Cardiac Function in Women with and Without Previous Assisted Reproductive Technology: A Prospective Observational Cohort Study
Abstract
1. Introduction
2. Materials and Methods
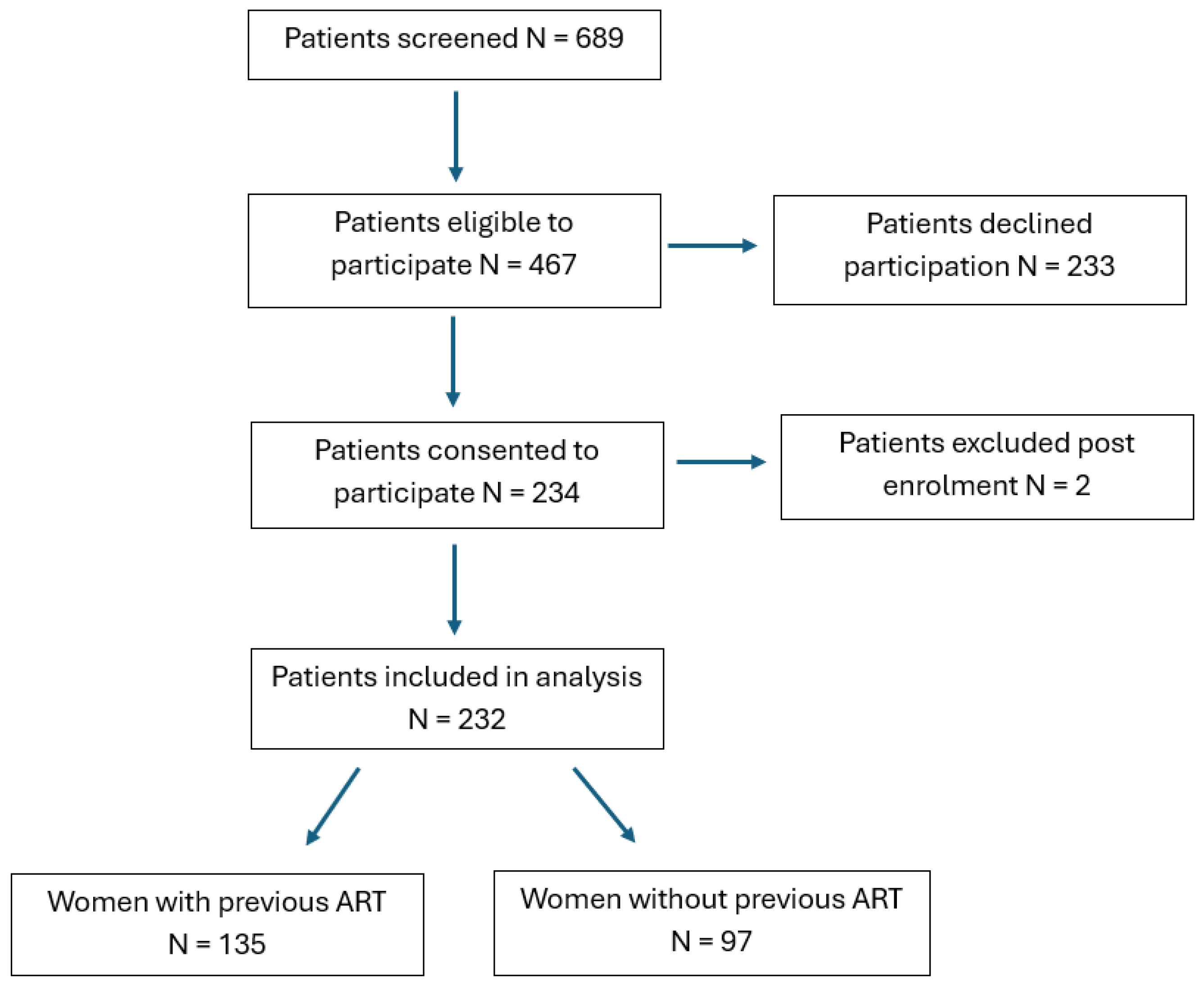
2.1. Study Population and Demographic Characteristics
2.2. Fertility Parameters
2.3. Blood Pressure and Transthoracic Echocardiography
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wyns, C.; De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.S.; Motrenko, T.; Smeenk, J.; Bergh, C.; Tandler-Schneider, A.; Rugescu, I.A.; Goossens, V. ART in Europe, 2018: Results generated from European registries by ESHRE. Hum. Reprod. Open 2022, 2022, hoac022. [Google Scholar] [CrossRef]
- Fauser, B.C. Towards the global coverage of a unified registry of IVF outcomes. Reprod. Biomed. Online 2019, 38, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Chih, H.J.; Elias, F.T.S.; Gaudet, L.; Velez, M.P. Assisted reproductive technology and hypertensive disorders of pregnancy: Systematic review and meta-analyses. BMC Pregnancy Childbirth 2021, 21, 449. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Liu, X.; Sheng, X.; Wang, H.; Gao, S. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: A meta-analysis of cohort studies. Fertil. Steril. 2016, 105, 73–85.e6. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 2007, 335, 974. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.D.; Malinowski, A.; Zhou, Q.; Yusuf, S.; Devereaux, P.J. Cardiovascular sequelae of preeclampsia/eclampsia: A systematic review and meta-analyses. Am. Heart J. 2008, 156, 918–930. [Google Scholar] [CrossRef]
- Perng, W.; Stuart, J.; Rifas-Shiman, S.L.; Rich-Edwards, J.W.; Stuebe, A.; Oken, E. Preterm birth and long-term maternal cardiovascular health. Ann. Epidemiol. 2015, 25, 40–45. [Google Scholar] [CrossRef]
- O’Kelly, A.C.; Michos, E.D.; Shufelt, C.L.; Vermunt, J.V.; Minissian, M.B.; Quesada, O.; Smith, G.N.; Rich-Edwards, J.W.; Garovic, V.D.; El Khoudary, S.R.; et al. Pregnancy and Reproductive Risk Factors for Cardiovascular Disease in Women. Circ. Res. 2022, 130, 652–672. [Google Scholar] [CrossRef]
- Apridonidze, T.; Essah, P.A.; Iuorno, M.J.; Nestler, J.E. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 1929–1935. [Google Scholar] [CrossRef]
- Ehrmann, D.A.; Liljenquist, D.R.; Kasza, K.; Azziz, R.; Legro, R.S.; Ghazzi, M.N. Prevalence and Predictors of the Metabolic Syndrome in Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2006, 91, 48–53. [Google Scholar] [CrossRef]
- Dayan, N.; Filion, K.B.; Okano, M.; Kilmartin, C.; Reinblatt, S.; Landry, T.; Basso, O.; Udell, J.A. Cardiovascular Risk Following Fertility Therapy: Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2017, 70, 1203–1213. [Google Scholar] [CrossRef]
