Topical Use of Tacrolimus in Corneal and Ocular Surface Pathologies: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Ethics and Registration
2.2. Search Strategy
2.3. Criteria for Literature Selection
- Randomized controlled trials (RCTs);
- Written in English;
- Human trials;
- Topical administration of the drug;
- Well-defined primary and secondary outcomes;
- Clear primary and/or secondary outcome data.
- Non-RCTs, reviews, observational studies, case reports, and editorial publications;
- Articles with only published abstracts;
- Non-English language articles;
- Non-human trials;
- Systemic administration of the drug;
- Ambiguous, unmentioned, or absent primary and/or secondary outcomes;
- Studies with unclear and poorly presented data.
2.4. Study Selection
2.5. Data Collection and Extraction
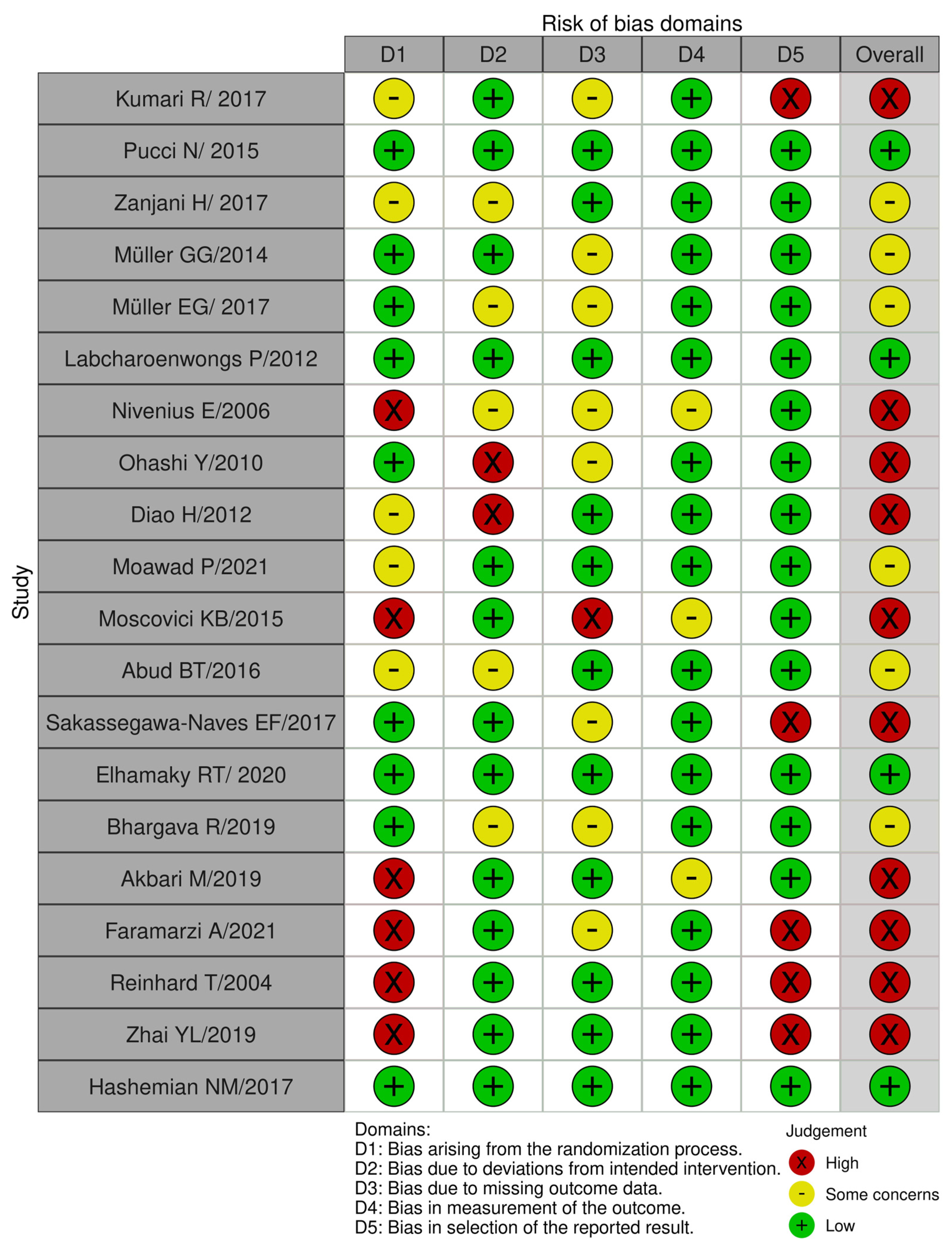
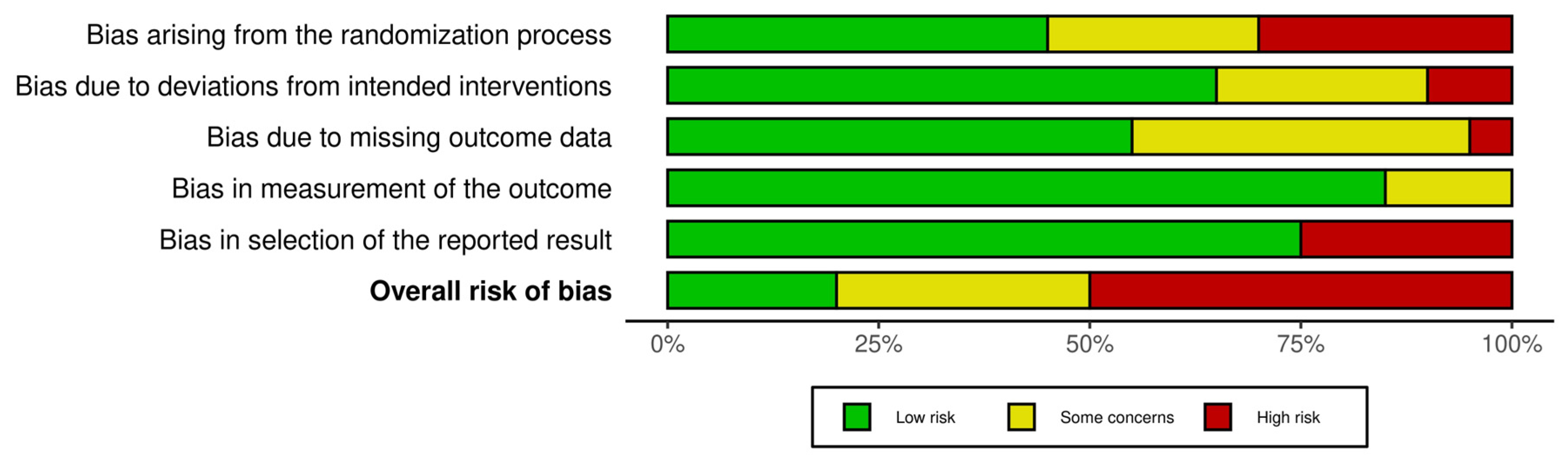
2.6. Risk-of-Bias Assessment
