Kinetics of Procalcitonin, CRP, IL-6, and Presepsin in Heart Transplant Patients Undergoing Induction with Thymoglobulin (rATG)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
2.4. Inflammatory Biomarker Descriptions
- C-reactive protein (CRP): CRP is an acute-phase protein produced by the liver in response to systemic inflammation. Its levels increase in response to both infections and non-infectious inflammatory conditions, making it a useful but non-specific marker [23].
- Presepsin: Presepsin is a soluble form of CD14 released into the bloodstream during bacterial infections as part of the innate immune response. It has shown promise as a marker for sepsis, particularly in critically-ill patients, and is gaining recognition for its ability to distinguish between SIRS and sepsis [23,28,29,30,31,32,33,34,35].
- Interleukin-6 (IL-6): IL-6 is a cytokine involved in the regulation of immune and acute-phase inflammatory responses. Elevated levels of IL-6 have been associated with both infection and systemic inflammation; however, its kinetics may provide valuable information on the progression of the inflammatory response in HTx patients [36,37,38,39,40,41,42].
2.5. Statistical Analysis
3. Results
3.1. Preoperative Characteristics
3.2. Intraoperative Variables
3.3. Postoperative Outcomes
3.4. Lasso Regression
3.5. Logistic Regression Analysis (Alternative to LASSO)
- Presepsin (POD1): 2.32 → Strong association with infection.
- IL-6 (POD3): 1.02 → Moderate association with infection.
- CRP (POD2): −0.91 → Unexpected negative correlation.
- PCT (POD5): 0.06 → No significant correlation.
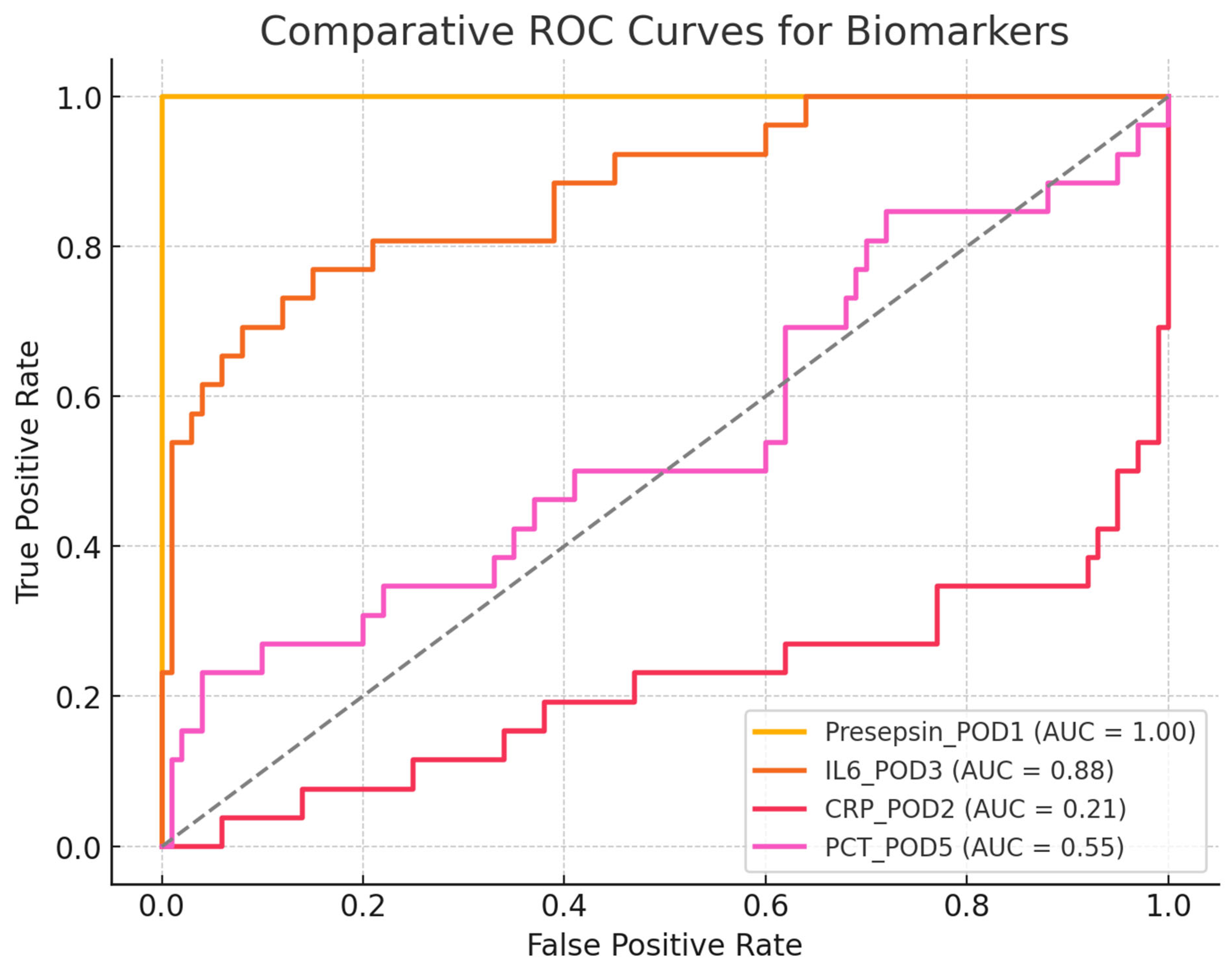
Comparative ROC-AUC Analysis
- Presepsin (POD1) = 1.00 → A perfect discriminator, though it requires validation in larger cohorts.
- IL-6 (POD3) = 0.89 → Good discriminative ability but less useful for early detection.
- CRP (POD2) = 0.58 → Poor discrimination, making it an unreliable marker for early infection.
- PCT (POD5) = 0.50 → No discriminative ability, performing no better than random chance.