- Udell, J.A.; Lu, H.; Redelmeier, D.A. Long-term cardiovascular risk in women prescribed fertility therapy. J. Am. Coll. Cardiol. 2013, 62, 1704–1712. [Google Scholar] [CrossRef]
- Westerlund, E.; Brandt, L.; Hovatta, O.; Wallén, H.; Ekbom, A.; Henriksson, P. Incidence of hypertension, stroke, coronary heart disease, and diabetes in women who have delivered after in vitro fertilization: A population-based cohort study from Sweden. Fertil. Steril. 2014, 102, 1096–1102. [Google Scholar] [CrossRef]
- Farland, L.V.; Grodstein, F.; Srouji, S.S.; Forman, J.P.; Rich-Edwards, J.; Chavarro, J.E.; Missmer, S.A. Infertility, fertility treatment, and risk of hypertension. Fertil. Steril. 2015, 104, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.P.; Barreto, A.T.F.; Neto, M.G.; Câmara, E.J.N.; Durães, A.R.; Roever, L.; Aras-Júnior, R. Prognostic power of conventional echocardiography in individuals without history of cardiovascular diseases: A systematic review and meta-analysis. Clinics 2021, 76, e2754. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, G.; Pizzino, F.; Paradossi, U.; Gueli, I.A.; Palazzini, M.; Gentile, P.; Di Spigno, F.; Ammirati, E.; Garascia, A.; Tedeschi, A.; et al. Charting the Unseen: How Non-Invasive Imaging Could Redefine Cardiovascular Prevention. J. Cardiovasc. Dev. Dis. 2024, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- Manau, D.; Fábregues, F.; Arroyo, V.; Jiménez, W.; Vanrell, J.A.; Balasch, J. Hemodynamic changes induced by urinary human chorionic gonadotropin and recombinant luteinizing hormone used for inducing final follicular maturation and luteinization. Fertil. Steril. 2002, 78, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, D.; Du Bois, E.F. A formula to estimate the approximate surface area if height and weight be known 1916. Nutrition 1989, 5, 303–311. [Google Scholar]
- Coelho Neto, M.A.; Ludwin, A.; Borrell, A.; Benacerraf, B.; Dewailly, D.; da Silva Costa, F.; Condous, G.; Alcazar, J.L.; Jokubkiene, L.; Guerriero, S.; et al. Counting ovarian antral follicles by ultrasound: A practical guide. Ultrasound Obstet. Gynecol. 2018, 51, 10–20. [Google Scholar] [CrossRef]
- National Institute for Clinical Excellence. Hypertension in Adults: Diagnosis and Management [NG136]; National Institute for Clinical Excellence: London, UK, 2023. [Google Scholar]
- Sahn, D.J.; DeMaria, A.; Kisslo, J.; Weyman, A. Recommendations regarding quantitation in M-mode echocardiography: Results of a survey of echocardiographic measurements. Circulation 1978, 58, 1072–1083. [Google Scholar] [CrossRef]
