Safety and Efficacy of Stereotactic Aspiration with Fibrinolysis for Supratentorial Spontaneous Intracerebral Hemorrhages: A Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
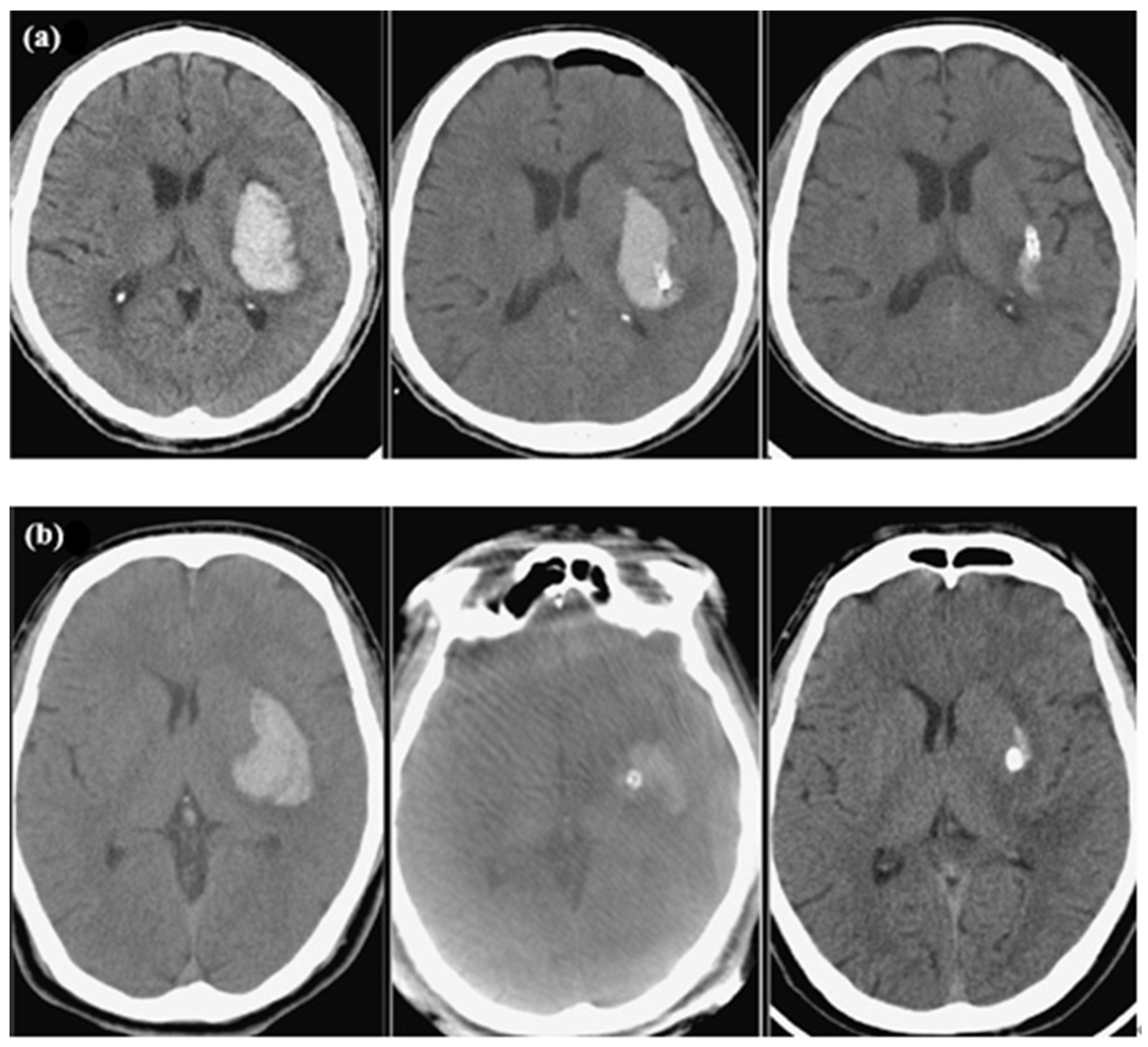
2.2. Measurements of Radiological Parameters
2.3. Surgical Procedure
2.4. Patient Care
2.5. Data Collection
2.6. Clinical Outcome Assessment
2.7. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICH | Intracerebral hemorrhage |
| MIS | Minimally invasive surgery |
| CT | Computed tomography |
| IVH | Intraventricular hemorrhage |
| mRS | Modified Rankin Scale |
| GCS | Glasgow Coma Scale |
| HTN | Hypertension |
| DM | Diabetes mellitus |
References
- Cho, D.Y.; Chen, C.C.; Lee, H.C.; Lee, W.Y.; Lin, H.L. Glasgow Coma Scale and hematoma volume as criteria for treatment of putaminal and thalamic intracerebral hemorrhage. Surg. Neurol. 2008, 70, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.; Hepburn, M.; Ziu, E.; Siddiq, F.; Qureshi, A.I. Modern Approaches to Evacuating Intracerebral Hemorrhage. Curr. Cardiol. Rep. 2018, 20, 132. [Google Scholar] [CrossRef] [PubMed]
- Sheth, K.N. Spontaneous Intracerebral Hemorrhage. N. Engl. J. Med. 2022, 387, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Zhang, M.; Li, W. Meta-analysis of stereotactic hematoma removal and craniotomy hematoma removal in the treatment of hypertensive intracerebral hemorrhage in the elderly. Medicine 2023, 102, e36533. [Google Scholar] [CrossRef]
- Chen, X.; Chen, W.; Ma, A.; Wu, X.; Zheng, J.; Yu, X.; Wang, Y.-X.J.; Wang, D. Frameless stereotactic aspiration and subsequent fibrinolytic therapy for the treatment of spontaneous intracerebral haemorrhage. Br. J. Neurosurg. 2011, 25, 369–375. [Google Scholar] [CrossRef]
- Thiex, R.; Rohde, V.; Rohde, I.; Mayfrank, L.; Zeki, Z.; Thron, A.; Gilsbach, J.M.; Uhl, E. Frame-based and frameless stereotactic hematoma puncture and subsequent fibrinolytic therapy for the treatment of spontaneous intracerebral hemorrhage. J. Neurol. 2004, 251, 1443–1450. [Google Scholar] [CrossRef]
- Bernotas, G.; Simaitis, K.; Bunevičius, A.; Tamašauskas, A. Safety and efficacy of stereotactic aspiration with fibrinolysis for deep-seated spontaneous intracerebral hemorrhages: A single-center experience. Medicina 2017, 53, 303–309. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Q.; Zhu, W.; Yin, Q.; Ma, M.; Fan, X.; Li, Y.; Ni, G.; Liu, C.; Liu, W.; et al. Stereotactic aspiration plus subsequent thrombolysis for moderate thalamic hemorrhage. World Neurosurg. 2012, 77, 122–129. [Google Scholar] [CrossRef]
