Integrating Clinical Parameters into Thyroid Nodule Malignancy Risk: A Retrospective Evaluation Based on ACR TI-RADS
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Patients’ Characteristics
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Russ, G.; Leboulleux, S.; Leenhardt, L.; Hegedüs, L. Thyroid Incidentalomas: Epidemiology, Risk Stratification with Ultrasound and Workup. Eur. Thyroid J. 2014, 3, 154–163. [Google Scholar] [CrossRef]
- Sosa, J.A.; Hanna, J.W.; Robinson, K.A.; Lanman, R.B. Increases in Thyroid Nodule Fine-Needle Aspirations, Operations, and Diagnoses of Thyroid Cancer in the United States. Surgery 2013, 154, 1420–1426; discussion 1426–1427. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Grani, G.; Lamartina, L.; Filetti, S.; Mandel, S.J.; Cooper, D.S. The Diagnosis and Management of Thyroid Nodules. JAMA 2018, 319, 914. [Google Scholar] [CrossRef] [PubMed]
- Kwong, N.; Medici, M.; Angell, T.E.; Liu, X.; Marqusee, E.; Cibas, E.S.; Krane, J.F.; Barletta, J.A.; Kim, M.I.; Larsen, P.R.; et al. The Influence of Patient Age on Thyroid Nodule Formation, Multinodularity, and Thyroid Cancer Risk. J. Clin. Endocrinol. Metab. 2015, 100, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Angell, T.E.; Maurer, R.; Wang, Z.; Kim, M.I.; Alexander, C.A.; Barletta, J.A.; Benson, C.B.; Cibas, E.S.; Cho, N.L.; Doherty, G.M.; et al. A Cohort Analysis of Clinical and Ultrasound Variables Predicting Cancer Risk in 20,001 Consecutive Thyroid Nodules. J. Clin. Endocrinol. Metab. 2019, 104, 5665–5672. [Google Scholar] [CrossRef]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef]
- Russ, G.; Bonnema, S.J.; Erdogan, M.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef]