- Hill, J.C.; Palma, R.A. Doppler tissue imaging for the assessment of left ventricular diastolic function: A systematic approach for the sonographer. J. Am. Soc. Echocardiogr. 2005, 18, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Al Saikhan, L.; Park, C.; Hardy, R.; Hughes, A. Prognostic implications of left ventricular strain by speckle-tracking echocardiography in the general population: A meta-analysis. Vasc. Health Risk Manag. 2019, 15, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Fujitake, E.; Jaspal, R.; Monasta, L.; Stampalija, T.; Lees, C. Acute cardiovascular changes in women undergoing in vitro fertilisation (IVF), a systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 248, 245–251. [Google Scholar] [CrossRef]
- La Sala, G.B.; Gaddi, O.; Bruno, G.; Brandi, L.; Cantarelli, M.; Salvatore, V.; Torelli, M.G.; Dall’asta, D. Noninvasive evaluation of cardiovascular hemodynamics during multiple follicular stimulation, late luteal phase and early pregnancy. Fertil. Steril. 1989, 51, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, X.; Zang, L.; Zhang, Q.; Li, J.; Zou, S. Correlation between steroid hormonal levels and cardiac function in women during controlled ovarian hyperstimulation. Endocrine 2013, 44, 784–789. [Google Scholar] [CrossRef]
- Weissman, A.; Lowenstein, L.; Tal, J.; Ohel, G.; Calderon, I.; Lightman, A. Modulation of heart rate variability by estrogen in young women undergoing induction of ovulation. Eur. J. Appl. Physiol. 2009, 105, 381–386. [Google Scholar] [CrossRef]
- Uckuyu, A.; Ciftci, C.F.; Ozcimen, E.E.; Ciftci, O.; Toprak, E.; Turhan, E. Effect of controlled ovarian hyperstimulation treatment on cardiac function. J. Reprod. Med. 2010, 55, 503–508. [Google Scholar]
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular Risks Associated with Gender and Aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef]
- Kammar-García, A.; Hernández-Hernández, M.E.; López-Moreno, P.; Ortíz-Bueno, A.M.; Martínez-Montaño, M.L. Relation of body composition indexes to cardiovascular disease risk factors in young adults. Semergen 2019, 45, 147–155. [Google Scholar] [CrossRef]
- Zafrir, B.; Salman, N.; Crespo-Leiro, M.G.; Anker, S.D.; Coats, A.J.; Ferrari, R.; Filippatos, G.; Maggioni, A.P.; Mebazaa, A.; Piepoli, M.F.; et al. Body surface area as a prognostic marker in chronic heart failure patients: Results from the Heart Failure Registry of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2016, 18, 859–868. [Google Scholar] [CrossRef]
| Variable | Total Population (n = 232) | Women with Previous ART (n = 135) | Women Without Previous ART (n = 97) | p-Value |
|---|---|---|---|---|
| Demographic details | ||||