- Vespa, P.; McArthur, D.; Miller, C.; O’Phelan, K.; Frazee, J.; Kidwell, C.; Saver, J.; Starkman, S.; Martin, N. Frameless stereotactic aspiration and thrombolysis of deep intracerebral hemorrhage is associated with reduction of hemorrhage volume and neurological improvement. Neurocrit. Care 2005, 2, 274–281. [Google Scholar] [CrossRef]
- Xiao, K.; Chu, H.; Chen, H.; Zhong, Y.; Zhong, L.; Tang, Y. Optimal time window for minimally invasive surgery in treating spontaneous intracerebral hemorrhage in the basal ganglia region: A multicenter and retrospective study. Br. J. Neurosurg. 2023, 37, 1061–1065. [Google Scholar] [CrossRef]
- Sirh, S.; Park, H.R. Optimal Surgical Timing of Aspiration for Spontaneous Supratentorial Intracerebral Hemorrhage. J. Cerebrovasc. Endovasc. Neurosurg. 2018, 20, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wang, J.; Chen, L.; Yan, W.; Gao, S.; Liu, Y.; Wang, X.; Dong, X.; Zhang, J.; Chen, S.; et al. Functional Outcome Analysis of Stereotactic Catheter Aspiration for Spontaneous Intracerebral Hemorrhage: Early or Late Hematoma Evacuation? J. Clin. Med. 2023, 12, 1533. [Google Scholar] [CrossRef]
- Kumar, S.; Madhariya, S.N.; Singh, D.; Agrawal, R.; Sahana, D.; Mourya, A. Comparison of Craniotomy and Stereotactic Aspiration Plus Thrombolysis in Isolated Capsulo-Ganglionic Hematoma: A Retrospective Analyses. Neurol. India 2022, 70, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, H.; Zhang, J.; Luo, M.; Wang, Q.; Zhao, Y.; Gan, Z.; Xu, B.; Chen, X.; The MISICH Study Team. Minimally invasive surgeries for spontaneous hypertensive intracerebral hemorrhage (MISICH): A multicenter randomized controlled trial. BMC Med. 2024, 22, 244. [Google Scholar] [CrossRef] [PubMed]
- Hannah, T.C.; Kellner, R.; Kellner, C.P. Minimally Invasive Intracerebral Hemorrhage Evacuation Techniques: A Review. Diagnostics 2021, 11, 576. [Google Scholar] [CrossRef]
- Montes, J.M.; Wong, J.H.; Fayad, P.B.; Awad, I.A. Stereotactic computed tomographic-guided aspiration and thrombolysis of intracerebral hematoma: Protocol and preliminary experience. Stroke 2000, 31, 834–840. [Google Scholar] [CrossRef]
- Hieber, M.; Lambeck, J.; Halaby, A.; Roelz, R.; Demerath, T.; Niesen, W.-D.; Bardutzky, J. Minimally-invasive bedside catheter haematoma aspiration followed by local thrombolysis in spontaneous supratentorial intracerebral haemorrhage: A retrospective single-center study. Front. Neurol. 2023, 14, 1188717. [Google Scholar] [CrossRef]