- Eidt, L.B.; de Oliveira, C.N.; De Lagos, Y.B.B.; Solera, G.L.M.; Izquierdo, R.; Meyer, E.L.d.S.; Mattevi, V.S.; Golbert, L. A Prospective Comparison of ACR-TIRADS and EU-TIRADS in Thyroid Nodule Assessment for FNA-US. Clin. Endocrinol. 2022, 98, 415–425. [Google Scholar] [CrossRef]
- Cibas, E.S.; Ali, S.Z. The Bethesda System for Reporting Thyroid Cytopathology. Am. J. Clin. Pathol. 2009, 132, 658–665. [Google Scholar] [CrossRef]
- Rago, T.; Fiore, E.; Scutari, M.; Santini, F.; Di Coscio, G.; Romani, R.; Piaggi, P.; Ugolini, C.; Basolo, F.; Miccoli, P.; et al. Male Sex, Single Nodularity, and Young Age Are Associated with the Risk of Finding a Papillary Thyroid Cancer on Fine-Needle Aspiration Cytology in a Large Series of Patients with Nodular Thyroid Disease. Eur. J. Endocrinol. 2010, 162, 763–770. [Google Scholar] [CrossRef]
- Belfiore, A.; La Rosa, G.L.; La Porta, G.A.; Giuffrida, D.; Milazzo, G.; Lupo, L.; Regalbuto, C.; Vigneri, R. Cancer Risk in Patients with Cold Thyroid Nodules: Relevance of Iodine Intake, Sex, Age, and Multinodularity. Am. J. Med. 1992, 93, 363–369. [Google Scholar] [CrossRef]
- Di Fermo, F.; Sforza, N.; Rosmarin, M.; Morosan Allo, Y.; Parisi, C.; Santamaria, J.; Pacenza, N.; Zuk, C.; Faingold, C.; Ferraro, F.; et al. Comparison of Different Systems of Ultrasound (US) Risk Stratification for Malignancy in Elderly Patients with Thyroid Nodules. Real World Experience. Endocrine 2020, 69, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Grani, G.; Brenta, G.; Trimboli, P.; Falcone, R.; Ramundo, V.; Maranghi, M.; Lucia, P.; Filetti, S.; Durante, C. Sonographic Risk Stratification Systems for Thyroid Nodules as Rule-out Tests in Older Adults. Cancers 2020, 12, 2458. [Google Scholar] [CrossRef] [PubMed]