2.7. Synthesis Methods
3. Results
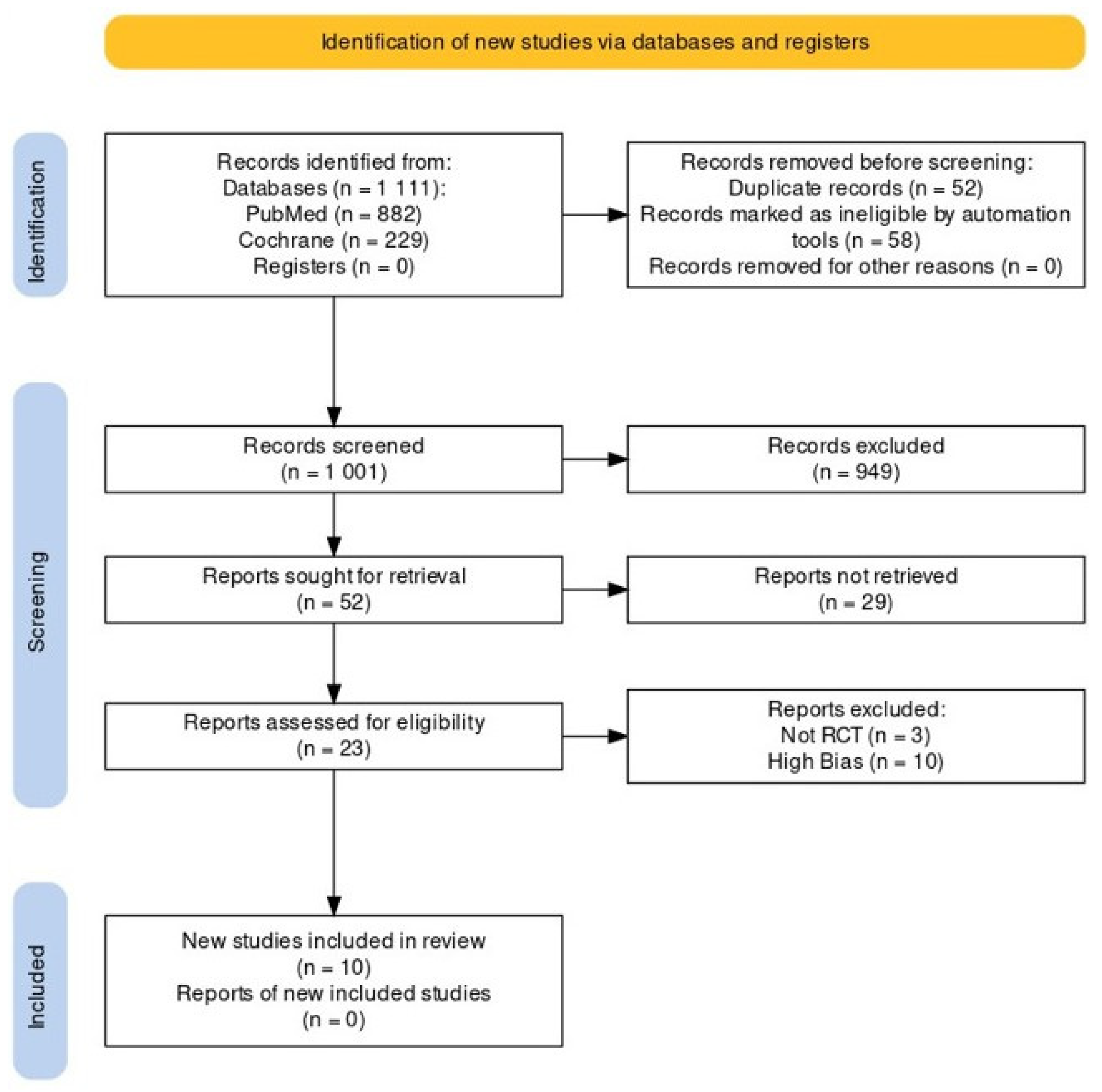
3.1. Search Results
3.2. Basic Data of the Included Studies
3.3. Assessment of Risk of Bias in the Included Studies
3.4. Summary of Findings and Qualitative Analysis of Results
- i.
- Vernal Keratoconjunctivitis (VKC)
- ii.
- Dry eye secondary to Sjögren syndrome
- iii.
- Ocular GVHD (Graft-Versus-Host-Disease)
- iv.
- Adenoviral corneal subepithelial infiltrates (SEIs)
- v.