3.6. Inflammatory Biomarker Kinetics
3.6.1. C-Reactive Protein (CRP)
3.6.2. Procalcitonin (PCT)
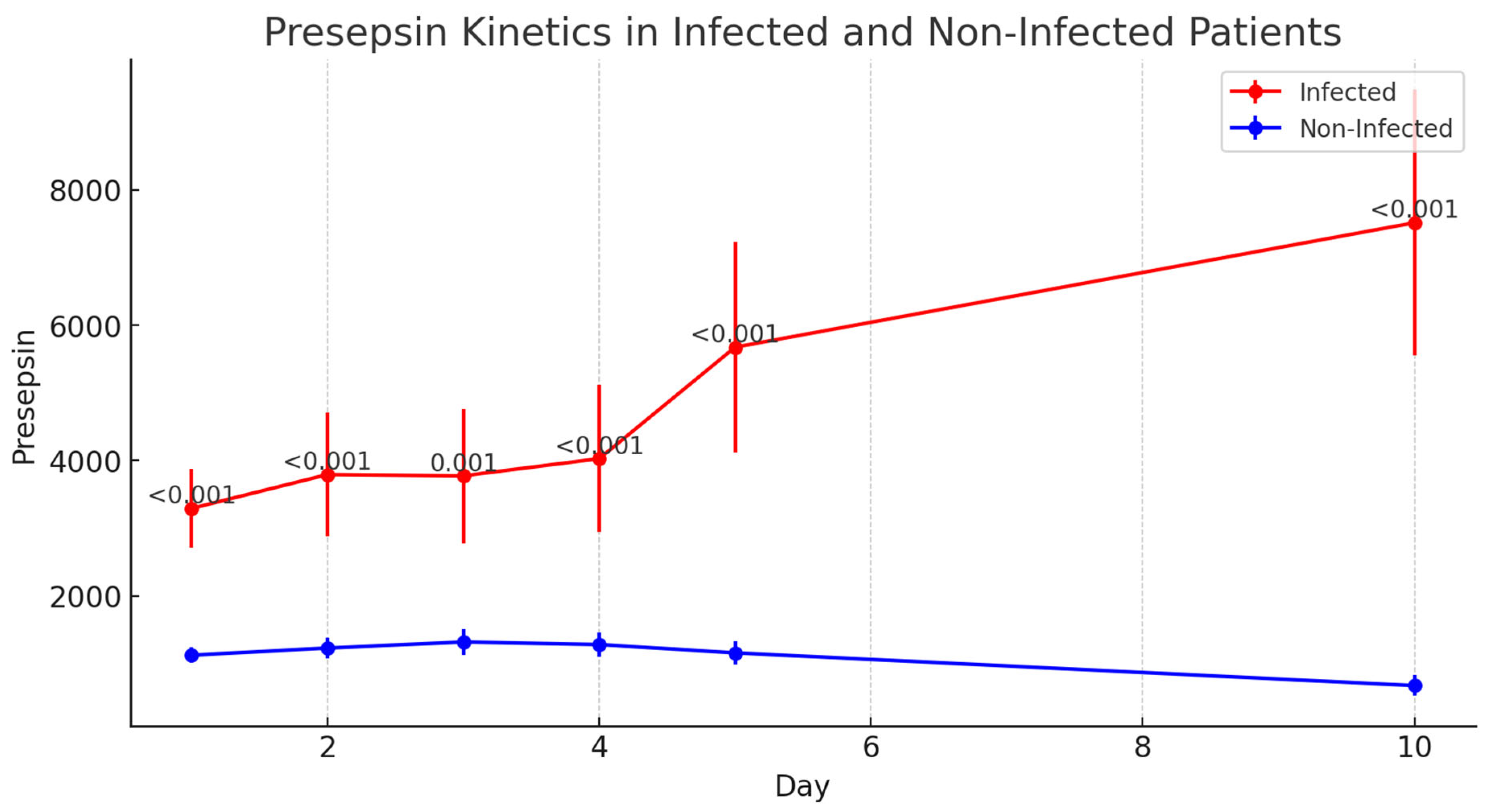
3.6.3. Presepsin
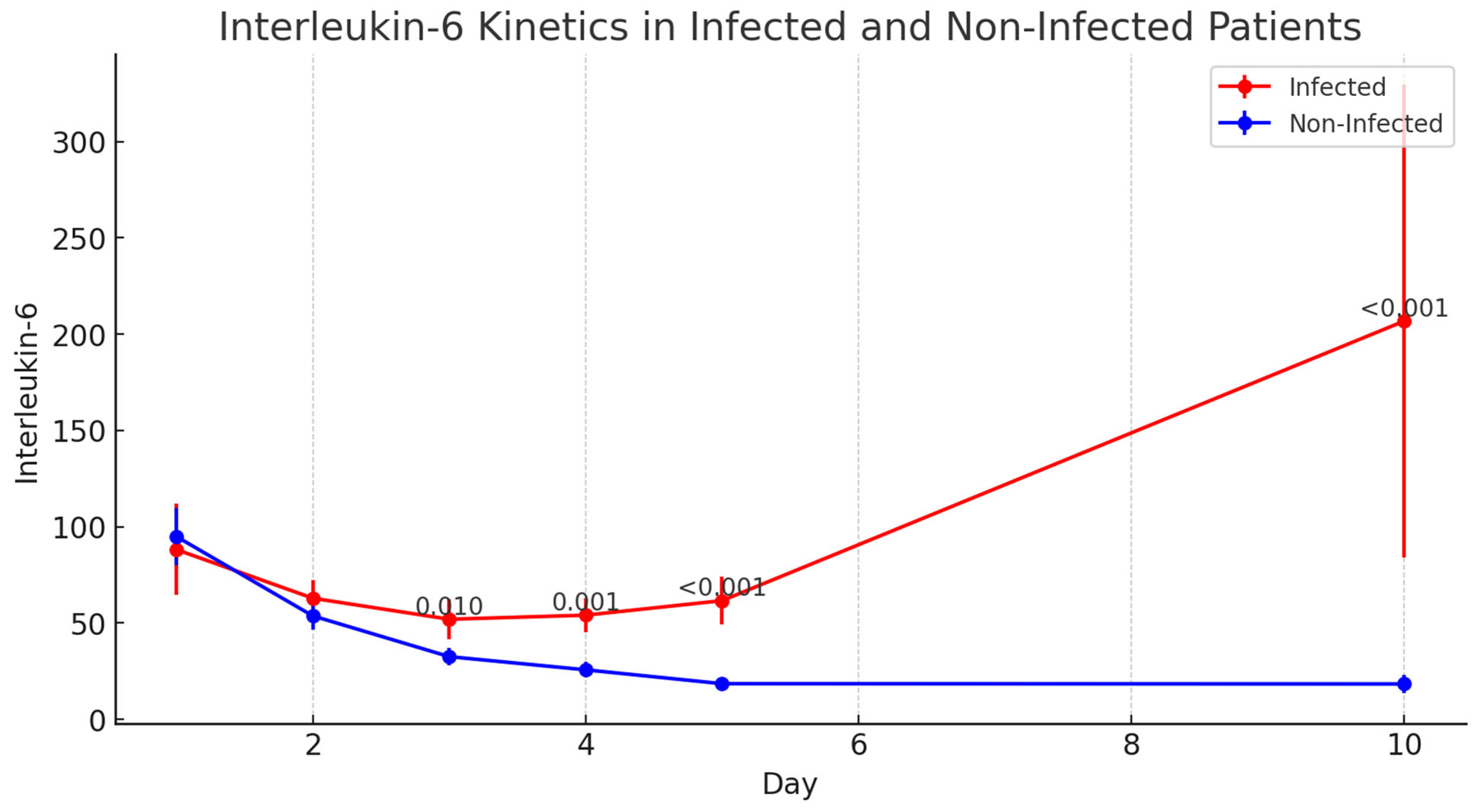
3.6.4. Interleukin-6 (IL-6)
- Presepsin: 1.00 (perfect discriminative ability);
- IL-6: 0.89 (excellent discriminative ability);
- CRP: 0.58 (low discriminative ability);
