Cardiac Resynchronization Therapy and Conduction System Pacing
Abstract
1. Introduction
2. Pathophysiology of Left Bundle Branch Block and Heart Failure
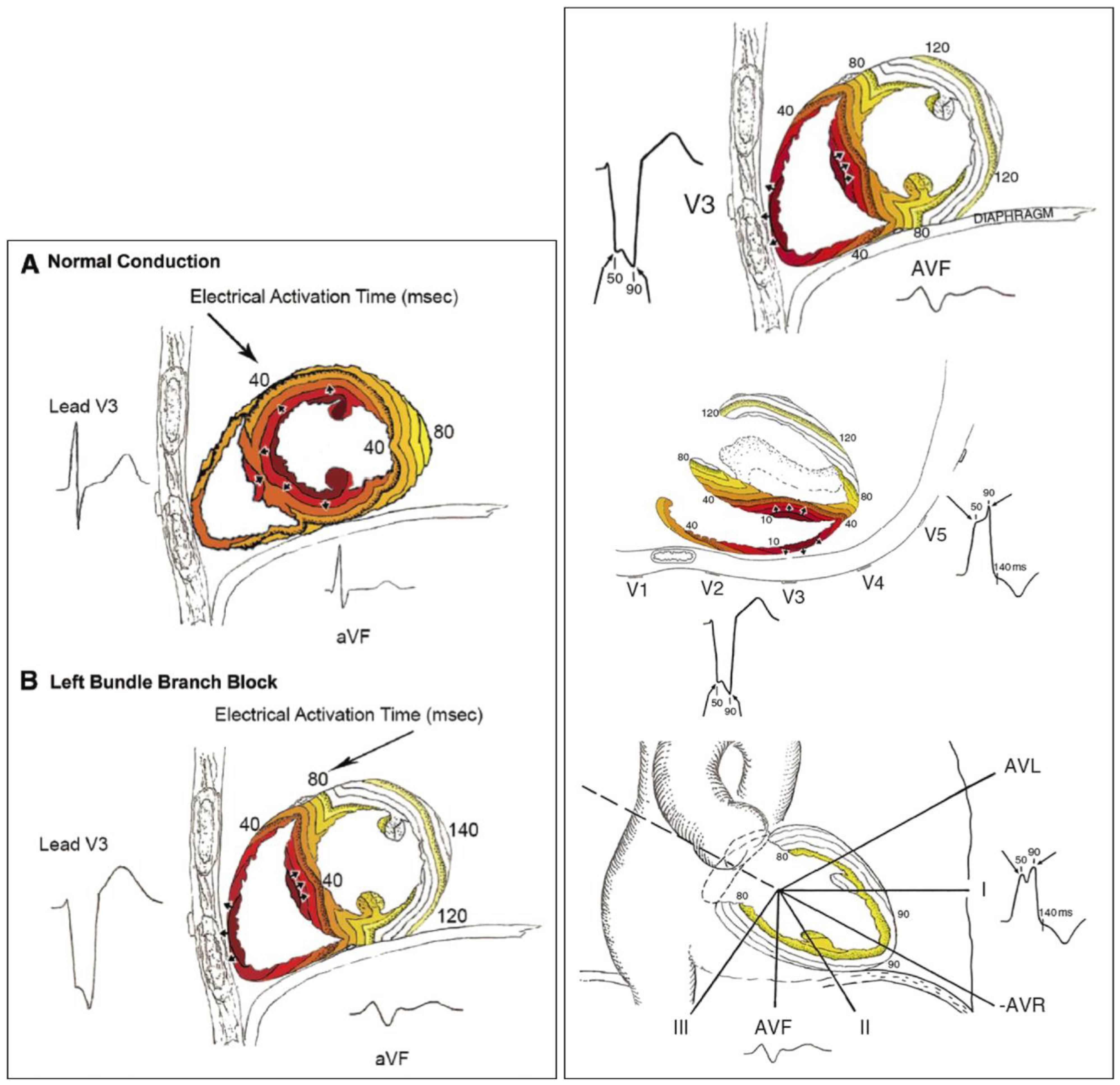
3. Left Bundle Branch: Electrocardiographic Criteria
4. Non-Electrocardiographic Assessment for Left Bundle Branch Block
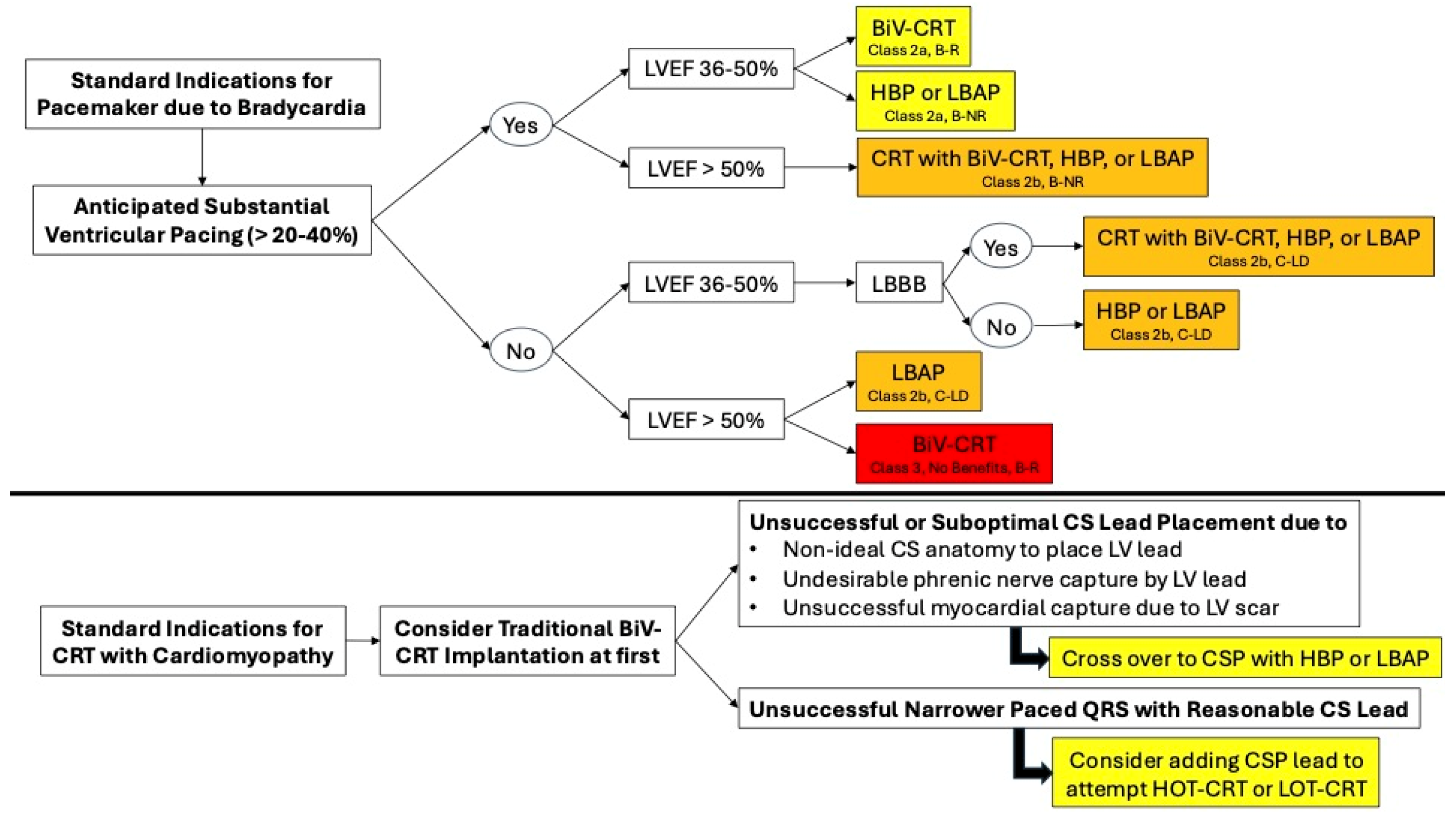
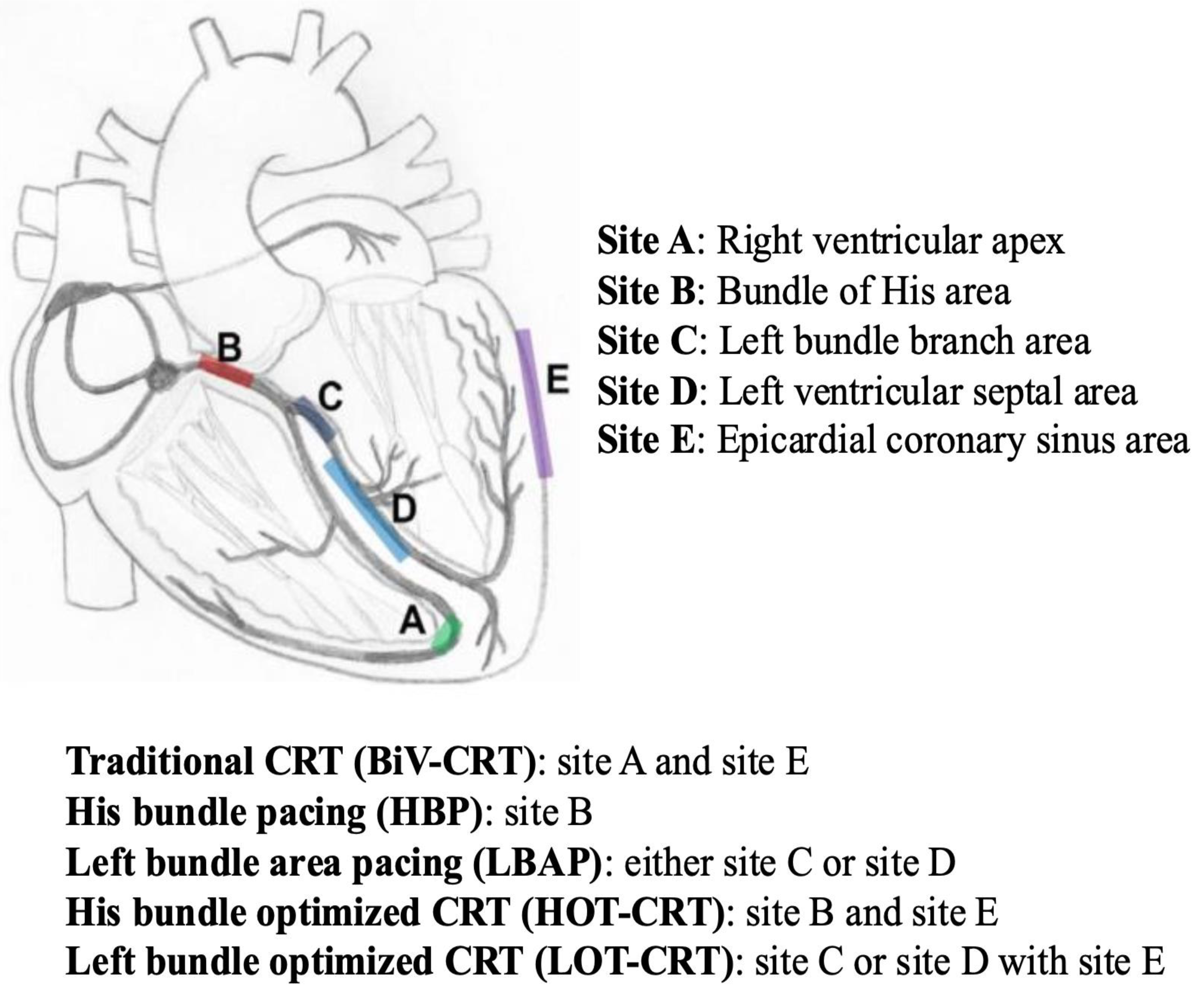
5. Biventricular Resynchronization Therapy with Traditional CRT via Transvenous CS Lead (BiV-CRT)