- Acute endothelial graft rejection after PKP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kino, T.; Hatanaka, H.; Hashimoto, M.; Nishiyama, M.; Goto, T.; Okuhara, M.; Kohsaka, M.; Aoki, H.; Imanaka, H. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J. Antibiot. 1987, 40, 1249–1255. [Google Scholar] [CrossRef]
- Parsons, W.H.; Sigal, N.H.; Wyvratt, M.J. FK-506–A novel immunosuppressant. Ann. N. Y. Acad. Sci. 1993, 685, 22–36. [Google Scholar] [CrossRef]
- Zhai, J.; Gu, J.; Yuan, J.; Chen, J. Tacrolimus in the treatment of ocular diseases. BioDrugs 2011, 25, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, T.; Assmann, T.; Homey, B. Tacrolimus: The drug for the turn of the millennium? Arch. Dermatol. 1999, 135, 574–580. [Google Scholar] [CrossRef]
- de Paulis, A.; Stellato, C.; Cirillo, R.; Ciccarelli, A.; Oriente, A.; Marone, G. Anti-inflammatory effect of FK-506 on human skin mast cells. J. Investig. Dermatol. 1992, 99, 723–728. [Google Scholar] [CrossRef]
- Shapiro, R.; Fung, J.J.; Jain, A.B.; Parks, P.; Todo, S.; Starzl, T.E. The side effects of FK 506 in humans. Transplant. Proc. 1990, 22, 35–36. [Google Scholar]
- Bertelmann, E.; Pleyer, U. Immunomodulatory therapy in ophthalmology-is there a place for topical application? Ophthalmologica 2004, 218, 359–367. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Pucci, N.; Caputo, R.; di Grande, L.; de Libero, C.; Mori, F.; Barni, S.; di Simone, L.; Calvani, A.; Rusconi, F.; Novembre, E. Tacrolimus vs. cyclosporine eyedrops in severe cyclosporine-resistant vernal keratoconjunctivitis: A randomized, comparative, double-blind, crossover study. Pediatr. Allergy Immunol. 2015, 26, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Zanjani, H.; Aminifard, M.N.; Ghafourian, A.; Pourazizi, M.; Maleki, A.; Arish, M.; Shahrakipoor, M.; Rohani, M.R.; Abrishami, M.; Zare, E.K.; et al. Comparative Evaluation of Tacrolimus Versus Interferon Alpha-2b Eye Drops in the Treatment of Vernal Keratoconjunctivitis: A Randomized, Double-Masked Study. Cornea 2017, 36, 675–678. [Google Scholar] [CrossRef]
- Müller, G.G.; Kara José, N.; de Castro, R.S. Topical tacrolimus 0.03% as sole therapy in vernal keratoconjunctivitis: A randomized double-masked study. Eye Contact Lens. 2014, 40, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Müller, E.G.; Santos, M.S.; Freitas, D.; Gomes, J.Á.; Belfort, R. Tacrolimus eye drops as monotherapy for vernal keratoconjunctivitis: A randomized controlled trial. Arq. Bras. Oftalmol. 2017, 80, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Labcharoenwongs, P.; Jirapongsananuruk, O.; Visitsunthorn, N.; Kosrirukvongs, P.; Saengin, P.; Vichyanond, P. A double-masked comparison of 0.1% tacrolimus ointment and 2% cyclosporine eye drops in the treatment of vernal keratoconjunctivitis in children. Asian Pac. J. Allergy Immunol. 2012, 30, 177–184. [Google Scholar]
- Moawad, P.; Shamma, R.; Hassanein, D.; Ragab, G.; El Zawahry, O. Evaluation of the effect of topical tacrolimus 0.03% versus cyclosporine 0.05% in the treatment of dry eye secondary to Sjogren syndrome. Eur. J. Ophthalmol. 2022, 32, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Abud, T.B.; Amparo, F.; Saboo, U.S.; Di Zazzo, A.; Dohlman, T.H.; Ciolino, J.B.; Hamrah, P.; Dana, R. A Clinical Trial Comparing the Safety and Efficacy of Topical Tacrolimus versus Methylprednisolone in Ocular Graft-versus-Host Disease. Ophthalmology 2016, 123, 1449–1457. [Google Scholar] [CrossRef]
