posterior Inferior Cerebellar Artery: A Review of the Literature. Int. J. Med. Sci. 2020, 17, 3005–3019. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R.H.; Horn, G.L., Jr.; Metwalli, Z.; Gopinath, S.P.; Benndorf, G. Endovascular Management of a Ruptured Aneurysm on a Posterior Inferior Cerebellar Artery with Extradural C2-Origin: Case Report and Literature Review. Neurointervention 2024, 19, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, E.; Brugerolles, P.; Premat, K.; Lenck, S.; Shotar, E.; Jacquens, A.; Degos, V.; Boch, A.L.; Carpentier, A.; Clarencon, F.; et al. Prepontine Cistern Filling Independently Predicts Poor Neurologic Outcomes in Ruptured PICA Aneurysms. Am. J. Neuroradiol. 2025, 46, 929–935. [Google Scholar] [CrossRef]
- Yang, M. Ruptured aneurysm at the meatal loop of the posterior inferior cerebellar artery: A case description. Quant. Imaging Med. Surg. 2024, 14, 4232237–4234237. [Google Scholar] [CrossRef]
- Brandel, M.G.; Plonsker, J.H.; Rennert, R.C.; Produturi, G.; Saripella, M.; Wali, A.R.; McCann, C.; Ravindra, V.M.; Santiago-Dieppa, D.R.; Pannell, J.S.; et al. Treatment of pediatric intracranial aneurysms: Institutional case series and systematic literature review. Childs Nerv. Syst. 2024, 40, 2419–2429. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Tang, Y.; You, W.; Jiang, Y.; Xu, Z.; Zhao, Y.; Liu, X.; Lv, J.; Liu, P.; Wei, H.; et al. Risk analysis of intracranial aneurysm rupture based on the arterial segment of origin. Front. Neurol. 2024, 15, 1339144. [Google Scholar] [CrossRef]
- Toader, C.; Serban, M.; Covache-Busuioc, R.-A.; Radoi, M.P.; Aljboor, G.S.R.; Glavan, L.-A.; Corlatescu, A.D.; Ilie, M.-M.; Gorgan, R.M. Navigating the Rare and Dangerous: Successful Clipping of a Superior Cerebellar Artery Aneurysm Against the Odds of Uncontrolled Hypertension. J. Clin. Med. 2024, 13, 7430. [Google Scholar] [CrossRef]
- Akly, M.S.P.; Vazquez, C.; Besada, C.H.; Rodriguez, M.J.; Conde, M.F.; Cajal, A.R.; Peuchot, V.A.; Dardik, D.; Baccanelli, M.M.; Serra, M.M. Prevalence of Intracranial Aneurysms in Hereditary Hemorrhagic Telangiectasia: Report from a Single Reference Center. Am. J. Neuroradiol. 2022, 43, 844–849. [Google Scholar] [CrossRef]
- Gokhale, V.S.; Nimmala, S.G.; Arkar, R. Posterior Inferior Cerebellar Artery Aneurysm Presenting With Severe Vertigo and Altered Sensorium. Cureus 2024, 16, e60869. [Google Scholar] [CrossRef]
- Garner, M.; Fries, F.; Kettner, M.; Haußmann, A.; Bachhuber, A.; Reith, W.; Yilmaz, U. Endovascular Treatment Strategies for Aneurysms of the Origin of the Posterior Inferior Cerebellar Artery. World Neurosurg. 2023, 172, e412–e417. [Google Scholar] [CrossRef]
- Chauhan, G.; Singh, V.; Prasad, S.N.; Phadke, R.V.; Neyaz, Z. Conventional and old endovascular techniques for vertebral aneurysms still work in the era of flow diversion. Egypt. J. Neurosurg. 2024, 39, 61. [Google Scholar] [CrossRef]
- Gaub, M.; Murtha, G.; Lafuente, M.; Webb, M.; Luo, A.; Birnbaum, L.A.; Mascitelli, J.R.; Al Saiegh, F. Flow Diversion for Endovascular Treatment of Intracranial Aneurysms: Past, Present, and Future Directions. J. Clin. Med. 2024, 13, 4167. [Google Scholar] [CrossRef]
- Zheng, Y.; Zheng, L.; Sun, Y.; Lin, D.; Wang, B.; Sun, Q.; Bian, L. Surgical Clipping of Previously Coiled Recurrent Intracranial Aneurysms: A Single-Center Experience. Front. Neurol. 2021, 12, 680375. [Google Scholar] [CrossRef]