- Iii, D.D.; Halabi, C.; DiNitto, J.; Mueller, K.; Fiorella, D.; Cooke, D.L.; Arthur, A.S. How to iGuide: Flat panel detector, CT-assisted, minimally invasive evacuation of intracranial hematomas. J. Neurointerv. Surg. 2022, 14, 522–526. [Google Scholar] [CrossRef]
- Akhigbe, T.; Okafor, U.; Sattar, T.; Rawluk, D.; Fahey, T. Stereotactic-Guided Evacuation of Spontaneous Supratentorial Intracerebral Hemorrhage: Systematic Review and Meta-Analysis. World Neurosurg. 2015, 84, 451–460. [Google Scholar] [CrossRef]
- Xu, F.; Tang, Z.; Luo, X.; Kang, H.; Hu, Q.; Wei; Zhu, S. No evidence of preoperative hematoma growth representing an increased postoperative rebleeding risk for minimally invasive aspiration and thrombolysis of ICH. Br. J. Neurosurg. 2010, 24, 268–274. [Google Scholar] [CrossRef]
- Scaggiante, J.; Zhang, X.; Mocco, J.; Kellner, C.P. Minimally Invasive Surgery for Intracerebral Hemorrhage. Stroke 2018, 49, 2612–2620. [Google Scholar] [CrossRef] [PubMed]
- Pradilla, G.; Ratcliff, J.J.; Hall, A.J.; Saville, B.R.; Allen, J.W.; Paulon, G.; McGlothlin, A.; Lewis, R.J.; Fitzgerald, M.; Caveney, A.F.; et al. Trial of Early Minimally Invasive Removal of Intracerebral Hemorrhage. N. Engl. J. Med. 2024, 390, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Polster, S.P.; Carrión-Penagos, J.; Lyne, S.B.; Gregson, B.A.; Cao, Y.; Thompson, R.E.; Stadnik, A.; Girard, R.; Money, P.L.; Lane, K.; et al. Intracerebral Hemorrhage Volume Reduction and Timing of Intervention Versus Functional Benefit and Survival in the MISTIE III and STICH Trials. Neurosurgery 2021, 88, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Awad, I.A.; Polster, S.P.; Carrión-Penagos, J.; Thompson, R.E.; Cao, Y.; Stadnik, A.; Money, P.L.; Fam, M.D.; Koskimäki, J.; Girard, R.; et al. Surgical Performance Determines Functional Outcome Benefit in the Minimally Invasive Surgery Plus Recombinant Tissue Plasminogen Activator for Intracerebral Hemorrhage Evacuation (MISTIE) Procedure. Neurosurgery 2019, 84, 1157–1168. [Google Scholar] [CrossRef]
- Al-Mufti, F.; Thabet, A.M.; Singh, T.; El-Ghanem, M.; Amuluru, K.; Gandhi, C.D. Clinical and Radiographic Predictors of Intracerebral Hemorrhage Outcome. Interv. Neurol. 2018, 7, 118–136. [Google Scholar] [CrossRef]
- Bhattathiri, P.S.; Gregson, B.; Prasad, K.S.; Mendelow, A.D.; Investigators, S. Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: Results from the STICH trial. Acta Neurochir. Suppl. 2006, 96, 65–68. [Google Scholar] [CrossRef]