- PCT: 0.50 (no discriminative ability, equivalent to a random test);
- Chi-square test for AUC comparison:
- Chi-square = 0.90;
- p-value = 0.83.
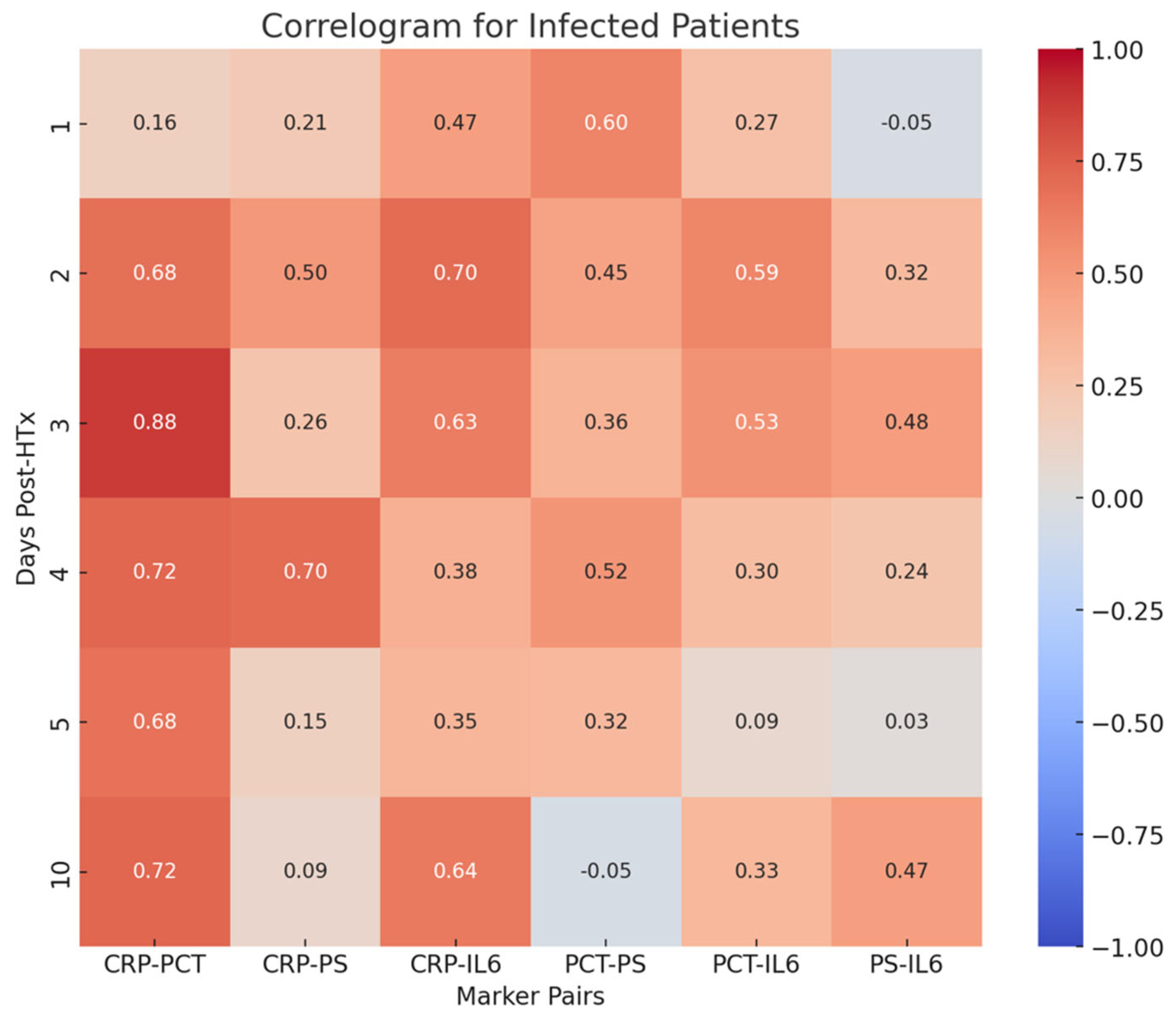
- PCT-CRP on the third, fourth, and tenth postoperative days (0.88, 0.72, 0.72, respectively);
- CRP-PS on the fourth postoperative day (0.70);
- CRP-IL-6 on the second postoperative day (0.70).