- Yang, Z.; Song, J.; Quan, K.; Li, P.; An, Q.; Shi, Y.; Liu, P.; Yu, G.; Tian, Y.; Zhou, L.; et al. Microsurgical treatment of posterior inferior cerebellar aneurysms based on angioarchitecture supplemented by high-resolution vessel wall MRI: A case series report. Stroke Vasc. Neurol. 2022, 7, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, D.; Sarma, P.; Deora, H.; Beniwal, M.; Vikas, V.; Rao, K.; Chandramouli, B.A.; Somanna, S. “Tailored” far lateral approach to anterior foramen magnum meningiomas—The importance of condylar preservation. Neurol. India 2019, 67, 142. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Guo, H.; Wei, J.; Liu, P.; Lv, M.; Li, Y. Posterior Inferior Cerebellar Artery Aneurysms: Comparison of Results of Surgical and Endovascular Managements at One Single Center. Neurol. India 2020, 68, 1115–1124. Available online: https://journals.lww.com/neur/fulltext/2020/68050/posterior_inferior_cerebellar_artery_aneurysms_.28.aspx (accessed on 5 March 2025). [CrossRef]
- Goertz, L.; Liebig, T.; Siebert, E.; Özpeynirci, Y.; Pennig, L.; Celik, E.; Schlamann, M.; Dorn, F.; Kabbasch, C. Treatment of Proximal Posterior Inferior Cerebellar Artery Aneurysms by Intrasaccular Flow Disruption: A Multicenter Experience. Am. J. Neuroradiol. 2022, 43, 1158–1163. [Google Scholar] [CrossRef]
- Sejkorová, A.; Petr, O.; Mulino, M.; Cihlář, J.; Hejčl, A.; Thomé, C.; Sameš, M.; Lanzino, G. Management of posterior inferior cerebellar artery aneurysms: What factors play the most important role in outcome? Acta Neurochir. 2017, 159, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-J.; Lee, Y.-J.; Kim, W.-B.; Kim, Y.-S.; Kim, T.-S.; Joo, S.-P. Intracranial–intracranial bypass strategies for the treatment of complex intracranial aneurysms: Anatomical characteristics and surgical intervention. Acta Neurochir. 2024, 166, 42. [Google Scholar] [CrossRef]
- You, W.; Meng, J.; Yang, X.; Zhang, J.; Jiang, G.; Yan, Z.; Gu, F.; Tao, X.; Chen, Z.; Wang, Z.; et al. Microsurgical Management of Posterior Circulation Aneurysms: A Retrospective Study on Epidemiology, Outcomes, and Surgical Approaches. Brain Sci. 2022, 12, 1066. [Google Scholar] [CrossRef]
- Toader, C.; Serban, M.; Covache-Busuioc, R.-A.; Radoi, M.P.; Aljboor, G.S.R.; Costin, H.P.; Ilie, M.-M.; Popa, A.A.; Gorgan, R.M. Single-Stage Microsurgical Clipping of Multiple Intracranial Aneurysms in a Patient with Cerebral Atherosclerosis: A Case Report and Review of Surgical Management. J. Clin. Med. 2025, 14, 269. [Google Scholar] [CrossRef]
- Xu, F.; Hong, Y.; Zheng, Y.; Xu, Q.; Leng, B. Endovascular treatment of posterior inferior cerebellar artery aneurysms: A 7-year single-center experience. J. Neurointerventional Surg. 2017, 9, 45–51. [Google Scholar] [CrossRef]
- Zedde, M.; Pascarella, R. Spontaneous Intracranial Vertebral Artery Dissection: A Rare Cause of Ischemic Stroke. J. Cardiovasc. Dev. Dis. 2025, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Wanhainen, A.; Herzeele, I.V.; Goncalves, F.B.; Montoya, S.B.; Berard, X.; Boyle, J.R.; D’Oria, M.; Prendes, C.F.; Karkos, C.D.; Kazimierczak, A.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 192–331. [Google Scholar] [CrossRef] [PubMed]
