Viability Test in Prediction of Response to Cardiac Resynchronization Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Nuclear Medicine Imaging, Data Reconstruction, and Image Analysis
2.2. Echocardiography
2.3. CRT Implantation
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HF | Heart failure |
| LVEF | Left ventricular ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| CRT | Cardiac resynchronization therapy |
| SPECT | Single-photon emission tomography |
| MPI | Myocardial perfusion imaging |
| MACE | Major adverse cardiovascular event |
| EDV | End-diastolic volume |
| ESV | End-systolic volume |
| SRS | Summed rest score |
| OR | Odds ratio |
| ROC curve | Receiver operating characteristic curve |
References
- Bax, J.J.; Delgado, V. Myocardial viability as integral part of the diagnostic and therapeutic approach to ischemic heart failure. J. Nucl. Cardiol. 2015, 22, 229–245. [Google Scholar] [CrossRef]
- Xu, Y.Z.; Cha, Y.M.; Feng, D.; Powell, B.D.; Wiste, H.J.; Hua, W.; Chareonthaitawee, P. Impact of myocardial scarring on outcomes of cardiac resynchronization therapy: Extent or location? J. Nucl. Med. 2012, 53, 47–54. [Google Scholar] [CrossRef]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Auricchio, A.; Stellbrink, C.; Sack, S.; Block, M.; Vogt, J.; Bakker, P.; Huth, C.; Schöndube, F. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J. Am. Coll. Cardiol. 2002, 39, 2026–2033. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002, 105, 539–542. [Google Scholar]
- Ypenburg, C.; Schalij, M.J.; Bleeker, G.B.; Steendijk, P.; Boersma, E.; Dibbets-Schneider, P.; Stokkel, M.P.; van der Wall, E.E.; Bax, J.J. Extent of viability to predict response to cardiac resynchronization therapy in ischemic heart failure patients. J. Nucl. Med. 2006, 47, 1565–1570. [Google Scholar]