- Walter, L.B.; Fernandes, P.M.; Strieder, D.L.; Scheinpflug, A.L.; Zanella, A.B.; Faccin, C.S.; Farenzena, M.; Xavier, L.F.; Zorzi, B.D.C.; Graudenz, M.S.; et al. Age-Related Variation in Malignant Cytology Rates of Thyroid Nodules: Insights from a Retrospective Observational Study Assessing the ACR TI-RADS. Eur. J. Endocrinol. 2023, 189, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Grani, G.; Stramazzo, I.; Locantore, P.; Virili, C.; Filardi, T.; Lecis, C.; Centello, R.; Cera, G.; Santaguida, M.G.; Gianfrilli, D.; et al. Validation of ACR TI-RADS Performance in Transition Age: Results from a Multicenter Retrospective Study by the TALENT Study Group. Endocrine 2024, 87, 1131–1140. [Google Scholar] [CrossRef]
- Shin, A.; Cho, S.; Jang, D.; Abe, S.K.; Saito, E.; Rahman, S.; Islam, R.; Sawada, N.; Shu, X.-O.; Koh, W.-P.; et al. Body Mass Index and Thyroid Cancer Risk: A Pooled Analysis of Half a Million Men and Women in the Asia Cohort Consortium. Thyroid 2022, 32, 306–314. [Google Scholar] [CrossRef]
- Paes, J.; Hua, K.; Nagy, R.J.; Kloos, R.T.; Jarjoura, D.; Ringel, M.D. The Relationship between Body Mass Index and Thyroid Cancer Pathology Features and Outcomes: A Clinicopathological Cohort Study. J. Clin. Endocrinol. Metab. 2010, 95, 4244–4250. [Google Scholar] [CrossRef]
- Fiore, E.; Vitti, P. Serum TSH and Risk of Papillary Thyroid Cancer in Nodular Thyroid Disease. J. Clin. Endocrinol. Metab. 2012, 97, 1134–1145. [Google Scholar] [CrossRef]
- Paparodis, R.D.; Bantouna, D.; Karvounis, E.; Imam, S.; Jaume, J.C. Higher TSH Is Not Associated with Thyroid Cancer Risk in the Presence of Thyroid Autoimmunity. J. Clin. Endocrinol. Metab. 2020, 105, e2389–e2397. [Google Scholar] [CrossRef]
- Falstie-Jensen, A.M.; Kjærsgaard, A.; Lorenzen, E.L.; Jensen, J.D.; Reinertsen, K.V.; Dekkers, O.M.; Ewertz, M.; Cronin-Fenton, D.P. Hypothyroidism and the Risk of Breast Cancer Recurrence and All-Cause Mortality—A Danish Population-Based Study. Breast Cancer Res. 2019, 21, 44. [Google Scholar] [CrossRef]