- Nordanstig, J.; Behrendt, C.-A.; Baumgartner, I.; Belch, J.; Bäck, M.; Fitridge, R.; Hinchliffe, R.; Lejay, A.; Mills, J.L.; Rother, U.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Asymptomatic Lower Limb Peripheral Arterial Disease and Intermittent Claudication. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 9–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Z.; Xiao, W.; Yang, Y.; Yan, Y.; Bai, L.; Quan, L.; Qi, T.; Liang, F. Near-Wall Slow Flow Contributes to Wall Enhancement of Middle Cerebral Artery Bifurcation Aneurysms on Vessel Wall MRI. Diagnostics 2024, 14, 2722. [Google Scholar] [CrossRef]
- Lampropoulos, D.S.; Hadjinicolaou, M. Investigating Hemodynamics in Intracranial Aneurysms with Irregular Morphologies: A Multiphase CFD Approach. Mathematics 2025, 13, 505. [Google Scholar] [CrossRef]
- Byun, J.; Park, W.; Park, J.C.; Ahn, J.S. Clinical Outcomes of Large (>10 mm) Unruptured Posterior Circulation Aneurysms and Their Predictors. J. Korean Neurosurg. Soc. 2020, 64, 39–50. [Google Scholar] [CrossRef]
- Körfer, D.; Erhart, P.; Dihlmann, S.; Hakimi, M.; Böckler, D.; Peters, A.S. Histopathological Characterization of Abdominal Aortic Aneurysms from Patients with Multiple Aneurysms Compared to Patients with a Single Abdominal Aortic Aneurysm. Biomedicines 2023, 11, 1311. [Google Scholar] [CrossRef]
- Ali, A.M.S.; Hannan, C.J.; Islim, A.I.; Mascitelli, J.R.; Javadpour, M. Surgical and Endovascular Treatment of Saccular Posterior Inferior Cerebellar Artery Aneurysms: Systematic Review and Meta-Analysis. World Neurosurg. 2022, 162, e168–e177. [Google Scholar] [CrossRef]
- Wiśniewski, K.; Tyfa, Z.; Tomasik, B.; Reorowicz, P.; Bobeff, E.J.; Posmyk, B.J.; Hupało, M.; Stefańczyk, L.; Jóźwik, K.; Jaskólski, D.J. Risk Factors for Recanalization after Coil Embolization. J. Pers. Med. 2021, 11, 793. [Google Scholar] [CrossRef]
- Park, H.R.; Kim, J.H.; Park, S.; Chang, J.-C.; Park, S.Q. National Trends in the Treatment of Ruptured Cerebral Aneurysms in Korea Using an Age-adjusted Method. J. Korean Med. Sci. 2020, 35, e323. [Google Scholar] [CrossRef]
- Fatehi, M.; Rizzuto, M.A.; Prakash, S.; Haw, C.; Gooderham, P.A.; Redekop, G.J. Functional Outcomes After Treatment of Posterior Inferior Cerebellar Artery Aneurysms. Cureus 2020, 12, e11746. [Google Scholar] [CrossRef]
- Peng, F.; Xia, J.; Zhang, F.; Lu, S.; Wang, H.; Li, J.; Liu, X.; Zhong, Y.; Guo, J.; Duan, Y.; et al. Intracranial aneurysm instability prediction model based on 4D-Flow MRI and HR-MRI. Neurotherapeutics 2024, 22, e00505. [Google Scholar] [CrossRef] [PubMed]
- Hachem, E.; Meliga, P.; Goetz, A.; Rico, P.J.; Viquerat, J.; Larcher, A.; Valette, R.; Sanches, A.F.; Lannelongue, V.; Ghraieb, H.; et al. Reinforcement learning for patient-specific optimal stenting of intracranial aneurysms. Sci. Rep. 2023, 13, 7147. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-H.; Choi, J.-I.; Ha, S.-K.; Lim, D.-J. Microsurgical clipping remains a viable option for the treatment of coilable ruptured middle cerebral artery aneurysms in the endovascular era. Neurosurg. Rev. 2025, 48, 38. [Google Scholar] [CrossRef] [PubMed]