- Elhamaky, T.R. Pentacam corneal densitometry-guided treatment of adenoviral corneal subepithelial infiltrates: A comparative study between transepithelial phototherapeutic keratectomy and topical tacrolimus. Int. Ophthalmol. 2021, 41, 67–77. [Google Scholar] [CrossRef]
- Bhargava, R.; Kumar, P. Comparison of the safety and efficacy of topical Tacrolimus (0.03%) versus dexamethasone (0.05%) for subepithelial infiltrates after adenoviral conjunctivitis. Indian J. Ophthalmol. 2019, 67, 594–598. [Google Scholar] [CrossRef]
- Hashemian, M.N.; Latifi, G.; Ghaffari, R.; Ghassemi, H.; Zarei-Ghanavati, M.; Mohammadi, S.F.; Yasseri, M.; Tafti, M.R.; Tafti, Z.F. Topical Tacrolimus as Adjuvant Therapy to Corticosteroids in Acute Endothelial Graft Rejection After Penetrating Keratoplasty: A Randomized Controlled Trial. Cornea 2018, 37, 307–312. [Google Scholar] [CrossRef]
- Kumari, R.; Saha, B.C.; Sinha, B.P.; Mohan, N. Tacrolimus versus Cyclosporine- Comparative Evaluation as First line drug in Vernal keratoconjuctivitis. Nepal. J. Ophthalmol. 2017, 9, 128–135. [Google Scholar] [CrossRef]
- Nivenius, E.; van der Ploeg, I.; Jung, K.; Chryssanthou, E.; van Hage, M.; Montan, P.G. Tacrolimus ointment vs steroid ointment for eyelid dermatitis in patients with atopic keratoconjunctivitis. Eye 2007, 21, 968–975. [Google Scholar] [CrossRef]
- Ohashi, Y.; Ebihara, N.; Fujishima, H.; Fukushima, A.; Kumagai, N.; Nakagawa, Y.; Namba, K.; Okamoto, S.; Shoji, J.; Takamura, E.; et al. A randomized, placebo-controlled clinical trial of tacrolimus ophthalmic suspension 0.1% in severe allergic conjunctivitis. J. Ocul. Pharmacol. Ther. 2010, 26, 165–174. [Google Scholar] [CrossRef]
- Diao, H.; She, Z.; Cao, D.; Wang, Z.; Lin, Z. Comparison of tacrolimus, fluorometholone, and saline in mild-to-moderate contact lens-induced papillary conjunctivitis. Adv. Ther. 2012, 29, 645–653. [Google Scholar] [CrossRef]
- Moscovici, B.K.; Holzchuh, R.; Sakassegawa-Naves, F.E.; Hoshino-Ruiz, D.R.; Albers, M.B.; Santo, R.M.; Hida, R.Y. Treatment of Sjögren’s syndrome dry eye using 0.03% tacrolimus eye drop: Prospective double-blind randomized study. Contact Lens Anterior Eye 2015, 38, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Sakassegawa-Naves, F.E.; Ricci, H.M.M.; Moscovici, B.K.; Miyamoto, D.A.; Chiacchio, B.B.; Holzchuh, R.; Santo, R.M.; Hida, R.Y. Tacrolimus Ointment for Refractory Posterior Blepharitis. Curr. Eye Res. 2017, 42, 1440–1444. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Soltani Moghadam, R.; Elmi, R.; Nosrati, A.; Taghiabadi, E.; Aghdami, N. Topical Tacrolimus as an adjunct to Conventional Therapy for Stromal Herpetic Keratitis: A Randomized Clinical Trial. J. Ophthalmic Vis. Res. 2019, 14, 400–411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faramarzi, A.; Abbasi, H.; Feizi, S.; Hadi, Y.; Azari, A.A.; Karimian, F.; Jafarinasab, M.R.; Kheiri, B. Topical 0.03% tacrolimus versus systemic mycophenolate mofetil as adjuncts to systemic corticosteroids for preventing graft rejection after repeat keratoplasty: One-year results of a randomized clinical trial. Eye 2021, 35, 2879–2888. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reinhard, T.; Mayweg, S.; Reis, A.; Sundmacher, R. Topical FK506 as immunoprophylaxis after allogeneic penetrating normal-risk keratoplasty: A randomized clinical pilot study. Transpl. Int. 2005, 18, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.Y.; Zhang, X.R.; Liu, H.; Ma, Y.; Xu, H.C. Observation of topical tacrolimus on high-risk penetrating keratoplasty patients: A randomized clinical trial study. Eye 2020, 34, 1600–1607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakagawa, H. Comparison of the efficacy and safety of 0.1% tacrolimus ointment with topical corticosteroids in adult patients with atopic dermatitis: Review of randomised, double-blind clinical studies conducted in Japan. Clin. Drug Investig. 2006, 26, 235–246. [Google Scholar] [CrossRef]
- Rasmussen, M.L.; Schou, M.G.; Bach-Holm, D.; Heegaard, S.; Jørgensen, C.A.; Kessel, L.; Wiencke, A.K.; Subhi, Y. Comparative efficacy of medical treatments for vernal keratoconjunctivitis in children and young adults: A systematic review with network meta-analyses. Acta Ophthalmol. 2022, 100, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; He, F.; Yang, Y.; Lin, W.; Qiu, W.; Meng, Q.; Zhang, J.; Zhou, Z. Therapeutic efficacy of tacrolimus in vernal keratoconjunctivitis: A meta-analysis of randomised controlled trials. Eur. J. Hosp. Pharm. 2022, 29, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Li, A.P.; Pan, Z.Q.; Luo, F.; Zou, L.H. Systematic review of penetrating keratoplasty rejection treated by immunosuppressants. Zhonghua Yan Ke Za Zhi 2010, 46, 1122–1127. [Google Scholar]
- Abudou, M.; Wu, T.; Evans, J.R.; Chen, X. Immunosuppressants for the prophylaxis of corneal graft rejection after penetrating keratoplasty. Cochrane Database Syst. Rev. 2015, 27, CD007603. [Google Scholar] [CrossRef] [PubMed]
| Author/Year | Country | Study Type | Patient Size | Age (T/C) | Condition |
|---|---|---|---|---|---|
| Pucci N/2015 [10] | Italy | RCT cros | 30/30 + 25/25 (part2) (eyes of total 30 and later 25 patients) | 9.05 ± 2.12 | Cyclosporine-resistant VKC |
| Zanjani H/2017 [11] | Iran | RCT | 20/20 | 10.9 ± 3.4/ 11.3 ± 5.4 | Resistant VKC |
| Müller GG/2014 [12] | Brazil | RCT pros | 11/10 | 10.8 ± 5.2/10 ± 2.8 | Refractory VKC |
| Müller EG/2017 [13] | Brazil | RCT pros | 8/8 | 10.8 ± 2.4/12.5 ± 2.3 | VKC |
| Labcharoenwongs P/2012 [14] | Thailand | RCT pros | 12/12 | 10.14 ± 2.60/9.07 ± 2.50 | VKC |
| Moawad P/2021 [15] | Egypt | RCT | 30/30 | 49.4 ± 12.92/44.9 ± 12.58 | Dry eye secondary to Sjögren syndrome |
| Abud BT/2016 [16] | USA | RCT pros | 24/16 | 54 ± 12/58 ± 11 | Ocular GVHD |
| Elhamaky RT/2020 [17] | Egypt | RCT pros | 21/22/20 (eyes), 12/12/11 (patients) | 36 ± 11.2/36.2 ± 10.2/38.2 ± 10.2 | Adenoviral corneal SEIs |
| Bhargava R/2019 [18] | India | RCT | 40/40 | 26 ± 4.1/25.4 ± 3.7 | Adenoviral corneal SEIs |
| Hashemian NM/2017 [19] | Iran | RCT pros | 17/14 (eyes and patients) | 46.3 ± 20.5/36.3 ± 15.7 | Acute endothelial graft rejection after PKP |
| Author/Year | Interventions (T/C) | Drug Conc. (T/C) | Galenic (T/C) | Frequency of Adminis. (T/C) | Duration | Re-Evaluations | Outcome Measures |
|---|---|---|---|---|---|---|---|