- Fiore, E.; Rago, T.; Latrofa, F.; Provenzale, M.A.; Piaggi, P.; Delitala, A.; Scutari, M.; Basolo, F.; Di Coscio, G.; Grasso, L.; et al. Hashimoto’s Thyroiditis Is Associated with Papillary Thyroid Carcinoma: Role of TSH and of Treatment with L-Thyroxine. Endocr.-Relat. Cancer 2011, 18, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, C.M.; Körmendiné Farkas, D.; Jørgensen, J.O.L.; Cronin-Fenton, D.; Sørensen, H.T. Benign Thyroid Diseases and Risk of Thyroid Cancer: A Nationwide Cohort Study. J. Clin. Endocrinol. Metab. 2018, 103, 2216–2224. [Google Scholar] [CrossRef] [PubMed]
- Krzentowska, A.; Konturek, A.; Gołkowski, F.; Merklinger-Gruchała, A.; Barczyński, M. Risk Stratification for Thyroid Malignancies in Chronic Lymphocytic Thyroiditis. Cancers 2025, 17, 1964. [Google Scholar] [CrossRef]
- Jackson, D.; Handelsman, R.S.; Farrá, J.C.; Lew, J.I. Increased Incidental Thyroid Cancer in Patients with Subclinical Chronic Lymphocytic Thyroiditis. J. Surg. Res. 2020, 245, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhu, X.; Cheng, F.; Zhou, B.; Zhu, X.; Zhu, L. Correlation between Thyroid Autoantibodies and the Risk of Thyroid Papillary Carcinoma. Gland Surg. 2020, 9, 950–955. [Google Scholar] [CrossRef]
- Srinivas, M.N.S.; Amogh, V.N.; Gautam, M.S.; Prathyusha, I.S.; Vikram, N.R.; Retnam, M.K.; Balakrishna, B.V.; Kudva, N. A Prospective Study to Evaluate the Reliability of Thyroid Imaging Reporting and Data System in Differentiation between Benign and Malignant Thyroid Lesions. J. Clin. Imaging Sci. 2016, 6, 5. [Google Scholar] [CrossRef]
- Grani, G.; Lamartina, L.; Ascoli, V.; Bosco, D.; Biffoni, M.; Giacomelli, L.; Maranghi, M.; Falcone, R.; Ramundo, V.; Cantisani, V.; et al. Reducing the Number of Unnecessary Thyroid Biopsies While Improving Diagnostic Accuracy: Toward the “Right” TIRADS. J. Clin. Endocrinol. Metab. 2018, 104, 95–102. [Google Scholar] [CrossRef]