- Sweid, A.; El Naamani, K.; Abbas, R.; Starke, R.M.; Badih, K.; El Hajjar, R.; Saad, H.; Hammoud, B.; Andrews, C.; Rahm, S.P.; et al. Clipping Could Be the Best Treatment Modality for Recurring Anterior Communicating Artery Aneurysms Treated Endovascularly. Neurosurgery 2022, 90, 627–635. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; la Rosa, C.C.-D.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Park, J.J.; Kim, B.J.; Youn, D.H.; Choi, H.J.; Jeon, J.P. A Preliminary Study of the Association between SOX17 Gene Variants and Intracranial Aneurysms Using Exome Sequencing. J. Korean Neurosurg. Soc. 2020, 63, 539–549. [Google Scholar] [CrossRef]
- Deora, H.; Nayak, N.; Dixit, P.; Vikas, V.; Rao, K.V.L.N.; Pruthi, N.; Srinivas, D.; Shukla, D.P.; Bhat, D.I.; Malla, B.R.; et al. Surgical Management and Outcomes of Aneurysms of Posterior Inferior Cerebellar Artery: Location-Based Approaches with Review of Literature. J. Neurosci. Rural Pract. 2020, 11, 34–43. [Google Scholar] [CrossRef]
- Pilipenko, Y.; Eliava, S.; Okishev, D.; Okisheva, E.; Spyrou, A. Vertebral artery and posterior inferior cerebellar artery aneurysms: Results of microsurgical treatment of eighty patients. Surg. Neurol. Int. 2019, 10, 227. [Google Scholar] [CrossRef]
- Kwon, B.; Song, Y.; Choi, Y.H.; Suh, D.C. Physiologic Flow Diversion Coiling Technique for Wide-Necked Aneurysms with an Asymmetric Bidirectional Flow at the Aneurysm Neck. Neurointervention 2022, 17, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Enriquez Marulanda, A.; Young, M.; Shutran, M.; Taussky, P.; Kicielinski, K.; Ogilvy, C.S. Acute Coiling with Delayed Flow Diversion for Posterior Communicating Segment Internal Carotid Artery Aneurysms: A Multicenter Case Series. Neurosurgery 2024, 94, 729–735. [Google Scholar] [CrossRef]
- Toader, C.; Serban, M.; Covache-Busuioc, R.-A.; Radoi, M.P.; Ciurea, A.V.; Dobrin, N. Comprehensive Management of a Giant Left Frontal AVM Coexisting with a Bilobed PComA Aneurysm: A Case Report Highlighting Multidisciplinary Strategies and Advanced Neurosurgical Techniques. J. Clin. Med. 2025, 14, 1232. [Google Scholar] [CrossRef] [PubMed]
- Civilla, L.; Dodier, P.; Palumbo, M.C.; Redaelli, A.C.L.; Koenigshofer, M.; Unger, E.; Meling, T.R.; Velinov, N.; Rössler, K.; Moscato, F. Development and assessment of case-specific physical and augmented reality simulators for intracranial aneurysm clipping. 3D Print. Med. 2024, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Rezaeitaleshmahalleh, M.; Tang, J.; Gemmette, J.; Pandey, A. Improving rupture status prediction for intracranial aneurysms using wall shear stress informatics. Acta Neurochir. 2025, 167, 15. [Google Scholar] [CrossRef]
- Sriamornrattanakul, K.; Akharathammachote, N.; Chonhenchob, A.; Mongkolratnan, A.; Niljianskul, N.; Phoominaonin, I.; Ariyaprakai, C. Far-lateral approach without C1 laminectomy for microsurgical treatment of vertebral artery and proximal posterior inferior cerebellar artery aneurysms: Experience from 48 patients. World Neurosurg. X 2023, 19, 100216. [Google Scholar] [CrossRef]
- Toader, C.; Eva, L.; Bratu, B.-G.; Covache-Busuioc, R.-A.; Costin, H.P.; Dumitrascu, D.-I.; Glavan, L.-A.; Corlatescu, A.D.; Ciurea, A.V. Intracranial Aneurysms and Genetics: An Extensive Overview of Genomic Variations, Underlying Molecular Dynamics, Inflammatory Indicators, and Forward-Looking Insights. Brain Sci. 2023, 13, 1454. [Google Scholar] [CrossRef]
- Giotta Lucifero, A.; Baldoncini, M.; Bruno, N.; Galzio, R.; Hernesniemi, J.; Luzzi, S. Shedding the Light on the Natural History of Intracranial Aneurysms: An Updated Overview. Medicina 2021, 57, 742. [Google Scholar] [CrossRef]