- Albuquerque, F.; Oliveira, A.F.; de Araújo Gonçalves, P.; Campante Teles, R.; de Sousa Almeida, M.; Gonçalves, M.; Lopes, P.M.; Cunha, G.J.L.; Presume, J.; Matos, D.; et al. Predicting obstructive coronary artery disease in heart failure with reduced ejection fraction: A practical clinical score. Rev. Port. Cardiol. 2023, 42, 21–28. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Roger, V.L. Epidemiology of heart failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Siddiqui, A.; Tasouli-Drakou, V.; Ringor, M.; DiCaro, M.V.; Yee, B.; Lei, K.; Tak, T. Recent Advances in Cardiac Resynchronization Therapy: Current Treatment and Future Direction. J. Clin. Med. 2025, 14, 889. [Google Scholar] [CrossRef]
- Auger, D.; Schalij, M.J.; Bax, J.J.; Delgado, V. Three-dimensional imaging in cardiac resynchronization therapy. Rev. Esp. Cardiol. 2011, 64, 1035–1044. [Google Scholar] [CrossRef]
- Cleland, J.G.; Daubert, J.C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L.; Cardiac Resynchronization-HeartFailure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N. Engl. J. Med. 2005, 352, 1539–1549. [Google Scholar] [CrossRef]
- Abraham, W.T.; Fisher, W.G.; Smith, A.L.; Delurgio, D.B.; Leon, A.R.; Loh, E.; Kocovic, D.Z.; Packer, M.; Clavell, A.L.; Hayes, D.L.; et al. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N. Engl. J. Med. 2002, 346, 1845–1853. [Google Scholar] [CrossRef]
- Bristow, M.R.; Saxon, L.A.; Boehmer, J.; Krueger, S.; Kass, D.A.; De Marco, T.; Carson, P.; DiCarlo, L.; DeMets, D.; White, B.G.; et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N. Engl. J. Med. 2004, 350, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.M.; Sanderson, J.E.; Gorcsan, J., 3rd. Echocardiography, dyssynchrony, and the response to cardiac resynchronization therapy. Eur. Heart J. 2010, 31, 2326–2337. [Google Scholar] [CrossRef]
- Sade, L.E.; Demir, O.; Atar, I.; Müderrisoglu, H.; Ozin, B. Effect of mechanical dyssynchrony and cardiac resynchronization therapy on left ventricular rotational mechanics. Am. J. Cardiol. 2008, 101, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.A.; Goodfield, N.E.R. Mechanical dyssynchrony and super-response to CRT. J. Nucl. Cardiol. 2022, 29, 1175–1177. [Google Scholar] [CrossRef] [PubMed]