4. Discussion
4.1. Limitations of CRP and PCT
4.2. IL-6: A Reliable Indicator of Systemic Inflammation
5. Future Directions
6. Conclusions
7. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HTx | Heart Transplantation |
| CRP | C-reactive Protein |
| PCT | Procalcitonin |
| IL-6 | Interleukin-6 |
| SIRS | Systemic Inflammatory Response Syndrome |
| rATG | Rabbit Anti-Thymocyte Globulin |
| POD | Postoperative Day |
| ICU | Intensive Care Unit |
| ECMO | Extracorporeal Membrane Oxygenation |
| MCS | Mechanical Circulatory Support |
| IQR | Interquartile Range |
| OR | Odds Ratio |
| CI | Confidence Interval |
| ROC | Receiver Operating Characteristic |
| AUC | Area Under the Curve |
| HF | Heart Failure |
| BMI | Body Mass Index |
| SD | Standard Deviation |
| LASSO | Least Absolute Shrinkage and Selection Operator |
| DNA | Deoxyribonucleic Acid |
| RNA | Ribonucleic Acid |
References
- Peled, Y.; Ducharme, A.; Kittleson, M.; Bansal, N.; Stehlik, J.; Amdani, S.; Saeed, D.; Cheng, R.; Clarke, B.; Dobbels, F.; et al. International Society for Heart and Lung Transplantation Guidelines for the Evaluation and Care of Cardiac Transplant Candidates-2024. J. Heart Lung Transplant. 2024, 43, 1529–1628.e54. [Google Scholar] [CrossRef] [PubMed]
- Velleca, A.; A Shullo, M.; Dhital, K.; Azeka, E.; Colvin, M.; DePasquale, E.; Farrero, M.; García-Guereta, L.; Jamero, G.; Khush, K.; et al. The International Society for Heart and Lung Transplantation (ISHLT) guidelines for the care of heart transplant recipients. J. Heart Lung Transplant. 2023, 42, e1–e141. [Google Scholar] [CrossRef] [PubMed]
- Tatum, R.; Briasoulis, A.; Tchantchaleishvili, V.; Massey, H.T. Evaluation of donor heart for transplantation. Heart Fail. Rev. 2022, 27, 1819–1827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sathianathan, S.; Bhat, G. Heart Transplant Donor Selection Guidelines: Review and Recommendations. Curr. Cardiol. Rep. 2022, 24, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Giovannico, L.; Fischetti, G.; Parigino, D.; Savino, L.; Di Bari, N.; Milano, A.D.; Padalino, M.; Bottio, T. Veno-Arterial Extracorporeal Membrane Oxygenation (VA-ECMO) Support in New Era of Heart Transplant. Transpl. Int. 2024, 37, 12981. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bifulco, O.; Bottio, T.; Caraffa, R.; Carrozzini, M.; Guariento, A.; Bejko, J.; Fedrigo, M.; Castellani, C.; Toscano, G.; Lorenzoni, G.; et al. Marginal versus Standard Donors in Heart Transplantation: Proper Selection Means Heart Transplant Benefit. J. Clin. Med. 2022, 11, 2665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, M.Y.; Khawaja, R.D.A.; Vargas, D. Heart Transplantation: Indications, Surgical Techniques, and Complications. Radiol. Clin. N. Am. 2023, 61, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Gómez-López, R.; Barge-Caballero, E.; Fernández-Ugidos, P.; Paniagua-Martin, M.J.; Barge-Caballero, G.; Couto-Mallón, D.; Solla-Buceta, M.; de Sierra, C.V.-G.; Aller-Fernández, V.; Fernández-Arias, L.; et al. In-Hospital Post-Operative Infection after Heart Transplantation: Epidemiology, Clinical Management, and Outcome. Surg. Infect. 2020, 21, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Eichenberger, E.M.; Satola, S.; Gupta, D.; Daneshmand, M.; Pouch, S. Management and outcomes of heart transplant candidates with bloodstream infection on temporary mechanical circulatory support. J. Heart Lung Transplant. 2023, 42, 1501–1504. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Moreno, S.; Lage, E.; Jiménez-Jambrina, M.; Ordóñez, A.; Borrego, J.; Hernández, A.; Cayuela, A.; Cisneros, J. Infections in cardiac transplant recipients: Clinical and microbiological characteristics and consequences. Transplant. Proc. 2006, 38, 2555–2557. [Google Scholar] [CrossRef] [PubMed]
- Alsaeed, M.; Husain, S. Infections in Heart and Lung Transplant Recipients. Crit. Care Clin. 2019, 35, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.A. Infection in organ transplantation. Am. J. Transplant. 2017, 4, 856–879. [Google Scholar] [CrossRef]