- Toader, C.; Tataru, C.P.; Munteanu, O.; Covache-Busuioc, R.-A.; Serban, M.; Ciurea, A.V.; Enyedi, M. Revolutionizing Neuroimmunology: Unraveling Immune Dynamics and Therapeutic Innovations in CNS Disorders. Int. J. Mol. Sci. 2024, 25, 13614. [Google Scholar] [CrossRef]
- Zou, X.; Liu, T.; Huang, Z.; Zhou, W.; Yuan, M.; Zhao, H.; Pan, Z.; Chen, P.; Shao, Y.; Hu, X.; et al. SOX17 is a Critical Factor in Maintaining Endothelial Function in Pulmonary Hypertension by an Exosome-Mediated Autocrine Manner. Adv. Sci. 2023, 10, 2206139. [Google Scholar] [CrossRef]
- Golombek, S.; Doll, I.; Kaufmann, L.; Lescan, M.; Schlensak, C.; Avci-Adali, M. A Novel Strategy for the Treatment of Aneurysms: Inhibition of MMP-9 Activity through the Delivery of TIMP-1 Encoding Synthetic mRNA into Arteries. Int. J. Mol. Sci. 2024, 25, 6599. [Google Scholar] [CrossRef]
- Akhtar, M.; Farooqi, H.A.; Nabi, R.; Hasan, H. Advancing aneurysm management: The potential of AI and machine learning in enhancing safety and predictive accuracy. Neurosurg. Rev. 2024, 47, 432. [Google Scholar] [CrossRef]
- Rugină, A.I.; Ungureanu, A.; Giuglea, C.; Marinescu, S.A. Artificial Intelligence in Breast Reconstruction: A Narrative Review. Medicina 2025, 61, 440. [Google Scholar] [CrossRef]
- Conte, N.; Gonçalves, T.T.; Louis, C.; Ikikame, J.; Góes Junior, A.M. de O. Surgical access to the distal cervical segment of the internal carotid artery and to a high carotid bifurcation—Integrative literature review and protocol proposal. J. Vasc. Bras. 2022, 21, e20210193. [Google Scholar] [CrossRef] [PubMed]
- de Macêdo, L.P.; Baptista-André, D.C.; Ugulino-Netto, A.; Franke, K.; Oliveira Eugênio, P.V.; Cezar-Junior, A.B.; Faquini, I.V.; de Carvalho-Júnior, E.V.; Almeida, N.S.; Azevedo-Filho, H.R.C. Management of a ruptured posterior inferior cerebellar artery (PICA) aneurysm with end-to-end in situ bypass: Case report. J. Cerebrovasc. Endovasc. Neurosurg. 2024, 26, 216–222. [Google Scholar] [CrossRef]
- Rennert, R.C.; Nguyen, V.N.; Abedi, A.; Atai, N.A.; Carey, J.N.; Tenser, M.; Amar, A.; Mack, W.J.; Russin, J.J. Combined open revascularization and endovascular treatment of complex intracranial aneurysms: Case series. Front. Neurol. 2023, 14, 1102496. [Google Scholar] [CrossRef] [PubMed]
- Kando, Y.; Shiiya, N.; Tsuda, K.; Washiyama, N.; Takahashi, D.; Yamashita, K. Radial artery vs saphenous vein grafts for sequential coronary bypass grafting as a second conduit for the left coronary territory. Gen. Thorac. Cardiovasc. Surg. 2022, 70, 862–870. [Google Scholar] [CrossRef]
- Andersen, F.T.; Petersen, J.; Kaspersen, A.E.; Pedersen, M.V.; Singer, G.; Madsen, M.H.A.; Lund, M.A.V.; Hasenkam, J.M.; Lading, T. Continuous assessment of tissue perfusion using quantitative indocyanine green fluorescence imaging during controlled hypo- and reperfusion. Surg. Endosc. 2025, 39, 5462–5477. [Google Scholar] [CrossRef] [PubMed]
- Peul, R.C.; Kharbanda, R.K.; Koning, S.; Kruiswijk, M.W.; Tange, F.P.; van den Hoven, P.; Vahrmeijer, A.L.; Klautz, R.J.M.; Hamming, J.F.; Hjortnaes, J.; et al. Intraoperative assessment of myocardial perfusion using near-infrared fluorescence and indocyanine green: A literature review. JTCVS Tech. 2025, 30, 81–93. [Google Scholar] [CrossRef]