- Rath, B.; Willy, K.; Wolfes, J.; Ellermann, C.; Reinke, F.; Köbe, J.; Eckardt, L.; Frommeyer, G. Predictors of response to cardiac resynchronization therapy in patients with chronic right ventricular pacing. Clin. Res. Cardiol. 2021, 110, 877–883. [Google Scholar] [CrossRef]
- Morishima, I.; Okumura, K.; Tsuboi, H.; Morita, Y.; Takagi, K.; Yoshida, R.; Nagai, H.; Tomomatsu, T.; Ikai, Y.; Terada, K.; et al. Impact of basal inferolateral scar burden determined by automatic analysis of 99mTc-MIBI myocardial perfusion SPECT on the long-term prognosis of cardiac resynchronization therapy. Europace 2017, 19, 573–580. [Google Scholar] [CrossRef]
- Harb, S.C.; Toro, S.; Bullen, J.A.; Obuchowski, N.A.; Xu, B.; Trulock, K.M.; Varma, N.; Rickard, J.; Grimm, R.; Griffin, B.; et al. Scar burden is an independent and incremental predictor of cardiac resynchronisation therapy response. Open Heart 2019, 6, e001067. [Google Scholar] [CrossRef]
- Arzola-Castaner, D.; Taub, C.; Kevin Heist, E.; Fan, D.; Haelewyn, K.; Mela, T.; Picard, M.H.; Ruskin, J.N.; Singh, J.P. Left ventricular lead proximity to an akinetic segment and impact on outcome of cardiac resynchronization therapy. J. Cardiovasc. Electrophysiol. 2006, 17, 623–627. [Google Scholar] [CrossRef]
- Adelstein, E.C.; Tanaka, H.; Soman, P.; Miske, G.; Haberman, S.C.; Saba, S.F.; Gorcsan, J., 3rd. Impact of scar burden by single-photon emission computed tomography myocardial perfusion imaging on patient outcomes following cardiac resynchronization therapy. Eur. Heart J. 2011, 32, 93–103. [Google Scholar] [CrossRef]
- Mele, D.; Luisi, G.A.; Malagù, M.; Laterza, A.; Ferrari, R.; Bertini, M. Echocardiographic evaluation of cardiac dyssynchrony: Does it still matter? Echocardiography 2018, 35, 707–715. [Google Scholar] [CrossRef]
- Bordachar, P.; Lafitte, S.; Réant, P.; Reuter, S.; Clementy, J.; Mletzko, R.U.; Siegel, R.M.; Goscinska-Bis, K.; Bowes, R.; Morgan, J.; et al. Low value of simple echocardiographic indices of ventricular dyssynchrony in predicting the response to cardiac resynchronization therapy. Eur. J. Heart Fail. 2010, 12, 588–592. [Google Scholar] [CrossRef]