- Hallevi, H.; Albright, K.C.; Aronowski, J.; Barreto, A.D.; Martin-Schild, S.; Khaja, A.M.; Gonzales, N.R.; Illoh, K.; Noser, E.A.; Grotta, J.C. Intraventricular hemorrhage: Anatomic relationships and clinical implications. Neurology 2008, 70, 848–852. [Google Scholar] [CrossRef]
- Hanley, D.F.; Thompson, R.E.; Rosenblum, M.; Yenokyan, G.; Lane, K.; McBee, N.; Mayo, S.W.; Bistran-Hall, A.J.; Gandhi, D.; Mould, W.A.; et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): A randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet 2019, 393, 1021–1032. [Google Scholar] [CrossRef]
| Total (n = 123) | |
|---|---|
| Age (years), mean ± SD | 53.8 ± 15.6 |
| Gender, n (%) | |
| Female | 37 (30.1%) |
| Male | 86 (69.9%) |
| Preoperative GCS score, mean ± SD | 11.3 ± 2.8 |
| Preoperative GCS score, n (%) | |
| GCS score 13–15 | 46 (37.4%) |
| GCS score 9–12 | 54 (43.9%) |
| GCS score 3–8 | 23 (18.7%) |
| Hypertension, n (%) | 84 (68.3%) |
| Diabetes, n (%) | 20 (16.3%) |
| Location, n (%) | |
| Basal ganglia | 103 (83.7%) |
| Thalamus | 12 (9.8%) |
| Lobar | 8 (6.5%) |
| Side, n (%) | |
| Right | 53 (43.1%) |
| Left | 70 (56.9%) |
| IVH, n (%) | 39 (31.7%) |
| Midline shift, n (%) | 80 (65%) |
| VP shunt, n (%) | 11 (8.9%) |
| Preoperative volume (mL), mean ± SD | 33.1 ± 17.9 |
| Preoperative volume (mL), n (%) | |
| <30 mL | 61 (49.6%) |
| 30–50 mL | 42 (34.1%) |
| >50 mL | 20 (16.3%) |
| Residual clot volume (mL), mean ± SD | 12.9 ± 13.5 |
| Residual ≤ 15 mL, n (%) | 81 (65.9%) |
| Residual percentage (%), mean ± SD | 36.7 ± 24 |
| Total dose of urokinase, mean ± SD | 5.5 ± 3 |
| Sepsis, n (%) | 11 (8.9%) |
| Discharge GCS score, mean ± SD | 13.3 ± 2.1 |
| Discharge GCS score, n (%) | |
| GCS score 13–15 | 86 (71.7%) |
| GCS score 9–12 | 28 (23.3%) |
| GCS score 3–8 | 6 (5%) |
| Time to evacuation (hrs), mean ± SD | 25.8 ± 43.8 |
| Time to evacuation (hrs), n (%) | |
| ≤6 h | 28 (22.8%) |
| 6–12 h | 43 (34.9%) |
| 12–24 h | 24 (19.5%) |
| >24 h | 28 (22.8%) |
| Evacuation percentage (%), mean ± SD | 63.3 ± 24 |
| Evacuation percentage (%), n (%) | |
| >75% reduction | 41 (33.3%) |
| 51–75% reduction | 48 (39%) |
| 26–50% reduction | 24 (19.5%) |
| ≤25% reduction | 10 (8.2%) |
| Discharge mRS, mean ± SD | 4.4 ± 1.1 |
| Discharge mRS, n (%) | |
| mRS 1–3 | 22 (17.9%) |
| mRS 4–6 | 101 (82.1%) |
| ICH score, mean ± SD | 1.5 ± 1.1 |
| ICH score, n (%) | |
| ICH score 0–2 | 99 (80.5%) |
| ICH score 3–4 | 24 (19.5%) |