- Ruan, V.; Czer, L.; Awad, M.; Kittleson, M.; Patel, J.; Arabia, F.; Esmailian, F.; Ramzy, D.; Chung, J.; De Robertis, M.; et al. Use of Anti-Thymocyte Globulin for Induction Therapy in Cardiac Transplantation: A Review. Transplant. Proc. 2017, 49, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Ansari, D.; Lund, L.H.; Stehlik, J.; Andersson, B.; Höglund, P.; Edwards, L.; Nilsson, J. Induction with anti-thymocyte globulin in heart transplantation is associated with better long-term survival compared with basiliximab. J. Heart Lung Transpl. 2015, 34, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, A.; Schulz, U.; Deuse, T.; Ruhpawar, A.; Schmitto, J.D.; Beiras-Fernandez, A.; Hirt, S.; Schweiger, M.; Kopp-Fernandes, L.; Barten, M.J. Thymoglobulin induction in heart transplantation: Patient selection and implications for maintenance immunosuppression. Transpl. Int. 2015, 28, 259–269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goldraich, L.A.; Leitão, S.A.T.; Scolari, F.L.; Marcondes-Braga, F.G.; Bonatto, M.G.; Munyal, D.; Harrison, J.; Ribeiro, R.V.P.; Azeka, E.; Piardi, D.; et al. A Comprehensive and Contemporary Review on Immunosuppression Therapy for Heart Transplantation. Curr. Pharm. Des. 2020, 26, 3351–3384. [Google Scholar] [CrossRef] [PubMed]
- Sutaria, N.; Sylvia, L.; DeNofrio, D. Immunosuppression and Heart Transplantation. Handb. Exp. Pharmacol. 2022, 272, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Demiralp, G.; Arrigo, R.T.; Cassara, C.; Johnson, M.R. Heart Transplantation-Postoperative Considerations. Crit. Care Clin. 2024, 40, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Mattei, M.F.; Redonnet, M.; Gandjbakhch, I.; Bandini, A.M.; Billes, A.; Epailly, E.; Guillemain, R.; Lelong, B.; Pol, A.; Treilhaud, M.; et al. Lower risk of infectious deaths in cardiac transplant patients receiving basiliximab versus anti-thymocyte globulin as induction therapy. J. Heart Lung Transpl. 2007, 26, 693–699. [Google Scholar] [CrossRef]
- Rafiei, M.; Kittleson, M.; Patel, J.; Osborne, A.; Chang, D.; Czer, L.; Reinsmoen, N.; Esmailian, F.; Kobashigawa, J. Anti-thymocyte gamma-globulin may prevent antibody production after heart transplantation. Transpl. Proc. 2014, 46, 3570–3574. [Google Scholar] [CrossRef]
- Aliabadi, A.; Grömmer, M.; Cochrane, A.; Salameh, O.; Zuckermann, A. Induction therapy in heart transplantation: Where are we now? Transpl. Int. 2013, 26, 684–695. [Google Scholar] [CrossRef] [PubMed]
- Beiras-Fernandez, A.; Chappell, D.; Hammer, C.; Beiras, A.; Reichart, B.; Thein, E. Impact of polyclonal anti-thymocyte globulins on the expression of adhesion and inflammation molecules after ischemia-reperfusion injury. Transpl. Immunol. 2009, 20, 224–228. [Google Scholar] [CrossRef]
- Huh, J.; Baines, L.; Talbot, D.; MacFie, C. Severe anti-thymocyte globulin-induced cytokine release syndrome in a renal transplant patient. Anaesth. Rep. 2021, 9, 16–19. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kaukonen, K.-M.; Bailey, M.; Pilcher, D.; Cooper, D.J.; Bellomo, R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N. Engl. J. Med. 2015, 372, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Binnie, A.; Lage, J.; Dos Santos, C.C. How can biomarkers be used to differentiate between infection and non-infectious causes of inflammation? In Evidence-Based Practice of Critical Care; Elsevier: Amsterdam, The Netherlands, 2020; pp. 319–324.e1. [Google Scholar] [CrossRef] [PubMed Central]
- RRyu, J.-A.; Yang, J.H.; Lee, D.; Park, C.-M.; Suh, G.Y.; Jeon, K.; Cho, J.; Baek, S.Y.; Carriere, K.C.; Chung, C.R.; et al. Clinical Usefulness of Procalcitonin and C-Reactive Protein as Outcome Predictors in Critically Ill Patients with Severe Sepsis and Septic Shock. PLoS ONE 2015, 10, e0138150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Knaus, H.A.; Rottner, T.; Baumann, C.K.; Cserna, J.; Mitterbauer, M.; Schulenburg, A.; Rabitsch, W.; Wohlfarth, P. Cytokine Release Syndrome during Antithymocyte Globulin/Anti-T Lymphocyte Globulin Serotherapy for Graft-versus-Host Disease Prophylaxis before Allogeneic Hematopoietic Stem Cell Transplantation: Incidence and Early Clinical Impact According to American Society of Transplantation and Cellular Therapy Grading Criteria. Transplant. Cell Ther. 2022, 28, e1–e260. [Google Scholar] [CrossRef] [PubMed]
- Memar, M.Y.; Baghi, H.B. Presepsin: A promising biomarker for the detection of bacterial infections. Biomed. Pharmacother. 2019, 111, 649–656. [Google Scholar] [CrossRef]
- Yaegashi, Y.; Shirakawa, K.; Sato, N.; Suzuki, Y.; Kojika, M.; Imai, S.; Takahashi, G.; Miyata, M.; Furusako, S.; Endo, S. Evaluation of a newly identified soluble CD14 subtype as a marker for sepsis. J. Infect. Chemother. 2005, 11, 234–238. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Liu, Y.N.; Wang, R.; Xie, L.X. The accuracy of presepsin (sCD14-ST) for the diagnosis of sepsis in adults: A meta-analysis. Crit. Care 2015, 19, 323. [Google Scholar] [CrossRef]