- Raisi-Estabragh, Z.; Kenawy, A.A.M.; Aung, N.; Cooper, J.; Munroe, P.B.; Harvey, N.C.; Petersen, S.E.; Khanji, M.Y. Variation in left ventricular cardiac magnetic resonance normal reference ranges: Systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 494–504. [Google Scholar] [CrossRef]
- Saeed, M.; Liu, H.; Liang, C.H.; Wilson, M.W. Magnetic resonance imaging for characterizing myocardial diseases. Int. J. Cardiovasc. Imaging 2017, 33, 1395–1414. [Google Scholar] [CrossRef]
- Chen, J.; Boogers, M.J.; Bax, J.J.; Soman, P.; Garcia, E.V. The use of nuclear imaging for cardiac resynchronization therapy. Curr. Cardiol. Rep. 2010, 12, 185–191. [Google Scholar] [CrossRef]
- Adelstein, E.C.; Saba, S. Scar burden by myocardial perfusion imaging predicts echocardiographic response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am. Heart J. 2007, 153, 105–112. [Google Scholar] [CrossRef]
- Bilchick, K.C.; Kuruvilla, S.; Hamirani, Y.S.; Ramachandran, R.; Clarke, S.A.; Parker, K.M.; Stukenborg, G.J.; Mason, P.; Ferguson, J.D.; Moorman, J.R.; et al. Impact of mechanical activation, scar, and electrical timing on cardiac resynchronization therapy response and clinical outcomes. J. Am. Coll. Cardiol. 2014, 63, 1657–1666. [Google Scholar] [CrossRef]
- Birnie, D.; DeKemp, R.A.; Ruddy, T.D.; Tang, A.S.; Guo, A.; Williams, K.; Wassenar, R.; Lalonde, M.; Beanlands, R.S. Effect of lateral wall scar on reverse remodeling with cardiac resynchronization therapy. Heart Rhythm 2009, 6, 1721–1726. [Google Scholar] [CrossRef]
- Bose, A.; Kandala, J.; Upadhyay, G.A.; Riedl, L.; Ahmado, I.; Padmanabhan, R.; Gewirtz, H.; Mulligan, L.J.; Singh, J.P. Impact of myocardial viability and left ventricular lead location on clinical outcome in cardiac resynchronization therapy recipients with ischemic cardiomyopathy. J. Cardiovasc. Electrophysiol. 2014, 25, 507–513. [Google Scholar] [CrossRef]