| Age (years) | 37.0 (34.0–40.0) | 38.0 (35.0–40.0) | 36.0 (33.5–39.0) | 0.01 |
| Height (cm) | 166.0 (161.0–170.0) | 165.0 (160.0–169.5) | 166.0 (161.0–170.00) | 0.69 |
| Weight (kg) | 66.3 (60.0–73.1) | 67.0 (59.8–74.8) | 65.3 (60.0–72.4) | 0.78 |
| Body Mass Index (kg/m2) | 24.2 (21.8–27.4) | 24.1 (21.3–28.3) | 24.3 (22.0–26.3) | 0.66 |
| Body Surface Area (m2) | 1.7 (1.6–1.8) | 1.7 (1.6–1.8) | 1.7 (1.6–1.8) | 0.80 |
| Race | 0.03 | |||
| White, n (%) | 194 (83.6) | 104 (77.0) | 90 (92.8) | |
| Black, n (%) | 14 (6.0) | 11 (8.1) | 3 (3.1) | |
| South Asian, n (%) | 15 (6.5) | 13 (9.6) | 2 (2.1) | |
| East Asian, n (%) | 3 (1.3) | 2 (1.5) | 1 (1.0) | |
| Other, n (%) | 6 (2.6) | 5 (3.7) | 1 (1.0) | |
| Smoking, n (%) | 3 (1.3) | 1 (0.7) | 2 (2.1) | 0.38 |
| Nulliparous, n (%) | 178 (76.7) | 91 (67.4) | 87 (89.7) | <0.001 |
| Variable | Total Population (n = 232) | Women with Previous ART (n = 135) | Women Without Previous ART (n = 97) | p-Value |
|---|---|---|---|---|
| ART variables | ||||
| Anti-Müllerian hormone (pmol/L) | 12.3 (6.9–19.9) | 12.4 (7.3–20.6) | 12.1 (6.4–18.2) | 0.53 |
| Endometrial thickness (mm) | 9.3 (7.7–11.1) | 9.2 (7.5–10.7) | 9.9 (8.0–113) | 0.07 |
| Antral follicle count | 13.0 (8.0–20.0) | 13.0 (8.0–20.5) | 14.0 (8.0–19.0) | 0.77 |
| Cardiac function variables | ||||
| Haemodynamic variables | ||||
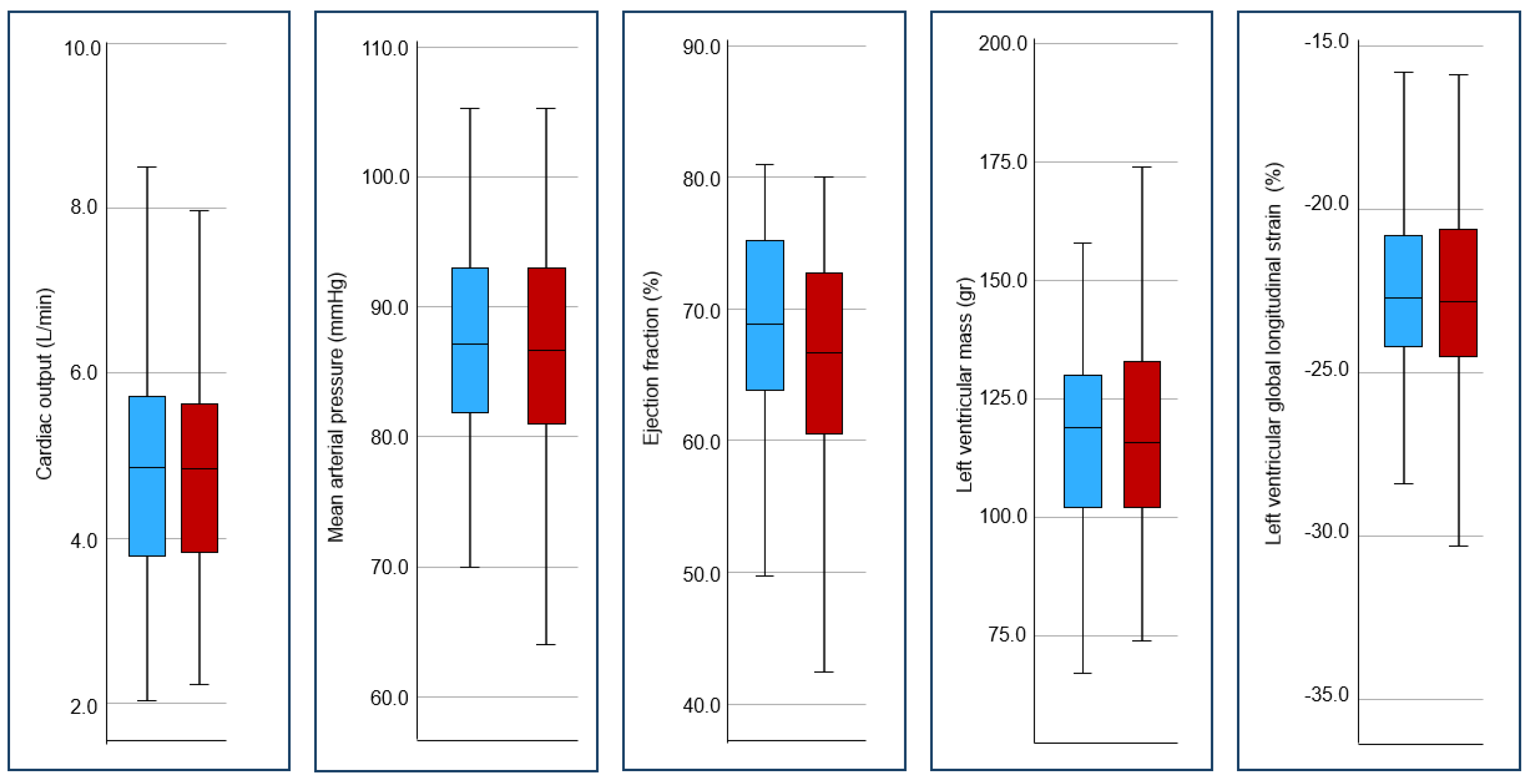
| Mean arterial pressure (mmHg) | 86.8 (81.0–93.0) | 86.7 (81.0–93.1) | 87.0 (81.1–93.0) | 0.61 |
| Left-ventricular outflow tract (mm) | 19.2 (18.5–20.5) | 19.4 (18.6–20.7) | 19.1 (18.5–20.4) | 0.40 |
| LV outflow tract velocity peak (cm/s) | 111.2 (97.7–124.6) | 109.1 (96.4–120.4) | 110.3 (96.3–128.0) | 0.20 |
| LV velocity time integral (cm/s) | 25.0 (21.7–27.6) | 24.8 (21.5–27.0) | 24.2 (20.9–28.0) | 0.80 |
| LV stroke volume (mL) | 71.6 (59.3–89.5) | 71.5 (60.9–89.2) | 71.5 (57.3– 89.5) | 0.58 |
| Heart rate (b/min) | 66.0 (59.0–74.0) | 65.0 (59.0–72.0) | 64.0 (59.0–74.0) | 0.70 |
| LV cardiac output (L/min) | 4.9 (3.9–5.7) | 4.8 (3.8–5.6) | 4.9 (3.8–5.7) | 0.82 |
| Peripheral vascular resistance (dynes/s/cm−5) | 1497.6 (1216.6–1780.7) | 1472.3 (1201.2–1754.2) | 1500.0 (1220.4–1859.8) | 0.52 |
| Left-ventricular systolic function | ||||
| Biplane LV end-diastolic volume (mL) | 72.6 (63.4–81.6) | 72.3 (64.3–79.8) | 72.6 (62.6–82.3) | 0.83 |
| Biplane LV end-systolic volume (mL) | 26.6 (23.2–31.1) | 27.3 (23.6–30.8) | 25.9 (22.8–32.0) | 0.19 |
| Biplane LV ejection fraction (%) | 63.8 (58.6–68.7) | 62.7 (58.0–67.7) | 64.6 (61.6–70.3) | 0.04 |
| LV Isovolumic contraction time (ms) | 58.0 (53.0–68.5) | 58.0 (51.5–69.0) | 58.0 (53.0–67.0) | 0.96 |