| Postoperative rebleeding, n (%) | 1 (0.8%) |
| Mortality, n (%) | 3 (2.4%) |
| Follow-up time (days), mean ± SD | 25.5 ± 11.6 |
| Discharge mRS | |||
|---|---|---|---|
| Variable | mRS 1–3 (n = 22) | mRS 4–6 (n = 101) | p Value |
| Age (years), mean ± SD | 48.3 ± 15.4 | 55 ± 15.4 | 0.061 |
| Gender, n (%) | 0.845 | ||
| Female | 7 (31.8%) | 30 (29.7%) | |
| Male | 15 (68.2%) | 71 (70.3%) | |
| Preoperative GCS score, mean ± SD | 13.2 ± 2 | 10.9 ± 2.8 | <0.001 ** |
| Preoperative GCS score, n (%) | <0.001 ** | ||
| GCS score 13–15 | 16 (72.7%) | 30 (29.7%) | |
| GCS score 9–12 | 6 (27.3%) | 48 (47.5%) | |
| GCS score 3–8 | 0 (0%) | 23 (22.8%) | |
| Hypertension, n (%) | 17 (77.3%) | 67 (66.3%) | 0.318 |
| Diabetes, n (%) | 2 (9.1%) | 18 (17.8%) | 0.524 |
| Location, n (%) | 0.082 | ||
| Basal ganglia | 19 (86.4%) | 84 (83.1%) | |
| Thalamus | 0 (0%) | 12 (11.9%) | |
| Lobar | 3 (13.6%) | 5 (5%) | |
| Side, n (%) | 0.482 | ||
| Right | 8 (36.4%) | 45 (44.6%) | |
| Left | 14 (63.6%) | 56 (55.4%) | |
| IVH, n (%) | 1 (4.5%) | 38 (37.6%) | 0.003 ** |
| Midline shift, n (%) | 7 (31.8%) | 73 (72.3%) | <0.001 ** |
| VP shunt, n (%) | 0 (0%) | 11 (10.9%) | 0.211 |
| Preoperative volume (mL), mean ± SD | 21 ± 11.6 | 35.8 ± 18 | <0.001 ** |
| Preoperative volume (mL), n (%) | 0.015 * | ||
| <30 mL | 17 (77.3%) | 44 (43.6%) | |
| 30–50 mL | 4 (18.2%) | 38 (37.6%) | |
| >50 mL | 1 (4.5%) | 19 (18.8%) | |
| Residual clot volume (mL), mean ± SD | 6.3 ± 6.4 | 14.4 ± 14.2 | 0.001 ** |
| Residual ≤ 15 mL, n (%) | 20 (90.9%) | 61 (60.4%) | 0.006 ** |
| Residual percentage (%), mean ± SD | 30.3 ± 27.8 | 38.1 ± 23 | 0.075 |
| Total dose of urokinase, mean ± SD | 4.9 ± 2.3 | 5.7 ± 3.1 | 0.347 |
| Sepsis, n (%) | 1 (4.5%) | 10 (9.9%) | 0.687 |
| Discharge GCS score, mean ± SD | 14.9 ± 0.4 | 13 ± 2.2 | <0.001 ** |
| Discharge GCS score, n (%) | 0.003 ** | ||
| GCS score 13–15 | 22 (100%) | 64 (65.3%) | |
| GCS score 9–12 | 0 (0%) | 28 (28.6%) | |
| GCS score 3–8 | 0 (0%) | 6 (6.1%) | |
| Time to evacuation (hrs), mean ± SD | 46.5 ± 62.9 | 21.2 ± 37.4 | 0.050 |
| Time to evacuation (hrs), n (%) | 0.009 ** | ||
| ≤48 h | 15 (68.2%) | 92 (91.1%) | |
| >48 h | 7 (31.8%) | 9 (8.9%) | |
| Evacuation percentage (%), mean ± SD | 69.7 ± 27.8 | 61.9 ± 23 | 0.075 |
| ICH score, mean ± SD | 0.5 ± 0.8 | 1.8 ± 1.1 | <0.001 ** |
| ICH score, n (%) | 0.072 | ||
| ICH score 0–2 | 21 (95.5%) | 78 (77.2%) | |
| ICH score 3–4 | 1 (4.5%) | 23 (22.8%) | |
| Postoperative rebleeding, n (%) | 0 (0%) | 1 (1%) | 1.000 |