- Fallavollita, J.A.; Heavey, B.M.; Luisi, A.J., Jr.; Michalek, S.M.; Baldwa, S.; Mashtare, T.L., Jr.; Hutson, A.D.; Dekemp, R.A.; Haka, M.S.; Sajjad, M.; et al. Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J. Am. Coll. Cardiol. 2014, 63, 141–149. [Google Scholar] [CrossRef]
- Barra, S.; Boveda, S.; Providencia, R.; Sadoul, N.; Duehmke, R.; Reitan, C.; Borgquist, R. Adding defibrillation therapy to cardiac resynchronization on the basis of the myocardial substrate. J. Am. Coll. Cardiol. 2017, 69, 1669–1678. [Google Scholar] [CrossRef]
- Leyva, F.; Zegard, A.; Umar, F.; Taylor, R.J.; Acquaye, E.; Gubran, C.; Chalil, S.; Patel, K.; Panting, J.; Marshall, H.; et al. Long-term clinical outcomes of cardiac resynchronization therapy with or without defibrillation: Impact of the aetiology of cardiomyopathy. Europace 2018, 20, 1804–1812. [Google Scholar] [CrossRef]
- Chen, J.; Garcia, E.V.; Bax, J.J.; Iskandrian, A.E.; Borges-Neto, S.; Soman, P. SPECT myocardial perfusion imaging for the assessment of left ventricular mechanical dyssynchrony. J. Nucl. Cardiol. 2011, 18, 685–694. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aalen, J.M.; Donal, E.; Larsen, C.K.; Duchenne, J.; Lederlin, M.; Cvijic, M.; Hubert, A.; Voros, G.; Leclercq, C.; Bogaert, J.; et al. Imaging predictors of response to cardiac resynchronization therapy: Left ventricular work asymmetry by echocardiography and septal viability by cardiac magnetic resonance. Eur. Heart J. 2020, 41, 3813–3823. [Google Scholar] [CrossRef]
- Larsen, C.K.; Smiseth, O.A.; Duchenne, J.; Galli, E.; Aalen, J.M.; Lederlin, M.; Bogaert, J.; Kongsgaard, E.; Linde, C.; Penicka, M.; et al. Cardiac Magnetic Resonance Identifies Responders to Cardiac Resynchronization Therapy with an Assessment of Septal Scar and Left Ventricular Dyssynchrony. J. Clin. Med. 2023, 12, 7182. [Google Scholar] [CrossRef]