- de Paule Adjiou, D.K.F.; Abbas, S.; Benali, O.; Alhassan, B.A.B.; El Manouni, O.; Kajeou, M.; El Ouahabi, A. PICA flow-related aneurysms and posterior fossa AVM: Rare association and challenging management: Case presentation and review of literature. Egypt. J. Neurosurg. 2024, 39, 13. [Google Scholar] [CrossRef]



| Category | Key Findings | Scientific Implications | Advantages | Limitations and Risks | References |
|---|---|---|---|---|---|
| Global Epidemiology and Regional Treatment Trends | PICA aneurysms account for 0.5–3% of all intracranial aneurysms. Higher prevalence in Asian populations, possibly due to genetic predisposition and increased screening. Microsurgical clipping remains more common in Asia, while endovascular coiling is preferred in North America and Europe. | Regional treatment disparities highlight the need for individualized approaches rather than a universal treatment paradigm. Genetic predispositions may play a role in regional aneurysm formation patterns. | Clipping provides definitive occlusion with lower recurrence rates. Coiling is minimally invasive with faster recovery. | High recurrence rates for coiling (30–40%), need for long-term follow-up, and risk of cranial nerve deficits post-clipping. | [32,33] |
| Hemodynamic and Biomechanical Risk Factors | PICA aneurysms develop in low-flow, high-shear stress regions, making them prone to rupture at smaller sizes. Flow stagnation, turbulent hemodynamics, and irregular wall shear stress increase rupture risk. Vessel wall MRI studies confirm higher prevalence of intra-aneurysmal thrombus and endothelial dysfunction in PICA aneurysms. | Small PICA aneurysms should not be observed conservatively based on size alone. Flow analysis using 4D-flow MRI and CFD modeling is increasingly used for risk assessment. | Advanced imaging (CFD, 4D-flow MRI) allows early rupture prediction. | High variability in patient-specific hemodynamics, difficult to establish universal treatment thresholds. | [34,35] |
| Microsurgical Clipping vs. Endovascular Coiling | Clipping achieves complete occlusion in 90–95% of cases with low recurrence. Coiling has a 30–40% recurrence rate, with 20–25% requiring retreatment. Stent-assisted coiling remains challenging due to small PICA caliber and high perforator risk. Flow diversion remains experimental for PICA aneurysms. | Microsurgical clipping remains the gold standard for broad-necked and deep-seated PICA aneurysms. Endovascular therapy is preferred for morphologically favorable aneurysms. Hybrid approaches (coiling before clipping) are being investigated. | Clipping is definitive with low recurrence. Coiling is less invasive, shorter recovery. | Clipping requires skilled microsurgical expertise, risks cranial nerve injury. Coiling has higher recurrence rates. | [36,37] |
| Genetic and Molecular Pathophysiology | SOX17, ELN, COL1A2, LOX mutations linked to posterior circulation aneurysms. Increased expression of MMP-9 and IL-6 correlates with aneurysm instability. Epigenetic modifications, such as TIMP3 hypermethylation, reduce vessel wall integrity. | Genetic screening may allow for early identification of high-risk individuals. Molecular-targeted therapies (MMP inhibitors, endothelial stabilizers) are in development. | Could enable personalized treatment strategies and early intervention. | Gene–environment interactions remain poorly understood. Targeted pharmacotherapy is not yet validated. | [38,39] |