- Tan, M.; Lu, Y.; Jiang, H.; Zhang, L. The diagnostic accuracy of procalcitonin and C-reactive protein for sepsis: A systematic review and meta-analysis. J. Cell Biochem. 2019, 120, 5852–5859. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Kim, E.H.; Kim, H.Y.; Ahn, J.G. Presepsin as a diagnostic marker of sepsis in children and adolescents: A systemic review and metaanalysis. BMC Infect. Dis. 2019, 19, 760. [Google Scholar] [CrossRef] [PubMed]
- Masson, S.; Caironi, P.; Fanizza, C.; Thomae, R.; Bernasconi, R.; Noto, A.; Oggioni, R.; Pasetti, G.S.; Romero, M.; Tognoni, G.; et al. Circulating presepsin (soluble CD14 subtype) as a marker of host response in patients with severe sepsis or septic shock: Data from the multicenter, randomized ALBIOS trial. Intensive Care Med. 2015, 41, 12–20, Corrected in Intensive Care Med. 2015, 41, 1736. [Google Scholar] [CrossRef] [PubMed]
- Abdelshafey, E.E.; Nasa, P.; Elgohary, A.E.; Khalil, M.F.; Rashwan, M.A.; Ghezala, H.B.; Tayar, A.A. Role of presepsin for diagnosis of sepsis and ICU mortality. A prospective controlled study. Indian. J. Crit. Care Med. 2021, 25, 153–157. [Google Scholar] [CrossRef]
- Pierrakos, C.; Velissaris, D.; Bisdorff, M.; Marshall, J.C.; Vincent, J.L. Biomarkers of sepsis: Time for a reappraisal. Crit. Care 2020, 24, 287. [Google Scholar] [CrossRef]
- Riedel, S.; Melendez, J.H.; An, A.T.; Rosenbaum, J.E.; Zenilman, J.M. Procalcitonin as a marker for the detection of bacteremia and sepsis in the emergency department. Am. J. Clin. Pathol. 2011, 135, 182–189. [Google Scholar] [CrossRef]
- Hatzistilianou, M. Diagnostic and prognostic role of procalcitonin in infections. Sci. World J. 2010, 10, 1941–1946. [Google Scholar] [CrossRef]
- Mierzchała-Pasierb, M.; Lipińska-Gediga, M. Sepsis diagnosis and monitoring-procalcitonin as standard, but what next? Anaesthesiol. Intensive. Ther. 2019, 51, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Kishimoto, T. IL-6: From its discovery to clinical applications. Int. Immunol. 2010, 22, 347–352. [Google Scholar] [CrossRef]
- Ruan, Q. IL-6 pathway and its inhibitors in heart failure. Pharmacol. Ther. 2019, 202, 41–55. [Google Scholar]
- Kumar, V. The role of IL-6 in the pathophysiology of inflammatory and autoimmune diseases. J. Immunol. 2020, 205, 123–131. [Google Scholar]
- Libermann, T.A.; Baltimore, D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell Biol. 1990, 10, 2327–2334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zazula, R.; Prucha, M.; Tyll, T.; Kieslichova, E. Induction of procalcitonin in liver transplant patients treated with anti-thymocyte globulin. Crit. Care 2007, 11, R131. [Google Scholar] [CrossRef]
- Brodska, H.; Drabek, T.; Malickova, K.; Kazda, A.; Vitek, A.; Zima, T.; Markova, M. Marked increase of procalcitonin after the administration of anti-thymocyte globulin in patients before hematopoietic stem cell transplantation does not indicate sepsis: A prospective study. Crit. Care 2009, 13, R37. [Google Scholar] [CrossRef]
- Sabat, R.; Höflich, C.; Döcke, W.; Kern, F.; Volk, H.-D.; Oppert, M.; Windrich, B.; Rosenberger, C.; Reinke, P.; Kaden, J. Massive elevation of procalcitonin plasma levels in the absence of infection in kidney transplant patients treated with pan-T-cell antibodies. Intensive. Care Med. 2001, 27, 987–991. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 1990, 265, 621–636. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- E Bianchi, M. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007, 81, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Taga, T.; Kishimoto, T. Interleukin-6 in biology and medicine. Adv. Immunol. 1993, 54, 1–78. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Kishimoto, T. IL-6 and NF-IL6 in acute-phase response and viral infection. Immunol. Rev. 1992, 127, 25–50. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.; Akira, S.; Taga, T. Interleukin-6 and its receptor: A paradigm for cytokines. Science 1992, 258, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Fishman, D.; Faulds, G.; Jeffery, R.; Mohamed-Ali, V.; Yudkin, J.S.; Humphries, S.; Woo, P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J. Clin. Investig. 1998, 102, 1369–1376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoshizaki, K.; Matsuda, T.; Nishimoto, N.; Kuritani, T.; Taeho, L.; Aozasa, K.; Nakahata, T.; Kawai, H.; Tagoh, H.; Komori, T.; et al. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman’s disease. Blood 1989, 74, 1360–1367. [Google Scholar] [CrossRef] [PubMed]