| LV ejection time (ms) | 294.0 (278.0–311.0) | 297.0 (281.0–312.5) | 292.0 (273.5–308.0) | 0.06 |
| LV myocardial performance index | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.25 |
| Left-atrial area (cm2) | 11.7 (9.9–12.9) | 11.1 (9.5–12.8) | 11.9 (10.4–13.2) | 0.09 |
| Left-atrial volume (mL) | 27.5 (21.8–33.2) | 26.6 (20.4–32.9) | 28.6 (24.3–33.0) | 0.11 |
| Left-ventricular diastolic function | ||||
| Mitral valve E-wave maximum velocity (cm/s) | 82.3 (72.3–92.2) | 80.1 (70.7–90.0) | 82.8 (72.8–93.9) | 0.24 |
| Mitral valve A-wave maximum velocity (cm/s) | 44.9 (33.8–54.4) | 44.9 (33.8–54.4) | 44.6 (32.5–54.7) | 0.87 |
| Mitral valve E/A-wave ratio | 1.8 (1.5–2.3) | 1.8 (1.5–2.3) | 1.8 (1.5–2.4) | 0.55 |
| Mitral valve e-lateral (cm/s) | 15.0 (12.4–17.3) | 14.9 (12.3–16.8) | 14.9 (12.6–17.6) | 0.41 |
| Mitral valve a-lateral (cm/s) | 9.4 (7.6–11.8) | 9.5 (7.6–11.9) | 9.2 (7.6–11.5) | 0.79 |
| Mitral valve s-lateral (cm/s) | 9.4 (8.0–11.7) | 9.5 (7.9–11.5) | 9.4 (8.4–11.6) | 0.74 |
| Mitral valve e-septal (cm/s) | 11.3 (10.0–13.3) | 11.3 (9.7–13.4) | 11.5 (10.3–13.1) | 0.35 |
| Mitral valve a-septal (cm/s) | 9.0 (7.9–10.7) | 9.0 (7.6–10.5) | 9.2 (8.2–10.9) | 0.27 |
| Mitral valve s-septal (cm/s) | 9.2 (7.9–10.4) | 9.3 (8.2–10.3) | 9.3 (7.8–10.7) | 0.71 |
| Isovolumic relaxation time (ms) | 67.0 (56.0–78.0) | 64.0 (53.0–76.5) | 67.0 (56.0–79.5) | 0.32 |
| Left-ventricular m-mode | ||||
| LV intraventricular septum in diastole (mm) | 10.2 (9.0–11.4) | 10.2 (9.0–11.8) | 10.1 (8.4–11.3) | 0.21 |
| LV end-diastolic diameter (mm) | 41.5 (38.4–45.2) | 41.8 (38.8–44.1) | 41.3 (38.2–45.9) | 0.41 |
| LV posterior wall in diastole (mm) | 9.5 (8.4–10.9) | 9.2 (8.2–10.5) | 9.9 (8.4–11.1) | 0.13 |
| LV end-systolic diameter (mm) | 26.0 (23.2–28.5) | 26.2 (23.8–28.6) | 25.7 (22.7–28.5) | 0.16 |
| LV mass (g) | 117.5 (102.2–132.8) | 116.0 (102.0–133.2) | 119.0 (103.0–130.0) | 0.82 |
| Global and Circumferential strain | ||||
| Average global longitudinal strain (%) | −22.7 (−24.2–20.6) | −22.8 (24.3–20.6) | −22.7 (−24.2–20.8) | 0.94 |
| Circumferential strain at the level of the mitral valve (%) | −26.1 (−31.5–20.1) | −26.9 (−31.0–20.3) | −24.4 (−31.3–19.8) | 0.40 |
| Circumferential strain at the level of the pectinate muscles (%) | −26.1 (−31.5–20.0) | −26.0 (−31.3–21.5) | −25.9 (−31.3–19.3) | 0.89 |
| Variable | Age | Body Mass Index | Body Surface Area | Race | Smoking | Nulliparous | Previous ART | Model p-Value | Model R-Squared |
|---|---|---|---|---|---|---|---|---|---|
| ART variables | |||||||||
| Anti-Müllerian hormone (pmol/L) | 0.004 | 0.01 | - | 0.49 | 0.31 | 0.37 | 0.59 | 0.01 | 0.08 |
| Endometrial thickness (mm) | 0.11 | 0.77 | - | 0.94 | 0.10 | 0.07 | 0.13 | 0.21 | 0.05 |