- Seifert, P.; Görges, R.; Zimny, M.; Kreissl, M.C.; Schenke, S. Interobserver Agreement and Efficacy of Consensus Reading in Kwak-, EU-, and ACR-Thyroid Imaging Recording and Data Systems and ATA Guidelines for the Ultrasound Risk Stratification of Thyroid Nodules. Endocrine 2019, 67, 143–154. [Google Scholar] [CrossRef]
- Gharib, H. Fine-Needle Aspiration Biopsy of the Thyroid: An Appraisal. Ann. Intern. Med. 1993, 118, 282. [Google Scholar] [CrossRef]
- Hoang, J.K.; Middleton, W.D.; Langer, J.E.; Schmidt, K.; Gillis, L.B.; Nair, S.S.; Watts, J.A.; Snyder, R.W.; Khot, R.; Rawal, U.; et al. Comparison of Thyroid Risk Categorization Systems and Fine-Needle Aspiration Recommendations in a Multi-Institutional Thyroid Ultrasound Registry. J. Am. Coll. Radiol. 2021, 18, 1605–1613. [Google Scholar] [CrossRef]
- Hoang, J.K.; Middleton, W.D.; Farjat, A.E.; Langer, J.E.; Reading, C.C.; Teefey, S.A.; Abinanti, N.; Boschini, F.J.; Bronner, A.J.; Dahiya, N.; et al. Reduction in Thyroid Nodule Biopsies and Improved Accuracy with American College of Radiology Thyroid Imaging Reporting and Data System. Radiology 2018, 287, 185–193. [Google Scholar] [CrossRef]
- Grani, G.; Lamartina, L.; Cantisani, V.; Maranghi, M.; Lucia, P.; Durante, C. Interobserver Agreement of Various Thyroid Imaging Reporting and Data Systems. Endocr. Connect. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Matrone, A.; Gambale, C.; Pieroni, E.; De Napoli, L.; Torregrossa, L.; Materazzi, G.; Elisei, R. Ultrasound Features and Risk Stratification System in NIFT-P and Other Follicular-Patterned Thyroid Tumors. Eur. J. Endocrinol. 2023, 189, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Luiz, R.; Assumpção, L.; Paiva, F.; Teixeira, S. Does a Three-Degree Hypoechogenicity Grading Improve Ultrasound Thyroid Nodule Risk Stratification and Affect the TI-RADS 4 Category? A Retrospective Observational Study. Arch. Endocrinol. Metab. 2023, 67, e000608. [Google Scholar] [CrossRef]