| Mortality, n (%) | 0 (0%) | 3 (3%) | 1.000 |
| Follow-up time (days), mean ± SD | 17.7 ± 8.3 | 27.2 ± 11.6 | <0.001 ** |
| (a) | |||
| Components of Regression Model | Odds Ratio | (95% CI) | p Value |
| Age | 1.03 | (1.00–1.07) | 0.070 |
| Gender | |||
| Female | Reference | ||
| Male | 1.10 | (0.41–2.98) | 0.845 |
| Preoperative GCS score | 0.67 | (0.53–0.85) | 0.001 ** |
| Hypertension | 0.58 | (0.20–1.71) | 0.322 |
| Diabetes | 2.17 | (0.46–10.12) | 0.325 |
| Side | |||
| Right | Reference | ||
| Left | 0.71 | (0.27–1.84) | 0.483 |
| IVH | 12.67 | (1.64–98.01) | 0.015 * |
| Midline shift | 5.59 | (2.06–15.15) | 0.001 ** |
| Preoperative volume (mL) | 1.08 | (1.03–1.14) | 0.001 ** |
| <30 mL | Reference | ||
| 30–50 mL | 3.67 | (1.14–11.86) | 0.030 * |
| >50 mL | 7.34 | (0.91–59.19) | 0.061 |
| Residual clot volume (mL) | 1.12 | (1.04–1.21) | 0.004 ** |
| Residual ≤ 15 mL | 0.15 | (0.03–0.69) | 0.014 * |
| Residual percentage (%) | 1.02 | (0.99–1.04) | 0.171 |
| Total dose of urokinase | 1.09 | (0.93–1.28) | 0.284 |
| Sepsis | 2.31 | (0.28–19.03) | 0.437 |
| Discharge GCS score | 0.14 | (0.04–0.47) | 0.002 ** |
| Time to evacuation (h) | 0.99 | (0.98–1.00) | 0.029 * |
| Evacuation percentage (%) | 0.99 | (0.96–1.01) | 0.171 |
| ICH score | 3.83 | (2.01–7.31) | <0.001 ** |
| Follow-up time (days) | 1.12 | (1.05–1.19) | 0.001 ** |
| (b) | |||
| Discharge mRS | p Value | ||
| mRS 1–3 (n = 22) | mRS4–6 (n = 101) | ||
| Preoperative GCS score < 12, n (%) | 4 (18.2%) | 55 (54.5%) | 0.002 ** |
| Preoperative volume (mL) > 24, n (%) | 5 (22.7%) | 75 (74.3%) | <0.001 ** |
| Residual clot volume (mL) > 4, n (%) | 9 (40.9%) | 80 (79.2%) | <0.001 ** |
| Follow-up time (days) > 20, n (%) | 7 (31.8%) | 74 (73.3%) | <0.001 ** |
| mRS, modified Rankin Scale; GCS, Glasgow Coma Scale | |||
| AUC | (95% CI) | p value | |
| Preoperative GCS score | 0.75 | (0.65–0.85) | <0.001 ** |
| Preoperative volume (mL) | 0.78 | (0.67–0.89) | <0.001 ** |
| Residual clot volume (mL) | 0.73 | (0.62–0.85) | 0.001 ** |
| Follow-up time (days) | 0.75 | (0.64–0.87) | <0.001 ** |
| (a) | Time to Evacuation (h) | p Value | |||
| ≤6 h (n = 28) | 6–12 h (n = 43) | 12–24 h (n = 24) | >24 h (n = 28) | ||
| Preoperative GCS score, mean ± SD | 9.9 ± 2.6 | 11.2 ± 2.9 | 11.5 ± 2.7 | 12.8 ± 2.3 | 0.001 ** |
| Preoperative volume (mL), mean ± SD | 36.9 ± 15.5 | 36.5 ± 21 | 31.1 ± 15.5 | 25.8 ± 15.1 | 0.035 * |
| Residual clot volume (mL), mean ± SD | 13.5 ± 10.5 | 15.4 ± 18.3 | 13.3 ± 10.5 | 8.3 ± 7.8 | 0.073 |