| Antral follicle count | <0.001 | 0.16 | - | 0.21 | 0.22 | 0.35 | 0.08 | <0.001 | 0.13 |
| Cardiac function variables | |||||||||
| Haemodynamic variables | |||||||||
| Mean arterial pressure (mmHg) | 0.95 | - | 0.001 | 0.97 | 0.99 | 0.97 | 0.53 | 0.13 | 0.06 |
| Left-ventricular outflow tract (mm) | 0.75 | - | <0.001 | 0.77 | 0.76 | 0.53 | 0.18 | 0.05 | 0.07 |
| LV outflow tract velocity peak (cm/s) | 0.70 | - | 0.02 | 0.11 | 0.78 | 0.32 | 0.41 | 0.02 | 0.08 |
| LV velocity time integral (cm/s) | 0.87 | - | 0.005 | 0.20 | 0.56 | 0.22 | 0.88 | 0.02 | 0.08 |
| LV stroke volume (mL) | 0.69 | - | <0.001 | 0.76 | 0.52 | 0.21 | 0.39 | <0.001 | 0.12 |
| Heart rate (b/min) | 0.39 | - | 0.95 | 0.14 | 0.07 | 0.82 | 0.32 | 0.22 | 0.05 |
| LV cardiac output (L/min) | 0.40 | - | <0.001 | 0.66 | 0.69 | 0.23 | 0.66 | <0.001 | 0.12 |
| Peripheral vascular resistance (dynes/s/cm−5) | 0.33 | - | 0.001 | 0.80 | 0.95 | 0.12 | 0.26 | 0.03 | 0.08 |
| Left-ventricular systolic function | |||||||||
| Biplane LV end-diastolic volume (mL) | 0.09 | - | <0.001 | 0.19 | 0.94 | 0.60 | 0.69 | <0.001 | 0.18 |
| Biplane LV end-systolic volume (mL) | 0.05 | - | <0.001 | 0.62 | 0.96 | 0.17 | 0.13 | 0.001 | 0.12 |
| Biplane LV ejection fraction (%) | 0.04 | - | 0.55 | 0.02 | 0.72 | 0.14 | 0.04 | 0.007 | 0.10 |
| LV Isovolumic contraction time (ms) | 0.42 | - | 0.51 | 0.13 | 0.59 | 0.98 | 0.91 | 0.42 | 0.04 |
| LV ejection time (ms) | 0.02 | - | 0.30 | 0.57 | 0.49 | 0.87 | 0.22 | 0.15 | 0.06 |
| LV myocardial performance index | 0.21 | - | 0.69 | 0.88 | 0.56 | 0.89 | 0.39 | 0.86 | 0.02 |
| Left-atrial area (cm2) | 0.51 | - | <0.001 | 0.48 | 0.55 | 0.31 | 0.12 | 0.002 | 0.11 |
| Left-atrial volume (mL) | 0.54 | - | <0.001 | 0.89 | 0.69 | 0.25 | 0.14 | 0.02 | 0.08 |
| Left-ventricular diastolic function | |||||||||
| Mitral valve E-wave maximum velocity (cm/s) | 0.49 | - | 0.24 | 0.04 | 0.68 | 0.78 | 0.34 | 0.17 | 0.05 |
| Mitral valve A-wave maximum velocity (cm/s) | 0.26 | - | 0.32 | 0.22 | 0.99 | 0.67 | 0.41 | 0.56 | 0.03 |
| Mitral valve E/A-wave ratio | 0.09 | - | 0.10 | 0.15 | 0.66 | 0.87 | 0.79 | 0.21 | 0.05 |
| Mitral valve e-lateral (cm/s) | 0.23 | - | 0.11 | 0.18 | 0.07 | 0.46 | 0.98 | 0.07 | 0.07 |
| Mitral valve a-lateral (cm/s) | 0.48 | - | 0.004 | 0.74 | 0.43 | 0.90 | 0.69 | 0.16 | 0.06 |
| Mitral valve s-lateral (cm/s) | 0.19 | - | 0.42 | 0.74 | 0.85 | 0.92 | 0.91 | 0.87 | 0.02 |
| Mitral valve e-septal (cm/s) | 0.08 | - | 0.28 | 0.53 | 0.45 | 0.91 | 0.92 | 0.53 | 0.04 |
| Mitral valve a-septal (cm/s) | 0.80 | - | 0.37 | 0.48 | 0.28 | 0.37 | 0.47 | 0.59 | 0.03 |
| Mitral valve s-septal (cm/s) | 0.31 | - | 0.02 | 0.05 | 0.16 | 0.78 | 0.31 | 0.41 | 0.08 |