- Bois, J.P.; Scott, C.; Chareonthaitawee, P.; Gibbons, R.J.; Rodriguez-Porcel, M. Phase analysis single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) detects dyssynchrony in myocardial scar and increases specificity of MPI. EJNMMI Res. 2019, 9, 11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verschure, D.O.; Verberne, H.J. Gated SPECT MPI and CT venography fusion: A new approach for appropriate CRT-pacemaker lead placement? J. Nucl. Cardiol. 2021, 28, 1446–1448. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; de Amorim Fernandes, F.; do Nascimento, E.A.; Garcia, E.V.; Mesquita, C.T.; Zhou, W. Incremental value of left ventricular shape parameters measured by gated SPECT MPI in predicting the super-response to CRT. J. Nucl. Cardiol. 2022, 29, 1537–1546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aupongkaroon, P.; Makarawate, P.; Chaosuwannakit, N. Comparison of radiation dose and its correlates between coronary computed tomography angiography and invasive coronary angiography in Northeastern Thailand. Egypt. Heart J. 2022, 74, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Carli, M.F.; Murthy, V.L. Cardiac PET/CT for the evaluation of known or suspected coronary artery disease. Radiographics 2011, 31, 1239–1254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grozdic Milojevic, I.; Tadic, M.; Sobic-Saranovic, D.; Milojevic, B.; Artiko, V.M. Myocardial perfusion stress test: Is it worth? Int. J. Cardiovasc. Imaging 2020, 36, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Guo, N.; Ge, Y.; Zhang, L.; Oudkerk, M.; Xie, X. Development and application of artificial intelligence in cardiac imaging. Br. J. Radiol. 2020, 93, 20190812. [Google Scholar] [CrossRef]
- Kolossváry, M.; Lin, A.; Kwiecinski, J.; Cadet, S.; Slomka, P.J.; Newby, D.E.; Dweck, M.R.; Williams, M.C.; Dey, D. Coronary Plaque Radiomic Phenotypes Predict Fatal or Nonfatal Myocardial Infarction: Analysis of the SCOT-HEART Trial. JACC Cardiovasc. Imaging 2025, 18, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Feeny, A.K.; Rickard, J.; Patel, D.; Toro, S.; Trulock, K.M.; Park, C.J.; LaBarbera, M.A.; Varma, N.; Niebauer, M.J.; Sinha, S.; et al. Machine Learning Prediction of Response to Cardiac Resynchronization Therapy: Improvement Versus Current Guidelines. Circ. Arrhythm. Electrophysiol. 2019, 12, e007316. [Google Scholar] [CrossRef]