| Post-operative and Long-Term Outcomes | Hydrocephalus occurs in 10–15% of ruptured PICA aneurysms, often requiring CSF diversion. Cranial nerve dysfunction (CN IX, X) occurs in 20–30% of cases post-clipping, but most resolve with rehabilitation. Microsurgical clipping offers better long-term occlusion than coiling. | Structured post-operative monitoring and early hydrocephalus detection are critical. Functional recovery is improved with early speech and balance rehabilitation. | Clipping provides lower recurrence, higher durability. | Post-operative cranial nerve deficits, risk of dysphagia and aspiration. | [40,41] |
| Hemodynamic Evolution and Flow Dynamics Post-Treatment | Coiling achieves immediate flow disruption but incomplete packing can lead to delayed aneurysm recanalization. Microsurgical clipping results in immediate, complete exclusion, eliminating flow-related aneurysm growth. Post-treatment flow alterations can induce unpredictable cerebrovascular resistance changes, requiring long-term follow-up. | Flow quantification using 4D-phase contrast MRI may help predict long-term hemodynamic changes post-treatment. | Allows real-time assessment of aneurysm recurrence. | High cost of imaging, unclear threshold for re-intervention. | [42,43] |
| Future Directions and Technological Innovations | AI-based aneurysm rupture prediction models integrating aneurysm morphology, wall shear stress, and genetic markers are under development. Augmented reality-assisted microsurgical navigation is being explored for improved clip positioning and perforator preservation. Miniaturized flow-diverting stents for small-caliber arteries remain experimental due to high thrombosis risk. | The future of PICA aneurysm management will involve hybrid surgical–endovascular strategies, real-time intraoperative imaging, and AI-driven risk stratification. | AI-driven analytics could allow for personalized treatment based on risk factors. | High costs, need for multi-left validation before widespread adoption. | [44,45,46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Șerban, M.; Toader, C.; Covache-Busuioc, R.-A. Ruptured Posterior Inferior Cerebellar Artery Aneurysms: Integrating Microsurgical Expertise, Endovascular Challenges, and AI-Driven Risk Assessment. J. Clin. Med. 2025, 14, 5374. https://doi.org/10.3390/jcm14155374
Șerban M, Toader C, Covache-Busuioc R-A. Ruptured Posterior Inferior Cerebellar Artery Aneurysms: Integrating Microsurgical Expertise, Endovascular Challenges, and AI-Driven Risk Assessment. Journal of Clinical Medicine. 2025; 14(15):5374. https://doi.org/10.3390/jcm14155374
Chicago/Turabian StyleȘerban, Matei, Corneliu Toader, and Răzvan-Adrian Covache-Busuioc. 2025. "Ruptured Posterior Inferior Cerebellar Artery Aneurysms: Integrating Microsurgical Expertise, Endovascular Challenges, and AI-Driven Risk Assessment" Journal of Clinical Medicine 14, no. 15: 5374. https://doi.org/10.3390/jcm14155374
APA StyleȘerban, M., Toader, C., & Covache-Busuioc, R.-A. (2025). Ruptured Posterior Inferior Cerebellar Artery Aneurysms: Integrating Microsurgical Expertise, Endovascular Challenges, and AI-Driven Risk Assessment. Journal of Clinical Medicine, 14(15), 5374. https://doi.org/10.3390/jcm14155374