| Isovolumic relaxation time (ms) | 0.45 | - | 0.90 | 0.61 | 0.95 | 0.64 | 0.47 | 0.85 | 0.02 |
| Left-ventricular m-mode | |||||||||
| LV intraventricular septum in diastole (mm) | 0.20 | - | 0.05 | 0.17 | 0.79 | 0.39 | 0.08 | 0.08 | 0.07 |
| LV end-diastolic diameter (mm) | 0.57 | - | <0.001 | 0.43 | 0.98 | 0.86 | 0.58 | <0.001 | 0.13 |
| LV posterior wall in diastole (mm) | 0.18 | - | 0.15 | 0.53 | 0.99 | 0.70 | 0.49 | 0.44 | 0.04 |
| LV end-systolic diameter (mm) | 0.68 | - | <0.001 | 0.47 | 0.16 | 0.94 | 0.65 | 0.001 | 0.11 |
| LV mass (g) | 0.27 | - | <0.001 | 0.75 | 0.94 | 0.39 | 0.37 | <0.001 | 0.15 |
| Global and Circumferential strain | |||||||||
| Average global longitudinal strain (%) | 0.51 | - | <0.001 | 0.002 | 0.85 | 0.30 | 0.86 | <0.001 | 0.12 |
| Circumferential strain at the level of the mitral valve (%) | 0.13 | - | 0.93 | 0.67 | 0.66 | 0.37 | 0.32 | 0.73 | 0.03 |
| Circumferential strain at the level of the pectinate muscles (%) | 0.14 | - | 0.74 | 0.89 | 0.80 | 0.11 | 0.97 | 0.76 | 0.03 |
| Variable | Age | Body Mass Index | Body Surface Area | Race | Smoking | Model p-Value | Model R-Squared | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef. B (SE) | p-Value | Coef. B (SE) | p-Value | Coef. B (SE) | p-Value | Coef. B (SE) | p-Value | Coef. B (SE) | p-Value | |||
| ART variables | ||||||||||||
| Anti-Müllerian hormone (pmol/L) | −0.65 (0.22) | 0.004 | −0.57 (0.23) | 0.02 | - | - | - | - | - | - | <0.001 | 0.06 |
| Antral follicle count | −0.75 (0.17) | <0.001 | - | - | - | - | - | - | - | - | <0.001 | 0.08 |
| Cardiac function variables | ||||||||||||
| Haemodynamic variables | ||||||||||||
| Mean arterial pressure (mmHg) | - | - | - | - | 15.13 (4.13) | <0.001 | - | - | - | - | <0.001 | 0.05 |
| LV outflow tract (mm) | - | - | - | - | 2.85 (0.77) | <0.001 | - | - | - | - | <0.001 | 0.06 |
| LV tract peak velocity (cm/s) | - | - | - | - | 27.74 (9.55) | 0.004 | - | - | - | - | 0.004 | 0.04 |
| LV velocity time integral (cm/s) | - | - | - | - | 7.58 (2.17) | <0.001 | - | - | - | - | <0.001 | 0.05 |
| LV stroke volume (mL) | - | - | - | - | 45.29 (8.83) | <0.001 | - | - | - | - | <0.001 | 0.10 |
| LV cardiac output (L/min) | - | - | - | - | 3.03 (0.62) | <0.001 | - | - | - | - | <0.001 | 0.10 |
| PVR (dynes/s/cm−5) | - | - | - | - | −778.85 (220.08) | <0.001 | - | - | - | - | <0.001 | 0.05 |
| Left-ventricular systolic function | ||||||||||||
| Biplane LV end-diastolic volume (mL) | - | - | - | - | 37.66 (6.03) | <0.001 | - | - | - | - | <0.001 | 0.15 |
| Biplane LV end-systolic volume (mL) | −0.23 (0.11) | 0.042 | - | - | 13.61 (3.02) | <0.001 | - | - | - | - | <0.001 | 0.09 |