- Grant, E.G.; Tessler, F.N.; Hoang, J.K.; Langer, J.E.; Beland, M.D.; Berland, L.L.; Cronan, J.J.; Desser, T.S.; Frates, M.C.; Hamper, U.M.; et al. Thyroid Ultrasound Reporting Lexicon: White Paper of the ACR Thyroid Imaging, Reporting and Data System (TIRADS) Committee. J. Am. Coll. Radiol. 2015, 12, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Teefey, S.A.; Middleton, W.D.; Reading, C.C.; Langer, J.E.; Beland, M.D.; Szabunio, M.M.; Desser, T.S. Effect of Decreasing the ACR TI-RADS Point Assignment for Punctate Echogenic Foci When They Occur in Mixed Solid and Cystic Thyroid Nodules. Am. J. Roentgenol. 2021, 216, 479–485. [Google Scholar] [CrossRef]
- Jasim, S.; Baranski, T.J.; Teefey, S.A.; Middleton, W.D. Investigating the Effect of Thyroid Nodule Location on the Risk of Thyroid Cancer. Thyroid 2020, 30, 401–407. [Google Scholar] [CrossRef]

| Age, Years | 48 (38–59) | |
| Male sex, n (%) | 233 (23.32) | |
| Nodule size (mm) | 16.1 (12–23) | |
| BMI (kg/m2) | 27.2 (23.9–32) | |
| Autoimmune thyroiditis, n (%) | 269 (23.85) | |
| Hypothyroidism, n (%) | 367 (32.54) | |
| Indication for FNA, n (%) | 705 (62.5) | |
| ACR TI-RADS categories, n (%) | Malignant histopathology, n (%) | |
| 1 | 1 (0.9) | 0 |
| 2 | 11 (1.1) | 0 |
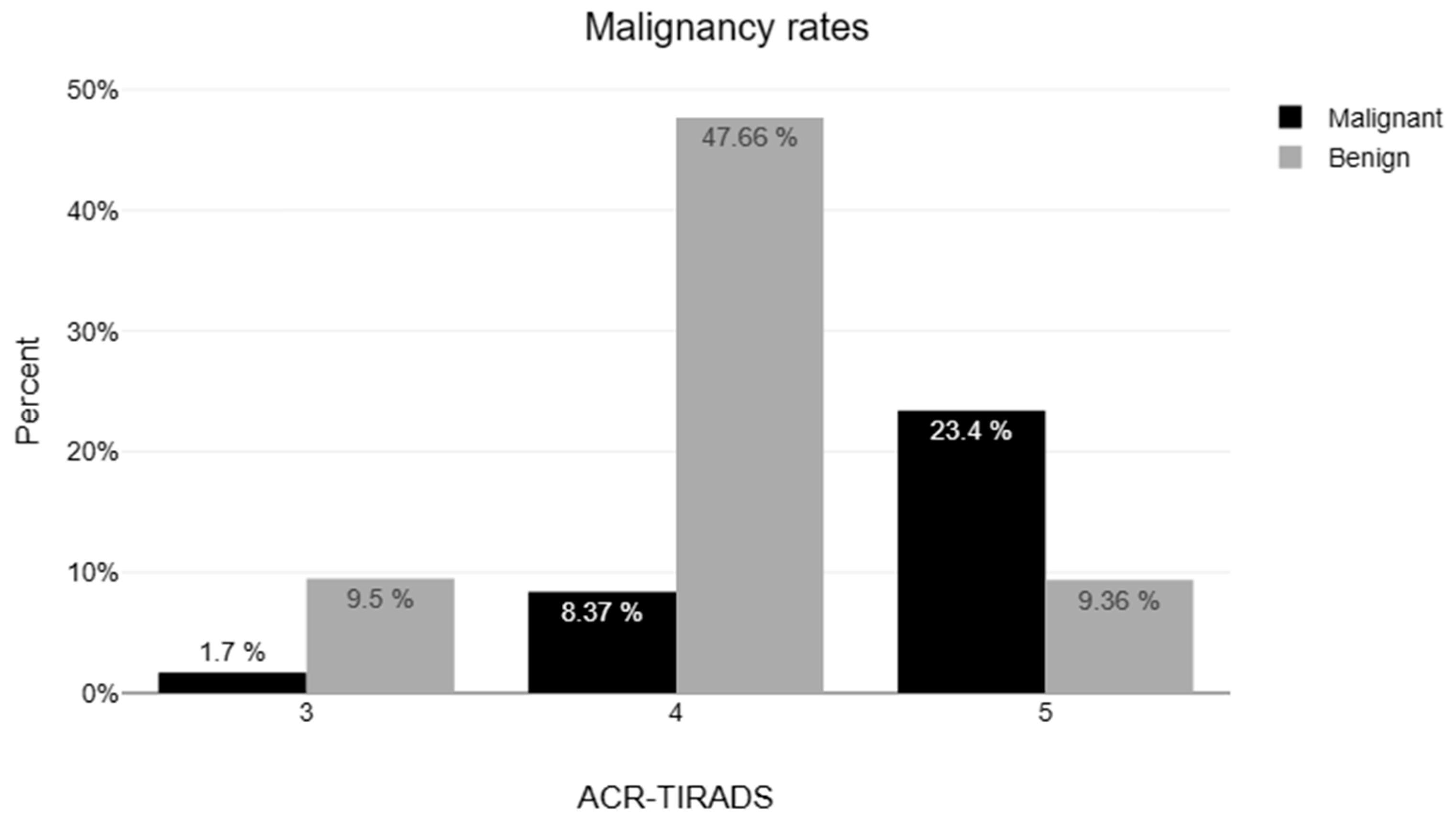
| 3 | 182 (17.7) | 16 (8.8) |
| 4 | 656 (63.8) | 145 (22.1) |
| 5 | 278 (27.1) | 206 (74.1) |
| Bethesda categories, n (%) | ||
| I | 11 (0.9) | 1 (9) |
| II | 531 (47.1) | 9 (1.7) |
| III | 262 (23.2) | 72 (27.5) |
| IV | 33 (2.9) | 5 (15.2) |
| V | 145 (12.8) | 136 (93.8) |
| VI | 146 (12.9) | 144 (98.6) |
| Bethesda Category | 20–39 y (n = 328) | 40–59 y (n = 528) | ≥60 y (n = 272) | p-Value |
|---|---|---|---|---|