| Total dose of urokinase, mean ± SD | 5.9 ± 2.7 | 5.3 ± 3.1 | 6 ± 3.3 | 5 ± 2.9 | 0.528 |
| Evacuation percentage (%), mean ± SD | 63.4 ± 22.1 | 62.7 ± 21.8 | 58.5 ± 25 | 68.5 ± 28 | 0.286 |
| Postoperative rebleeding, n (%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (3.6%) | 0.650 |
| (b) | Time to Evacuation (h) | p Value | |||
| ≤48 h (n = 107) | >48 h (n = 16) | ||||
| Preoperative GCS score, mean ± SD | 11.1 ± 2.8 | 12.8 ± 2.4 | 0.022 * | ||
| Preoperative volume (mL), mean ± SD | 34.8 ± 17.8 | 21.8 ± 14.8 | 0.003 ** | ||
| Residual clot volume (mL), mean ± SD | 14.3 ± 13.8 | 3.9 ± 4.6 | <0.001** | ||
| Evacuation percentage (%), mean ± SD | 60.6 ± 23.5 | 81.5 ± 19.1 | <0.001** | ||
| Evacuation Percentage, % | p Value | ||||
|---|---|---|---|---|---|
| >75% (n = 41) | 51–75% (n = 48) | 26–50% (n = 24) | ≤25% (n = 10) | ||
| Preoperative volume (mL), mean ± SD | 29.3 ± 16.3 | 34.7 ± 16 | 34.4 ± 17.6 | 38.1 ± 30.4 | 0.364 |
| Total dose of urokinase, mean ± SD | 5.1 ± 2.2 | 6.1 ± 2.8 | 5 ± 3.9 | 6.1 ± 3.6 | 0.274 |
| Time to evacuation (hrs), mean ± SD | 47.3 ± 68.6 | 13.4 ± 12.3 | 16.6 ± 18.6 | 18.7 ± 15.8 | 0.118 |
| Postoperative rebleeding, n (%) | 0 (0%) | 1 (2.1%) | 0 (0%) | 0 (0%) | 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-N.; Shen, C.-C.; Yang, M.-Y.; Cheng, W.-Y.; Lai, C.-M. Safety and Efficacy of Stereotactic Aspiration with Fibrinolysis for Supratentorial Spontaneous Intracerebral Hemorrhages: A Single-Center Experience. J. Clin. Med. 2025, 14, 3636. https://doi.org/10.3390/jcm14113636
Chang C-N, Shen C-C, Yang M-Y, Cheng W-Y, Lai C-M. Safety and Efficacy of Stereotactic Aspiration with Fibrinolysis for Supratentorial Spontaneous Intracerebral Hemorrhages: A Single-Center Experience. Journal of Clinical Medicine. 2025; 14(11):3636. https://doi.org/10.3390/jcm14113636
Chicago/Turabian StyleChang, Chia-Ning, Chiung-Chyi Shen, Meng-Yin Yang, Wen-Yu Cheng, and Chih-Ming Lai. 2025. "Safety and Efficacy of Stereotactic Aspiration with Fibrinolysis for Supratentorial Spontaneous Intracerebral Hemorrhages: A Single-Center Experience" Journal of Clinical Medicine 14, no. 11: 3636. https://doi.org/10.3390/jcm14113636
APA StyleChang, C.-N., Shen, C.-C., Yang, M.-Y., Cheng, W.-Y., & Lai, C.-M. (2025). Safety and Efficacy of Stereotactic Aspiration with Fibrinolysis for Supratentorial Spontaneous Intracerebral Hemorrhages: A Single-Center Experience. Journal of Clinical Medicine, 14(11), 3636. https://doi.org/10.3390/jcm14113636