| Pucci N/2015 [10] | Tacrolimus/ cyclosporine A | 0.1%/1% | e-d/e-d | One drop/time, 3 times daily | 3 (+1) + 3 W (2 parts) | 0 W, 3 W, 7 W | Signs, symptoms, quality of life score, side effects |
| Zanjani H/2017 [11] | Tacrolimus +placebo/ interferon α-2b + placebo | 0.005%/ 1,000,000 IU/mL | e-d/e-d | Two drops/time | NA (2 M?) | 0 W, 2 W, 1 M, 2 M | Signs, symptoms, side effects |
| Müller GG/2014 [12] | Tacrolimus + placebo/tacrolimus + olopatadine | 0.03%/0.03% + 0.1% | oint/oint + e/d | Twice daily | 30 d | 0 W, 30 d | Signs, symptoms, clinical impression of the progress, self-assessment, safety, side effects |
| Müller EG/2017 [13] | Tacrolimus/sodium cromoglycate | 0.03%/4% | e-d/e-d | 3 times daily | 90 d | 0 d, 15 d, 30 d, 45 d, 90 d | Signs, symptoms, safety, side effects |
| Labcharoenwongs P/2012 [14] | Tacrolimus + placebo/cyclosporine + placebo | 0.1%/2% | oint/e-d | Twice daily + 4 times daily/ 4 times daily + twice daily | 8 W | 0 W, 2 W, 4 W, 8 W | Symptoms, signs, side effects |
| Moawad P/2021 [15] | Tacrolimus +placebo/ cyclosporine +placebo | 0.03%/0.05% | e-d/e-d | Twice daily | 6 M | 0 d, 90 d, 180 d | Symptoms, frequency of artificial tears, TBUT, staining scores, Schirmer, meibum quality and expressibility |
| Abud BT/2016 [16] | Tacrolimus/methylprednisolone | 0.05%/0.5% | e-d/e-d | Twice daily | 10 W | 0 W, 5 W, 10 W | Side effects, symptoms, staining scores, TBUT, Schirmer, HLA-DR, ICAM-1 |
| Elhamaky RT/2020 [17] | Tacrolimus/Te-PTK with MMC/control group | 0.03%/0.02% (MMC) | oint/surgery | Once daily | 2–6 M | 0 W, 1 W, 1 M, 3 M, 6 M, 12 M | Changes in corneal densitometry, changes in corneal HOA, subjective evaluations of effectiveness, objective evaluations of effectiveness |
| Bhargava R/2019 [18] | Tacrolimus/Dexamethasone | 0.03%/0.05% | oint/oint | Twice daily | 6 M | (side effects 1 M, 3 M, 6 M), (IOP 1 M, 2 M, 3 M, 4 M, 5 M, 6 M) | Symptom score, SEI score, VA, IOP, side effects |
| Hashemian NM/2017 [19] | Tacrolimus +Prednisolone/Placebo+ Prednisolone | 0.05% | e-d/e-d | 4 times daily until rejection reversal and then 0.01% every 6 h | Until rejection reversal and then for 3 months | 1 d, 3 d, 1 w, and every 3 to 5 days until reversal. Then every 2 w until 2 M, monthly for 3 M, and every 3 M thereafter. | Rejection reversal or treatment failure, time to rejection reversal, recurrence of rejection |
| Study | Patient Population | Condition Treated | Key Findings |
|---|---|---|---|
| Pucci N et al. (2015) [10] | 30 patients | Cyclosporine-resistant VKC | Significant improvement in signs and symptoms |
| Zanjani H et al. (2017) [11] | 40 patients | Resistant VKC | Effective as InterferonA-2b |
| Muller GG et al. (2014) [12] | 21 patients | Refractory VKC | No significant difference with the addition of olopatadine |
| Muller EG et al. (2017) [13] | 16 patients | VKC | Superior to sodium cromoglycate |
| Labcharoenwongs P et al. (2012) [14] | 24 patients | VKC | Effective as cyclosporine |
| Moawad P et al. (2021) [15] | 60 patients | Dry eye secondary to Sjogren syndrome | Improved symptoms and signs similar to cyclosporine |
| Abud BT et al. (2016) [16] | 40 patients | Ocular GVHD | Tacrolimus more effective than methylprednisolone in reducing corneal staining |