| Biplane LV ejection fraction (%) | 0.31 (0.13) | 0.021 | - | - | - | - | - | - | - | - | 0.021 | 0.02 |
| LV ejection time (ms) | 1.21 (0.45) | 0.008 | - | - | - | - | - | - | - | - | 0.008 | 0.03 |
| Left-atrial area (cm2) | - | - | - | - | 4.42 (1.03) | <0.001 | - | - | - | - | <0.001 | 0.08 |
| Left-atrial volume (mL) | - | - | - | - | 14.84 (3.94) | <0.001 | - | - | - | - | <0.001 | 0.06 |
| Left-ventricular diastolic function | ||||||||||||
| Mitral valve e-lateral (cm/s) | - | - | - | - | - | - | - | - | −4.46 (1.98) | 0.025 | 0.025 | 0.02 |
| Mitral valve a-lateral (cm/s) | - | - | - | - | 4.22 (1.37) | 0.002 | - | - | - | - | 0.002 | 0.04 |
| Mitral valve s-septal (cm/s) | - | - | - | - | 2.18 (0.95) | 0.022 | - | - | - | - | 0.022 | 0.02 |
| Left-ventricular m-mode | ||||||||||||
| LV intraventricular septum in diastole (mm) | - | - | - | - | 1.87 (0.89) | 0.037 | - | - | - | - | 0.037 | 0.02 |
| LV end-diastolic diameter (mm) | - | - | - | - | 11.63 (2.18) | <0.001 | - | - | - | - | <0.001 | 0.11 |
| LV end-systolic diameter (mm) | - | - | - | - | 8.23 (1.90) | <0.001 | - | - | - | - | <0.001 | 0.08 |
| LV mass (g) | - | - | - | - | 64.98 (10.77) | <0.001 | - | - | - | - | <0.001 | 0.14 |
| Global and Circumferential strain | ||||||||||||
| Average global longitudinal strain (%) | - | - | - | - | 5.17 (1.41) | <0.001 | * | 0.002 | - | - | <0.001 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baird, F.; Kakouri, E.; Huluta, I.; Sarris, I.; Sunkara, S.K.; Nicolaides, K.H.; Kametas, N. Cardiac Function in Women with and Without Previous Assisted Reproductive Technology: A Prospective Observational Cohort Study. J. Clin. Med. 2025, 14, 5366. https://doi.org/10.3390/jcm14155366
Baird F, Kakouri E, Huluta I, Sarris I, Sunkara SK, Nicolaides KH, Kametas N. Cardiac Function in Women with and Without Previous Assisted Reproductive Technology: A Prospective Observational Cohort Study. Journal of Clinical Medicine. 2025; 14(15):5366. https://doi.org/10.3390/jcm14155366
Chicago/Turabian StyleBaird, Freya, Eleni Kakouri, Iulia Huluta, Ippokratis Sarris, Sesh K. Sunkara, Kypros H. Nicolaides, and Nick Kametas. 2025. "Cardiac Function in Women with and Without Previous Assisted Reproductive Technology: A Prospective Observational Cohort Study" Journal of Clinical Medicine 14, no. 15: 5366. https://doi.org/10.3390/jcm14155366
APA StyleBaird, F., Kakouri, E., Huluta, I., Sarris, I., Sunkara, S. K., Nicolaides, K. H., & Kametas, N. (2025). Cardiac Function in Women with and Without Previous Assisted Reproductive Technology: A Prospective Observational Cohort Study. Journal of Clinical Medicine, 14(15), 5366. https://doi.org/10.3390/jcm14155366