| Benign (I–II) | 110 (33.5%) | 272 (51.5%) | 160 (58.9%) | <0.001 |
| Indeterminate (III–IV) | 94 (28.7%) | 141 (26.7%) | 60 (22.1%) | 0.116 |
| Malignant (V–VI) | 124 (37.8%) | 115 (21.8%) | 52 (19.1%) | <0.001 |
| Confirmed malignancy * | 150 (45.7%) | 153 (28.9%) | 64 (23.5%) | <0.001 |
| Malignancy | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| Yes (n = 367) | No (n = 761) | Coefficient B | Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p | |
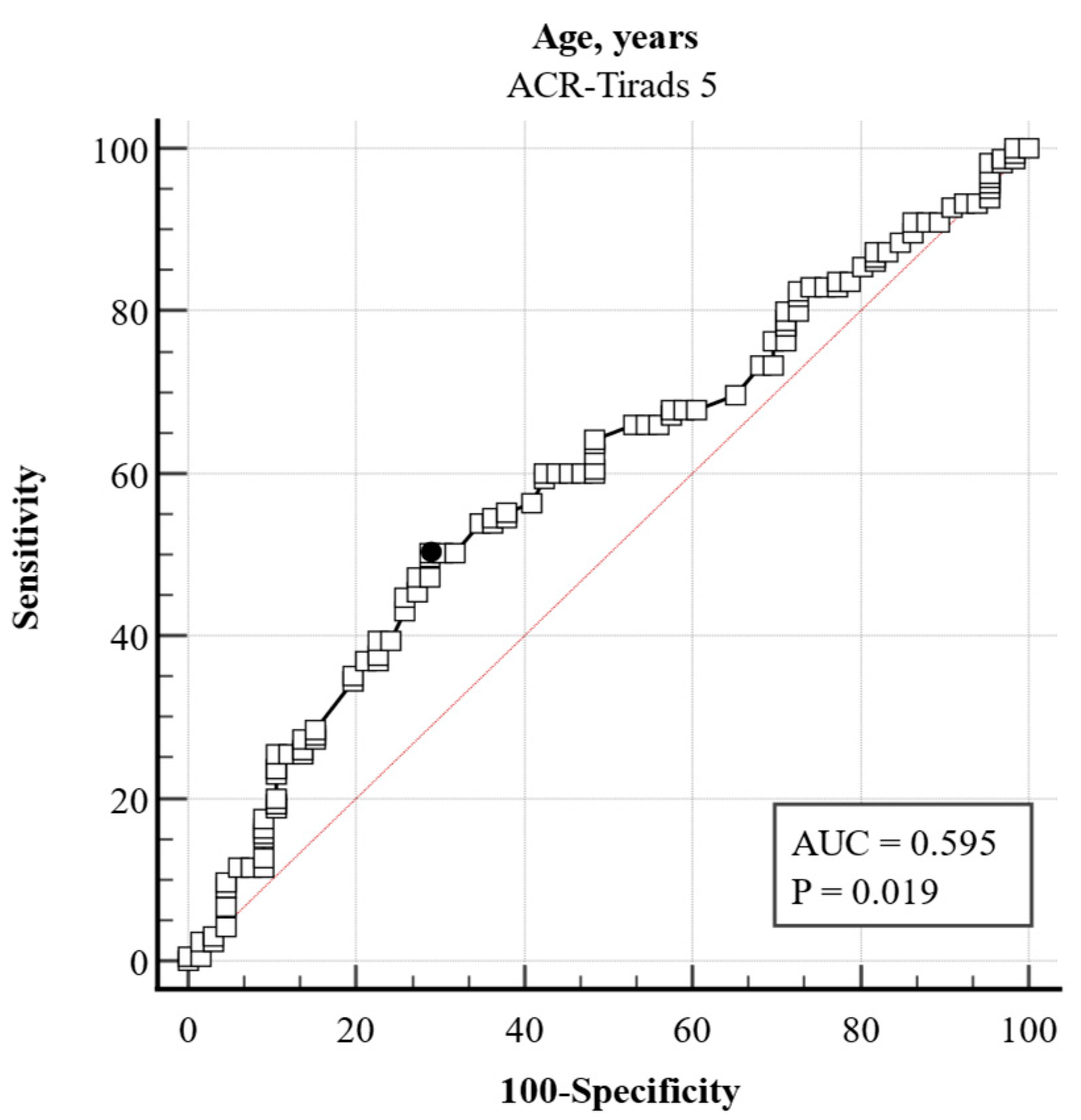
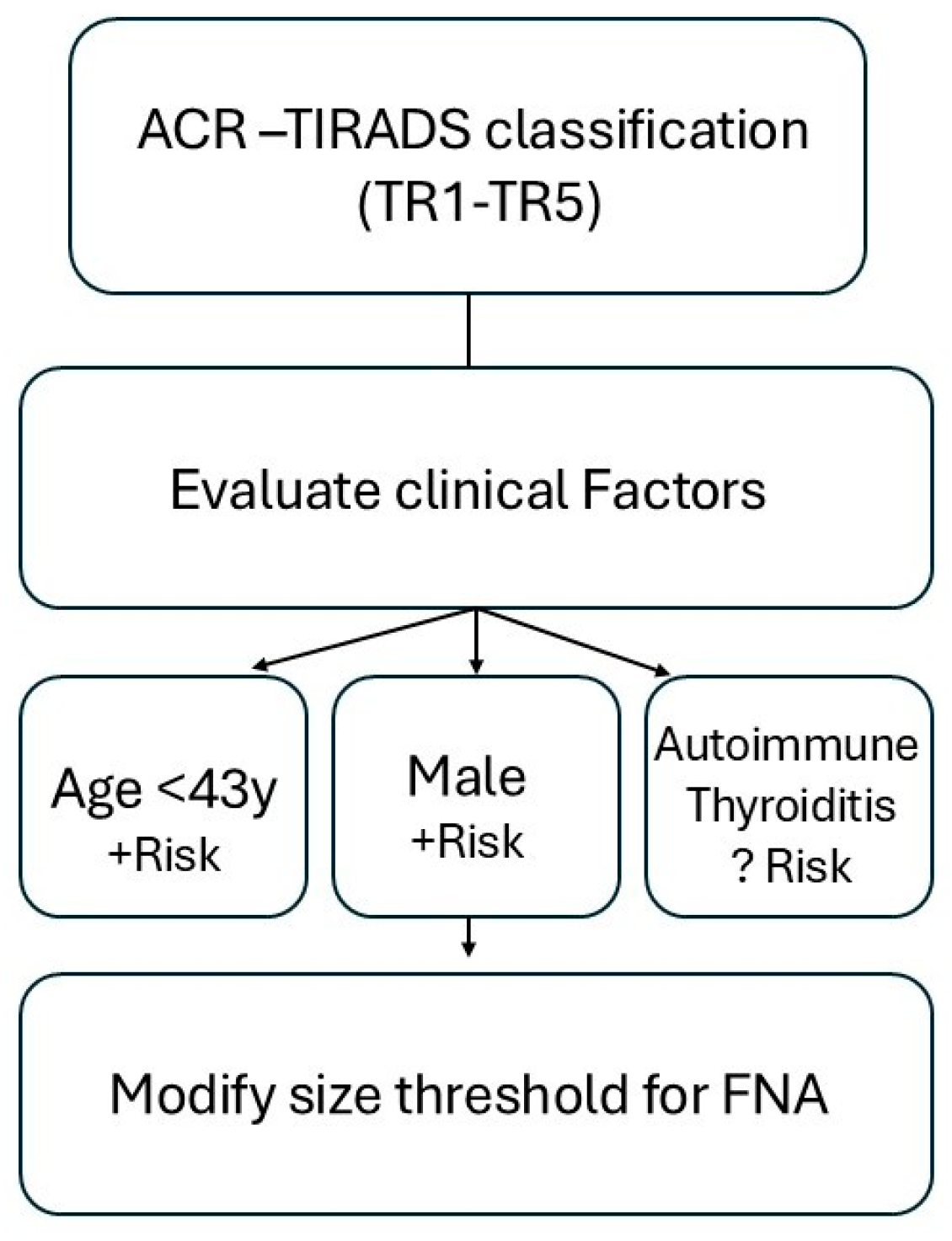
| Age, years (median, IQR) | 44 (35–55) | 50 (40–60) | −0.02 | 0.98 (0.97–0.99) | <0.001 | 0.98 (0.97–0.99) | <0.001 |
| Nodule size, mm | 13 (10.65–19.2) | 18 (13–24) | −0.06 | 0.94 (0.93–0.96) | <0.001 | 0.96 (0.95–0.99) | 0.01 |
| Male sex, N * | 92/324 (28.4) | 141/677 (20.8) | 0.38 | 1.47 (1.10–1.95) | 0.009 | 1.42 (0.99–2.01) | 0.051 |
| AT, n (%) | 92 (25.1) | 177 (23.3) | 0.504 | ||||
| Hypothyroidism, n (%) | 112 (30.5) | 255 (33.5) | 0.315 | ||||
| BMI, kg/m2 | 27.00 (23.6–32.42) | 27.34 (23.92–31.96) | 0.814 | ||||
| Coefficient B | Std. Error | z | OR | 95% CI | p | |
|---|---|---|---|---|---|---|
| Age, years | −0.03 | 0.01 | 4.19 | 0.97 | 0.96–0.98 | <0.001 |
| Hypothyroidism | −0.52 | 0.24 | 2.18 | 0.6 | 0.37–0.95 | 0.029 |
| Male sex | 0.14 | 0.24 | 0.58 | 1.15 | 0.72–1.83 | 0.564 |
| BMI, kg/m2 | 0.02 | 0.02 | 0.92 | 1.02 | 0.98–1.05 | 0.359 |
| AT | −0.19 | 0.26 | 0.72 | 0.83 | 0.5–1.38 | 0.472 |
| Nodule size, mm | 0.01 | 0.01 | 0.84 | 1.01 | 0.98–1.04 | 0.401 |
| Variable | Coefficient | Std. Error | Wald | OR | 95% CI | p |
|---|---|---|---|---|---|---|
| Nodule size, mm | 0.16250 | 0.049951 | 10.5829 | 1.176 | 1.066–1.297 | 0.001 |
| Variable | Coefficient | Std. Error | Wald | OR | 95% CI | p |
|---|---|---|---|---|---|---|