| Variable | Unit | Overall | Infected | Non-Infected | p | |
|---|---|---|---|---|---|---|
| Preoperative data | ||||||
| Population | N, % | 126 (100.0) | 26 (20.6) | 100 (79.4) | - | |
| Age | Years | Median [1–3 IQR] | 62 [51–65] | 60 [49–64] | 63 [52–66] | 0.166 |
| Male gender | N, % | 96 (76.2) | 22 (84.6) | 74 (74.0) | 0.716 | |
| Body mass index | kg/m2 | Mean ± SD | 25.9 ± 3.2 | 26.2 ± 2.2 | 25.8 ± 3.3 | 0.692 |
| Body surface area (DuBois) | m2 | Mean ± SD | 1.86 ± 0.19 | 1.92 ± 0.18 | 1.84 ± 0.19 | 0.224 |
| Heart failure | N, % | |||||
| Intermacs class 1–2 | 70 (55.6) | 24 (92.3) | 46 (46.0) | 0.004 | ||
| HF—acute onset | 14 (11.1) | 10 (38.5) | 4 (4.0) | 0.003 | ||
| Risk factors | N, % | |||||
| Hypertension | 96 (76.2) | 20 (76.9) | 76 (76.0) | 1.000 | ||
| Diabetes | 24 (19.0) | 4 (15.4) | 20 (20.0) | 1.000 | ||
| Dyslipidemia | 84 (66.7) | 16 (61.5) | 68 (68.0) | 0.912 | ||
| Extracardiac arteriopathy | 42 (33.3) | 10 (38.5) | 32 (32.0) | 0.912 | ||
| Previous cardiac surgery | 32 (25.4) | 4 (15.4) | 28 (28.0) | 0.486 | ||
| Renal replacement therapy | 20 (15.9) | 12 (46.2) | 8 (8.0) | 0.003 | ||
| Admitted to ICU | N, % | 52 (41.3) | 22 (84.6) | 30 (30.0) | <0.001 | |
| ICU stay > 96 h | 48 (38.1) | 22 (84.6) | 26 (26.0) | <0.001 | ||
| Mechanical circulatory support | N, % | |||||
| ECMO | 26 (20.6) | 18 (69.2) | 8 (8.0) | <0.001 | ||
| Impella | 6 (4.8) | 6 (23.1) | 0 (0.0) | 0.007 | ||
| C-reactive protein | mg/L | Median [1–3 IQR] | 5.0 [4.0–35.0] | 133.0 [29.0–174.9] | 4.0 [4.0–10.6] | <0.001 |
| Variable | Unit | Overall | Infected | Non-Infected | p | |
|---|---|---|---|---|---|---|
| Intraoperative Data | ||||||
| Duration | Min | Median [1–3 IQR] | ||||
| Graft ischemic time | 210 [185–240] | 240 [190–268] | 209 [183–227] | 0.116 | ||
| Cardiopulmonary bypass | 182 [162–199] | 193 [168–225] | 179 [160–198] | 0.281 | ||
| Transfusion | N, % | |||||
| Whole blood | 116 (92.1) | 26 (100.0) | 90 (90.0) | 0.574 | ||
| Plasma | 122 (96.8) | 26 (100.0) | 96 (96.0) | 1.000 | ||
| Platelets | 44 (34.9) | 16 (61.5) | 28 (28.0) | 0.053 | ||
| Pharmacological support | N, % | |||||
| Epinephrine | 124 (98.4) | 26 (100.0) | 98 (98.0) | 1.000 | ||
| Norepinephrine | 122 (96.8) | 26 (100.0) | 96 (96.0) | 1.000 | ||
| Nitric oxide | 122 (96.8) | 26 (100.0) | 96 (96.0) | 1.000 | ||
| Mechanical circulatory support | N, % | |||||
| ECMO | 26 (20.6) | 12 (46.2) | 14 (14.0) | 0.030 | ||
| Variable | Unit | Overall | Infected | Non-Infected | p | |
|---|---|---|---|---|---|---|
| Postoperative Inflammatory Markers | ||||||
| C-reactive protein | mg/L | Median [1–3 IQR] | ||||
| POD-1 | 85.7 [53.9–130.5] | 89.4 [64.1–132.0] | 84.0 [52.8–127.5] | 0.502 | ||
| POD-2 | 126.7 [103.6–155.6] | 104.5 [63.1–132.0] | 131.3 [106.9–155.7] | 0.079 | ||
| POD-3 | 87.9 [64.2–118.4] | 83.8 [60.0–125.5] | 88.1 [65.1–116.0] | 0.616 | ||
| POD-4 | 52.2 [34.3–73.2] | 58.0 [29.5–129.7] | 52.2 [35.4–67.7] | 0.659 | ||
| POD-5 | 33.4 [23.4–48.2] | 41.8 [26.5–91.1] | 32.9 [23.1–45.8] | 0.245 | ||
| POD-10 | 19.8 [10.3–51.6] | 72.4 [31.5–157.0] | 15.4 [9.0–30.8] | <0.001 | ||
| C-reactive protein—AUC | mg/L | Mean ± SD | 5054.6 ± 2343.0 | 1294.1 ± 380.4 | 3715.0 ± 2107.9 | 0.020 |
| Procalcitonin | ng/mL | Median [1–3 IQR] | ||||
| POD-1 | 24.8 [10.9–57.5] | 20.0 [11.0–63.0] | 26.1 [11.0–44.2] | 0.728 | ||
| POD-2 | 18.7 [7.4–45.8] | 20.0 [5.7–42.0] | 18.0 [7.9–46.4] | 0.939 | ||
| POD-3 | 15.9 [5.5–34.4] | 15.9 [4.1–30.5] | 17.3 [5.7–34.8] | 1.000 | ||
| POD-4 | 9.8 [3.9–26.5] | 8.6 [3.0–34.4] | 10.1 [3.9–24.6] | 0.926 | ||
| POD-5 | 5.4 [1.9–12.2] | 5.7 [2.2–29.0] | 5.3 [1.9–11.7] | 0.552 | ||
| POD-10 | 0.7 [0.2–1.3] | 2.7 [0.6–4.6] | 0.7 [0.2–0.9] | 0.001 | ||
| Procalcitonin—AUC | ng/mL | Mean ± SD | 1616.9 ± 1110.3 | 303.2 ± 149.3 | 1128.2 ± 813.6 | 0.035 |
| Presepsin | pg/mL | Median [1–3 IQR] | ||||
| POD-1 | 1168 [686–1789] | 2671 [1894–3123] | 907 [605–1394] | <0.001 | ||
| POD-2 | 1084 [667–1898] | 2843 [1795–3453] | 794 [637–1426] | <0.001 | ||
| POD-3 | 875 [548–2082] | 2978 [1739–3453] | 807 [518–1592] | <0.001 | ||
| POD-4 | 842 [496–2331] | 2512 [2135–3976] | 677 [456–1868] | <0.001 | ||
| POD-5 | 741 [362–2656] | 3134 [2645–5355] | 612 [328–1366] | <0.001 | ||