- Sabouri, M.; Hajianfar, G.; Hosseini, Z.; Amini, M.; Mohebi, M.; Ghaedian, T.; Madadi, S.; Rastgou, F.; Oveisi, M.; Bitarafan Rajabi, A.; et al. Myocardial Perfusion SPECT Imaging Radiomic Features and Machine Learning Algorithms for Cardiac Contractile Pattern Recognition. J. Digit. Imaging 2023, 36, 497–509. [Google Scholar] [CrossRef]
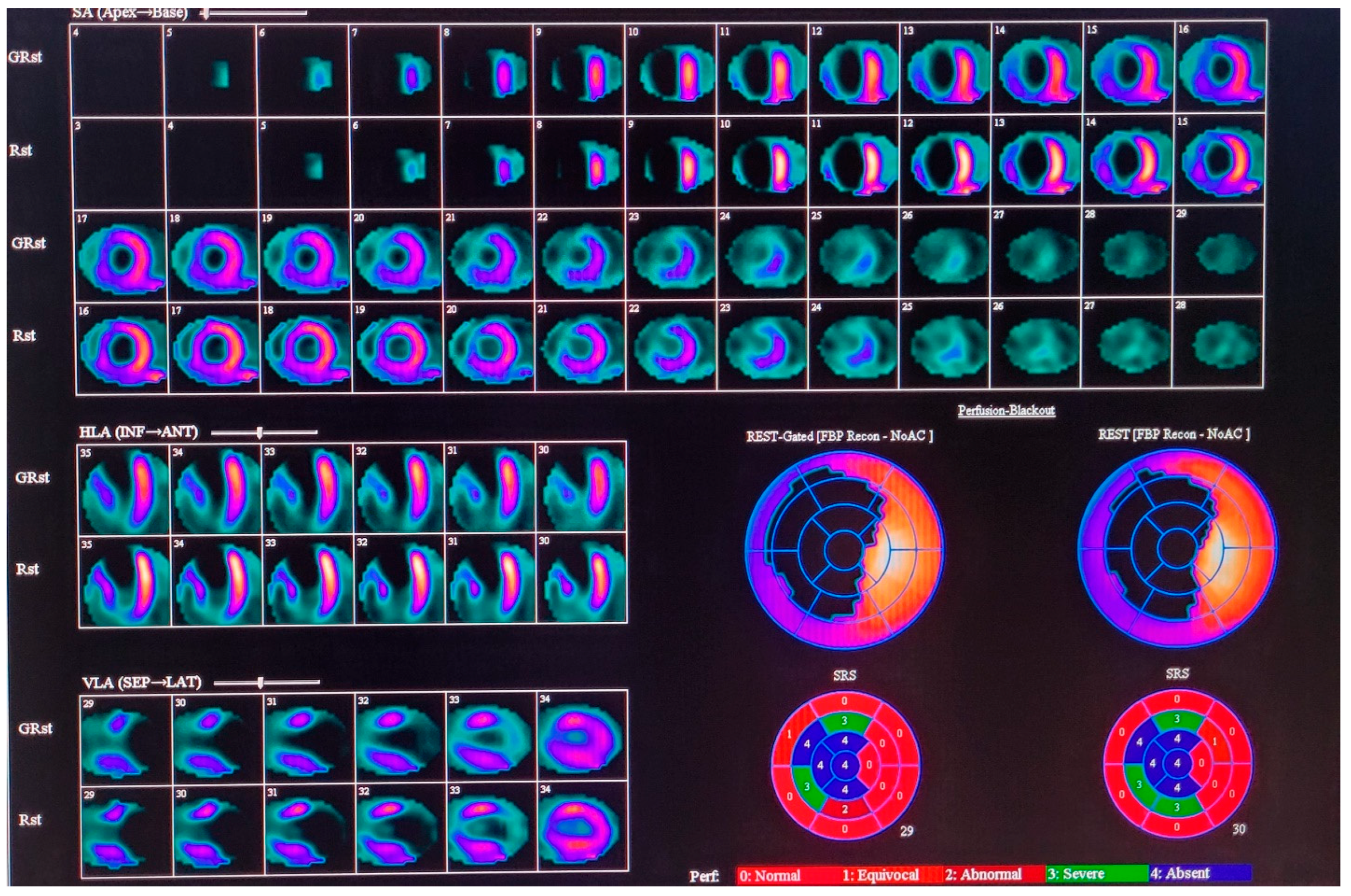
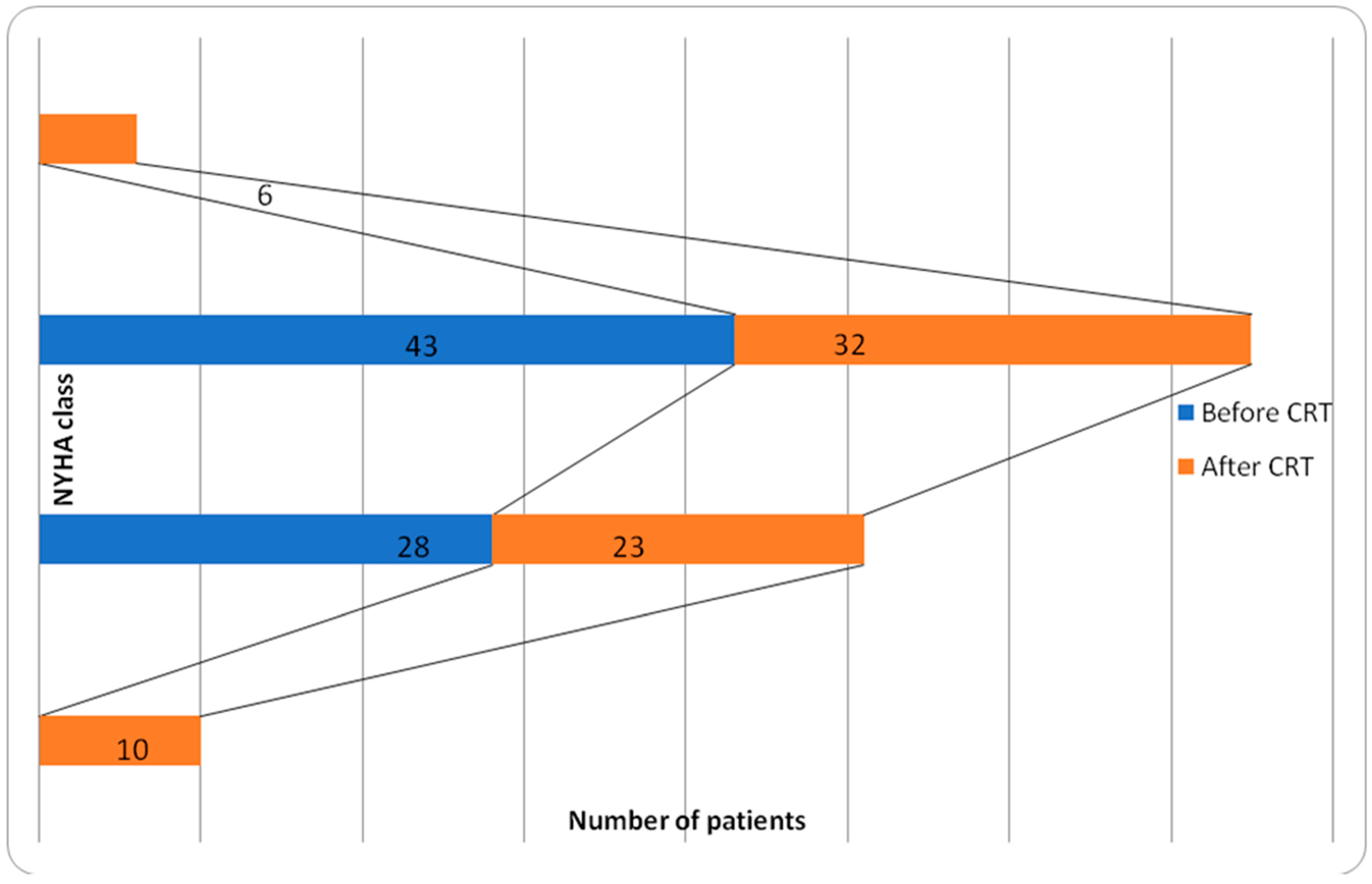
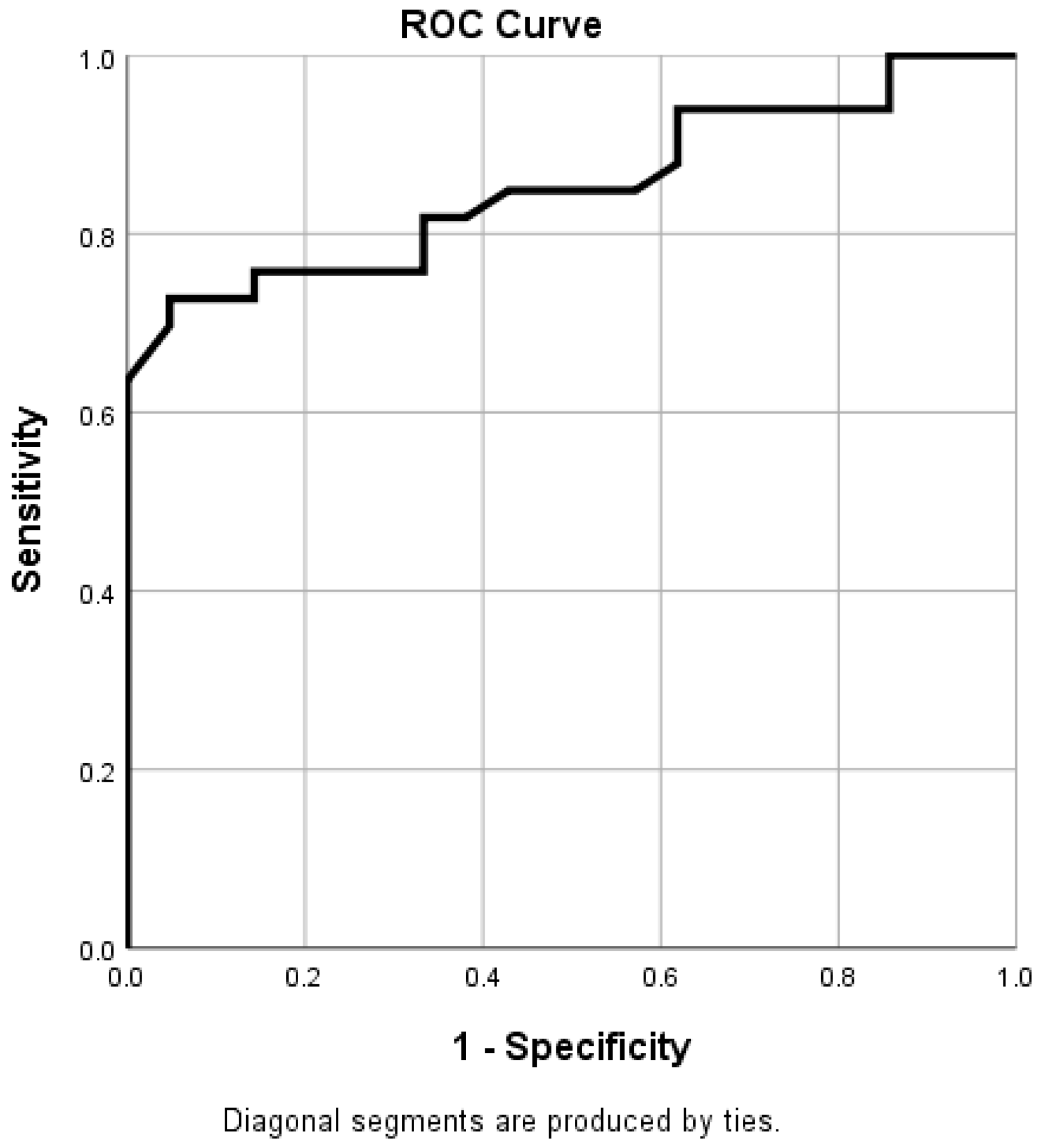
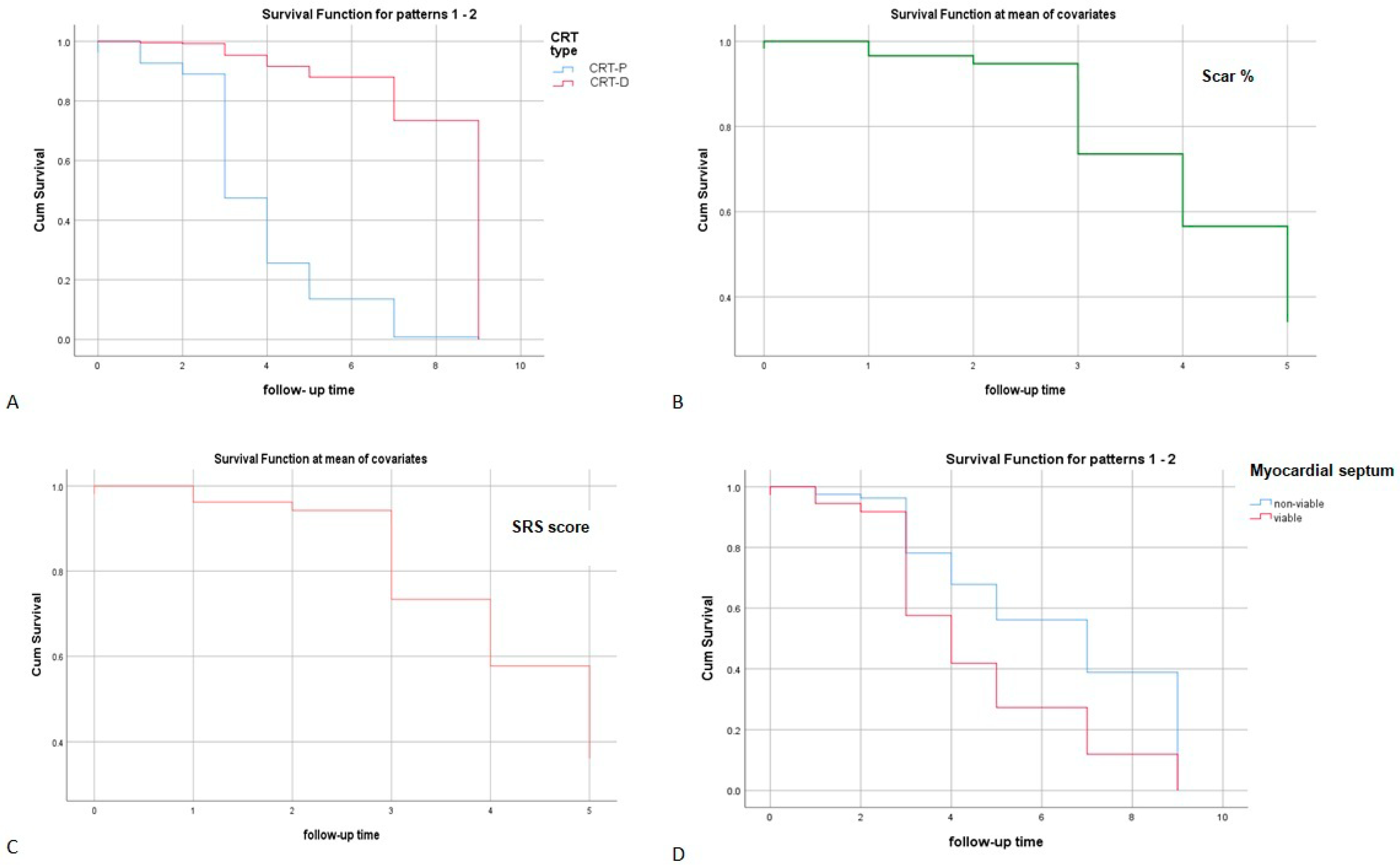
| Variable | Overall Value |
|---|---|
| Age | 66.26 ± 9.25 |
| Male gender | 61 (85%) |
| NYHA class | II 40% (28 pts) III 60% (43 pts) |
| QRS duration (ms) | 164 ± 21 |
| True LBBB | 50 (70%) |
| EF% (ultrasound measurement) | 24.93 ± 7.84 |
| Medication * | |
| Diuretics | 93% |
| ACE inhibitors | 82% |
| Beta-blockers | 53% |
| Variable | Overall Value |
|---|---|
| LVEF % | 26.67 ± 7.71 |
| LVEDV | 277.34 ± 93.17 |
| LVESV | 208.21 ± 83.48 |
| SRS | 25.02 ± 11.29 |
| Scar % | 44.53 ± 20.94 |
| Variable | CRT Responders (%) | CRT Non-Responders (%) | p |
|---|---|---|---|
| Age | 66.96 ± 9.21 | 66.76 ± 8.45 | 0.900 |
| Male gender | 84% | 87% | 0.786 |
| Atrial fibrillation | 22% | 24% | 0.810 |
| Medication * | |||
| Diuretics | 92% | 93% | 0.970 |
| ACE inhibitors | 87% | 89% | 0.830 |
| Beta-blockers | 58% | 56% | 0.860 |
| Variable | Responder | Non-Responder | p |
|---|---|---|---|
| SPECT parameters | |||
| LVEF% | 26.20 ± 8.76 | 27.21 ± 7.49 | 0.690 |
| LVEDV | 265.81 ± 113.43 | 281.22 ± 82.49 | 0.651 |
| LVESV | 200.75 ± 102.71 | 210.11 ± 73.44 | 0.760 |
| SRS | 17.57 ± 6.34 | 30.78 ± 10.13 | 0.001 |
| Scar % | 32.31 ± 14.73 | 55.03 ± 16.53 | 0.001 |
| EF % (ultrasound measurement) | 25.08 ± 8.81 | 25.81 ± 7.05 | 0.600 |
| Periprocedural complications | 3 (12%) | 1 (2%) | 0.294 |
| Univariate | Analysis | Multivariate | Analysis | |||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | p | OR | 95% CI | p |
| Gender | 0.661 | 0.186–2.345 | 0.521 | 0.000 | 0.000–1.100 | 0.884 |