| Age | −0.043998 | 0.011991 | 13.4629 | 0.9570 | 0.934–0.979 | 0.001 |
| Hypothyroidism | −0.98317 | 0.39491 | 6.1981 | 0.3741 | 0.172–0.811 | 0.012 |
| Variable | Coefficient | Std. Error | Wald | Odds Ratio | 95% CI | p |
|---|---|---|---|---|---|---|
| Age | −0.020841 | 0.010382 | 4.0295 | 0.9794 | 0.9596–0.9995 | 0.044 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelopoulos, N.; Androulakis, I.; Askitis, D.P.; Valvis, N.; Paparodis, R.D.; Petkova, V.; Boniakos, A.; Zianni, D.; Rizoulis, A.; Bantouna, D.; et al. Integrating Clinical Parameters into Thyroid Nodule Malignancy Risk: A Retrospective Evaluation Based on ACR TI-RADS. J. Clin. Med. 2025, 14, 5352. https://doi.org/10.3390/jcm14155352
Angelopoulos N, Androulakis I, Askitis DP, Valvis N, Paparodis RD, Petkova V, Boniakos A, Zianni D, Rizoulis A, Bantouna D, et al. Integrating Clinical Parameters into Thyroid Nodule Malignancy Risk: A Retrospective Evaluation Based on ACR TI-RADS. Journal of Clinical Medicine. 2025; 14(15):5352. https://doi.org/10.3390/jcm14155352
Chicago/Turabian StyleAngelopoulos, Nikolaos, Ioannis Androulakis, Dimitrios P. Askitis, Nicolas Valvis, Rodis D. Paparodis, Valentina Petkova, Anastasios Boniakos, Dimitra Zianni, Andreas Rizoulis, Dimitra Bantouna, and et al. 2025. "Integrating Clinical Parameters into Thyroid Nodule Malignancy Risk: A Retrospective Evaluation Based on ACR TI-RADS" Journal of Clinical Medicine 14, no. 15: 5352. https://doi.org/10.3390/jcm14155352
APA StyleAngelopoulos, N., Androulakis, I., Askitis, D. P., Valvis, N., Paparodis, R. D., Petkova, V., Boniakos, A., Zianni, D., Rizoulis, A., Bantouna, D., Jaume, J. C., & Livadas, S. (2025). Integrating Clinical Parameters into Thyroid Nodule Malignancy Risk: A Retrospective Evaluation Based on ACR TI-RADS. Journal of Clinical Medicine, 14(15), 5352. https://doi.org/10.3390/jcm14155352