| POD-10 | 270 [125–1971] | 3746 [3147–10,545] | 174 [112–720] | <0.001 | ||
| Presepsin—AUC | pg/mL | Mean ± SD | 1255 ± 14,237 | 53,138 ± 16,198 | 56,018 ± 11,820 | 0.732 |
| Interleukin-6 | pg/mL | Median [1–3 IQR] | ||||
| POD-1 | 59.1 [35.5–97.8] | 60.6 [55.7–93.1] | 57.8 [34.8–98.6] | 0.610 | ||
| POD-2 | 37.5 [25.0–80.7] | 56.1 [33.2–89.3] | 32.1 [22.4–68.8] | 0.091 | ||
| POD-3 | 22.9 [18.4–45.2] | 35.1 [27.1–61.5] | 20.5 [15.6–33.4] | 0.010 | ||
| POD-4 | 18.3 [12.5–45.6] | 48.9 [27.1–77.8] | 16.6 [11.2–28.1] | 0.001 | ||
| POD-5 | 13.8 [9.4–37.5] | 51.3 [37.8–86.1] | 12.8 [8.6–18.2] | <0.001 | ||
| POD-10 | 8.5 [6.5–24.5] | 63.7 [25.0–112.0] | 8.0 [6.1–14.3] | <0.001 | ||
| Interleukin-6—AUC | pg/mL | Mean ± SD | 3349.1 ± 1677.2 | 987.5 ± 591.5 | 1999.7 ± 1476.0 | 0.150 |
| Variable | Coefficient | OR | Lower CI | Upper CI | p |
|---|---|---|---|---|---|
| Preoperative C-reactive protein | 1.05 | 2.85 | 1.03 | 7.87 | 0.04 |
| Bleeding requiring surgical revision | 0.95 | 2.57 | 0.93 | 7.11 | 0.07 |
| Preoperative ICU stay >96 h | 0.51 | 1.67 | 0.6 | 4.61 | 0.32 |
| Interleukin-6 (POD5) | 0.46 | 1.58 | 0.57 | 4.36 | 0.38 |
| Presepsin (POD1) | 0.33 | 1.38 | 0.5 | 3.82 | 0.53 |
| Presepsin (POD5) | 0.15 | 1.16 | 0.42 | 3.2 | 0.78 |
| Graft ischemic time | 0.12 | 1.12 | 0.41 | 3.1 | 0.82 |
| HF—acute onset | −0.07 | 0.93 | 0.34 | 2.58 | 0.9 |
| Interleukin-6 (POD2) | −0.12 | 0.89 | 0.32 | 2.46 | 0.82 |
| Interleukin-6 (POD1) | −0.37 | 0.69 | 0.25 | 1.91 | 0.48 |
| Age | −0.60 | 0.55 | 0.2 | 1.52 | 0.25 |
| C-reactive protein (POD2) | −0.61 | 0.54 | 0.2 | 1.5 | 0.24 |
| Variable | Unit | Overall | Infected | Non-Infected | p | |
|---|---|---|---|---|---|---|
| Postoperative Data | ||||||
| In-hospital mortality | N, % | 30 (23.8) | 16 (61.5) | 14 (14.0) | 0.001 | |
| Cause of death | N, % | |||||
| Cardiac | 8 (6.3) | 2 (7.7) | 6 (6.0) | 1.000 | ||
| Infection-associated | 12 (9.5) | 12 (46.2) | 0 (0.0) | <0.001 | ||
| Duration | Median [1–3 IQR] | |||||
| ICU-stay | Days | 6 [5–18] | 23 [12–37] | 6 [5–8] | 0.001 | |
| Hospital stay | Days | 31 [26–98] | 44 [23–56] | 28 [23–37] | 0.333 | |
| Mechanical ventilation | Hours | 31 [26–98] | 267 [99–360] | 29 [24–50] | <0.001 | |
| Mechanical circulatory support | N, % | |||||
| ECMO | 34 (27.0) | 14 (53.8) | 20 (20.0) | 0.036 | ||
| Stroke | N, % | |||||
| Ischemic | 2 (1.6) | 2 (7.7) | 0 (0.0) | 0.206 | ||
| Hemorragic | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 | ||
| Renal replacement therapy | N, % | 62 (49.2) | 24 (92.3) | 38 (38.0) | <0.001 | |
| Bleeding requiring surgical revision | N, % | 18 (14.3) | 12 (46.2) | 6 (6.0) | 0.002 | |
| Transfusion | N, % | |||||
| Whole blood | 118 (93.7) | 26 (100.0) | 92 (92.0) | 0.572 | ||
| Plasma | 122 (96.8) | 26 (100.0) | 96 (96.0) | 1.000 | ||
| Platelets | 44 (34.9) | 16 (61.5) | 28 (28.0) | 0.05 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovannico, L.; Santobuono, V.E.; Fischetti, G.; Mazzone, F.; Parigino, D.; Savino, L.; Alfeo, M.; Milano, A.D.; Guaricci, A.I.; Ciccone, M.M.; et al. Kinetics of Procalcitonin, CRP, IL-6, and Presepsin in Heart Transplant Patients Undergoing Induction with Thymoglobulin (rATG). J. Clin. Med. 2025, 14, 5369. https://doi.org/10.3390/jcm14155369
Giovannico L, Santobuono VE, Fischetti G, Mazzone F, Parigino D, Savino L, Alfeo M, Milano AD, Guaricci AI, Ciccone MM, et al. Kinetics of Procalcitonin, CRP, IL-6, and Presepsin in Heart Transplant Patients Undergoing Induction with Thymoglobulin (rATG). Journal of Clinical Medicine. 2025; 14(15):5369. https://doi.org/10.3390/jcm14155369
Chicago/Turabian StyleGiovannico, Lorenzo, Vincenzo Ezio Santobuono, Giuseppe Fischetti, Federica Mazzone, Domenico Parigino, Luca Savino, Maria Alfeo, Aldo Domenico Milano, Andrea Igoren Guaricci, Marco Matteo Ciccone, and et al. 2025. "Kinetics of Procalcitonin, CRP, IL-6, and Presepsin in Heart Transplant Patients Undergoing Induction with Thymoglobulin (rATG)" Journal of Clinical Medicine 14, no. 15: 5369. https://doi.org/10.3390/jcm14155369
APA StyleGiovannico, L., Santobuono, V. E., Fischetti, G., Mazzone, F., Parigino, D., Savino, L., Alfeo, M., Milano, A. D., Guaricci, A. I., Ciccone, M. M., Padalino, M., & Bottio, T. (2025). Kinetics of Procalcitonin, CRP, IL-6, and Presepsin in Heart Transplant Patients Undergoing Induction with Thymoglobulin (rATG). Journal of Clinical Medicine, 14(15), 5369. https://doi.org/10.3390/jcm14155369