| Elhamaky RT (2020) [17] | 35 patients | Adenoviral corneal SEIs | Tacrolimus and Te-PTK both effective in reducing corneal densitometry and aberrations |
| Bhargava R et al. (2019) [18] | 80 patients | Adenoviral corneal SEIs | Tacrolimus more effective than dexamethasone in improving SEI and symptoms |
| Hashemian NM et al. (2017) [19] | 31 patients | Acute endothelial graft rejection after PKP | Tacrolimus with prednisolone significantly reduced time to rejection reversal and recurrence of rejection episodes |
| Study Reference | Condition Treated | Comparison | Side Effects in Tacrolimus Group | Side Effects in Control Group |
|---|---|---|---|---|
| Pucci N et al. (2015) [10] | Cyclosporine-resistant VKC | TAC 0.1% vs. Cyclosporine | Burning sensation, stinging, pain on administration | Burning sensation, stinging, pain on administration |
| Zanjani H et al. (2017) [11] | Resistant VKC | TAC 0.005% vs. InterferonA-2b | No significant side effects observed | No significant side effects observed |
| Muller GG et al. (2014) [12] | Refractory VKC | TAC 0.03% vs. TAC 0.03% + Olopatadine | Burning sensation (in 80% of patients) | Burning sensation (in 80% of patients) |
| Muller EG et al. (2017) [13] | VKC | TAC 0.03% vs. Sodium cromoglycate | Burning sensation | No significant side effects observed |
| Labcharoenwongs P et al. (2012) [14] | VKC | TAC 0.1% vs. Cyclosporine | Burning sensation, declining course that became non-significant | Burning sensation, declining course that became non-significant |
| Moawad P et al. (2021) [15] | Dry eye secondary to Sjogren syndrome | TAC 0.03% vs. Cyclosporine | No significant side effects observed | No significant side effects observed |
| Abud BT et al. (2016) [16] | Ocular GVHD | TAC 0.05% vs. Methylprednisolone | Burning sensation | Increased IOP |
| Elhamaky RT (2020) [17] | Adenoviral corneal SEIs | TAC 0.03% vs. Te-PTK with MMC | Burning sensation, foreign body sensation | Burning sensation, foreign body sensation |
| Bhargava R et al. (2019) [18] | Adenoviral corneal SEIs | TAC 0.03% vs. Dexamethasone | Burning sensation | Increased IOP |
| Hashemian NM et al. (2017) [19] | Acute endothelial graft rejection after PKP | TAC 0.05% + Prednisolone vs. Prednisolone alone | No significant side effects observed | No significant side effects observed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katonis, G.; Tzamalis, A.; Tsinopoulos, I.; Ziakas, N. Topical Use of Tacrolimus in Corneal and Ocular Surface Pathologies: A Systematic Review. J. Clin. Med. 2025, 14, 5347. https://doi.org/10.3390/jcm14155347
Katonis G, Tzamalis A, Tsinopoulos I, Ziakas N. Topical Use of Tacrolimus in Corneal and Ocular Surface Pathologies: A Systematic Review. Journal of Clinical Medicine. 2025; 14(15):5347. https://doi.org/10.3390/jcm14155347
Chicago/Turabian StyleKatonis, Georgios, Argyrios Tzamalis, Ioannis Tsinopoulos, and Nikolaos Ziakas. 2025. "Topical Use of Tacrolimus in Corneal and Ocular Surface Pathologies: A Systematic Review" Journal of Clinical Medicine 14, no. 15: 5347. https://doi.org/10.3390/jcm14155347
APA StyleKatonis, G., Tzamalis, A., Tsinopoulos, I., & Ziakas, N. (2025). Topical Use of Tacrolimus in Corneal and Ocular Surface Pathologies: A Systematic Review. Journal of Clinical Medicine, 14(15), 5347. https://doi.org/10.3390/jcm14155347