| Age | 0.984 | 0.926–1.046 | 0.602 | 1.575 | 0.640–3.850 | 0.320 |
| EF | 1.009 | 0.946–1.077 | 0.780 | 0.410 | 0.080–1.930 | 0.260 |
| EDV | 0.997 | 0.988–1.006 | 0.509 | 1.400 | 0.840–2.330 | 0.197 |
| ESV | 0.996 | 0.985–1.007 | 0.456 | 0.654 | 0.314–1.240 | 0.195 |
| SRS | 0.919 | 0.865–0.978 | 0.008 | 0.917 | 0.205–4.640 | 0.889 |
| Anterior scar localization | 0.059 | 0.006–0.552 | 0.013 | 0.977 | 0.000–1.600 | 0.976 |
| Septal scar localization | 0.315 | 0.106–0.992 | 0.048 | 0.001 | 0.130–4.000 | 0.225 |
| Inferior scar localization | 0.436 | 0.052–3.630 | 0.440 | 1106.9 | 0.000–8.000 | 0.897 |
| Lateral scar localization | 0.167 | 0.022–1.250 | 0.080 | 82,039 | 0.000–8.100 | 0.893 |
| Inferolateral scar localization | 0.000 | 0.00–2.130 | 0.986 | 0.000 | 0.000–8.520 | 0.953 |
| Apical scar localization | 0.11 | 0.023–0.537 | 0.020 | 0.000 | 0.100–6.010 | 0.780 |
| Scar % | 0.953 | 0.924–0.984 | 0.003 | 0.917 | 0.271–7.900 | 0.889 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grozdic Milojevic, I.; Radovanovic, N.N.; Petrovic, J.; Sobic-Saranovic, D.; Artiko, V. Viability Test in Prediction of Response to Cardiac Resynchronization Therapy. J. Clin. Med. 2025, 14, 5341. https://doi.org/10.3390/jcm14155341
Grozdic Milojevic I, Radovanovic NN, Petrovic J, Sobic-Saranovic D, Artiko V. Viability Test in Prediction of Response to Cardiac Resynchronization Therapy. Journal of Clinical Medicine. 2025; 14(15):5341. https://doi.org/10.3390/jcm14155341
Chicago/Turabian StyleGrozdic Milojevic, Isidora, Nikola N. Radovanovic, Jelena Petrovic, Dragana Sobic-Saranovic, and Vera Artiko. 2025. "Viability Test in Prediction of Response to Cardiac Resynchronization Therapy" Journal of Clinical Medicine 14, no. 15: 5341. https://doi.org/10.3390/jcm14155341
APA StyleGrozdic Milojevic, I., Radovanovic, N. N., Petrovic, J., Sobic-Saranovic, D., & Artiko, V. (2025). Viability Test in Prediction of Response to Cardiac Resynchronization Therapy. Journal of Clinical Medicine, 14(15), 5341. https://doi.org/10.3390/jcm14155341