6. Guideline Recommendations for Traditional or BiV-CRT Implantation
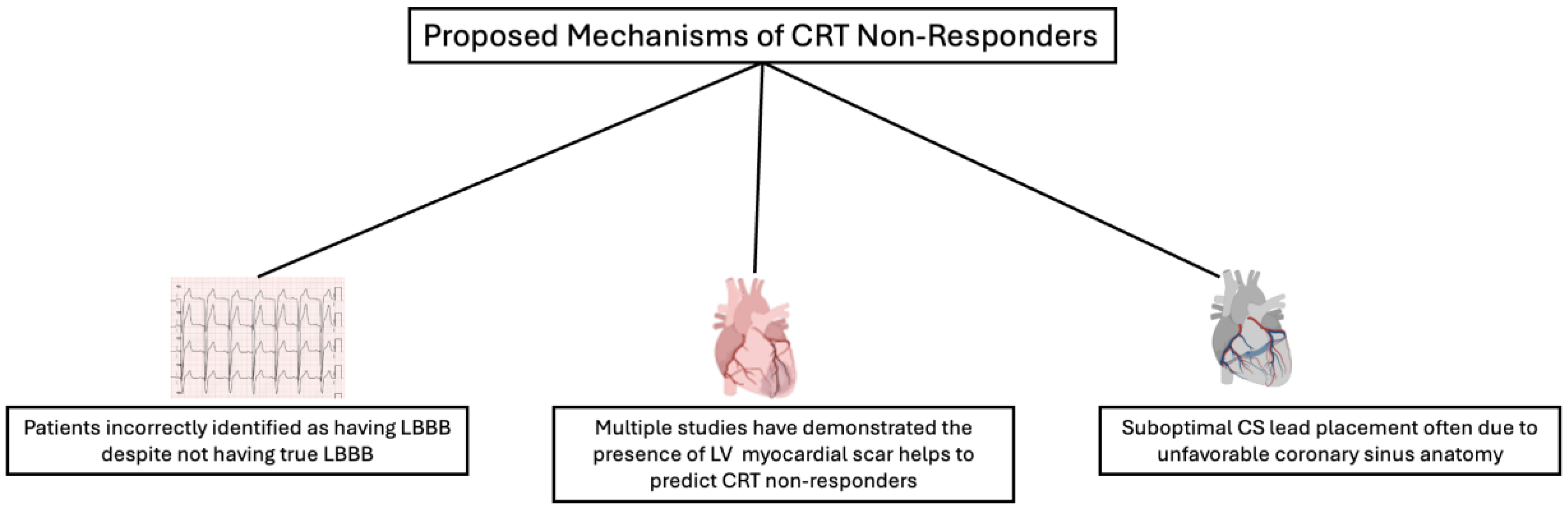
7. Suggested Mechanism of Traditional BiV-CRT Non-Responders
- Lack of true substrate for CRT
- 2.
- Presence and size of LV myocardial scarring
- 3.
- CS lead position
8. His-Bundle Pacing (HBP)
9. Left Bundle Area Pacing (LBAP)
10. Ongoing Randomized Clinical Trials for Conduction System Pacing
- Patients requiring pacing treatment for high-degree AVB (Table 6);
- Patients requiring CRT therapy following the standard indications (Table 7);
- Patients with permanent AF with slow ventricular response and/or requiring AV nodal ablation for better rate control (Table 8);
- Patients with acute post-procedural AVB secondary to transcatheter aortic valve implantation (Table 9).
11. Uncertainty with CSP and Interests in Future Investigations
- Optimal criteria for determining a successfully captured cardiac conduction system during the CSP implantation, especially for the LBAP system;
- Differences in clinical outcomes between proximal and distal LBAP;
- Differences in clinical outcomes between LBAP and LV septal pacing;
- Delayed activation of the right ventricle with LBAP, and whether anodal capture of the LBAP lead is good or bad in patients with concurrent RV failure;
- Benefits of CSP in patients with diastolic heart failure;
- Safety and long-term outcomes of the CSP implantation in patients with interventricular septal disease such as infiltrative cardiomyopathy like cardiac sarcoidosis;
- Safety and short/long-term outcomes of transvenous lead extraction of CSP leads.
12. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strauss, D.G.; Selvester, R.H.; Wagner, G.S. Defining Left Bundle Branch Block in the Era of Cardiac Resynchronization Therapy. Am. J. Cardiol. 2011, 107, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T. Right Bundle Branch Block: Current Considerations. Curr. Cardiol. Rev. 2021, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Baldasseroni, S.; Opasich, C.; Gorini, M.; Lucci, D.; Marchionni, N.; Marini, M.; Campana, C.; Perini, G.; Deorsola, A.; Masotti, G.; et al. Left Bundle-Branch Block Is Associated with Increased 1-Year Sudden and Total Mortality Rate in 5517 Outpatients with Congestive Heart Failure: A Report from the Italian Network on Congestive Heart Failure. Am. Heart J. 2002, 143, 398–405. [Google Scholar] [CrossRef]
- Vernooy, K.; Verbeek, X.A.A.M.; Peschar, M.; Crijns, H.J.G.M.; Arts, T.; Cornelussen, R.N.M.; Prinzen, F.W. Left Bundle Branch Block Induces Ventricular Remodelling and Functional Septal Hypoperfusion. Eur. Heart J. 2005, 26, 91–98. [Google Scholar] [CrossRef]
- Kléber, A.G.; Rudy, Y. Basic Mechanisms of Cardiac Impulse Propagation and Associated Arrhythmias. Physiol. Rev. 2004, 84, 431–488. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, J.; Huntjens, P.R.; Prinzen, F.W.; Delhaas, T.; Lumens, J. Septal Flash and Septal Rebound Stretch Have Different Underlying Mechanisms. Am. J. Physiol.-Heart Circ. Physiol. 2016, 310, H394–H403. [Google Scholar] [CrossRef]
- Surkova, E.; Badano, L.P.; Bellu, R.; Aruta, P.; Sambugaro, F.; Romeo, G.; Migliore, F.; Muraru, D. Left Bundle Branch Block: From Cardiac Mechanics to Clinical and Diagnostic Challenges. EP Eur. 2017, 19, 1251–1271. [Google Scholar] [CrossRef]
- Auffret, V.; Martins, R.P.; Daubert, C.; Leclercq, C.; Le Breton, H.; Mabo, P.; Donal, E. Idiopathic/Iatrogenic Left Bundle Branch Block–Induced Reversible Left Ventricle Dysfunction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 3177–3188. [Google Scholar] [CrossRef]
- Kusumoto, F.M.; Schoenfeld, M.H.; Barrett, C.; Edgerton, J.R.; Ellenbogen, K.A.; Gold, M.R.; Goldschlager, N.F.; Hamilton, R.M.; Joglar, J.A.; Kim, R.J.; et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients with Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2019, 140, e382–e482. [Google Scholar] [CrossRef]
- Treger, J.S.; Allaw, A.B.; Razminia, P.; Roy, D.; Gampa, A.; Rao, S.; Beaser, A.D.; Yeshwant, S.; Aziz, Z.; Ozcan, C.; et al. A Revised Definition of Left Bundle Branch Block Using Time to Notch in Lead I. JAMA Cardiol. 2024, 9, 449–456. [Google Scholar] [CrossRef]
- Dutta, A.; Alqabbani, R.R.M.; Hagendorff, A.; Tayal, B. Understanding the Application of Mechanical Dyssynchrony in Patients with Heart Failure Considered for CRT. J. Cardiovasc. Dev. Dis. 2024, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Ghio, S.; Constantin, C.; Klersy, C.; Serio, A.; Fontana, A.; Campana, C.; Tavazzi, L. Interventricular and Intraventricular Dyssynchrony Are Common in Heart Failure Patients, Regardless of QRS Duration. Eur. Heart J. 2004, 25, 571–578. [Google Scholar] [CrossRef]
- Tayal, B.; Gorcsan, J.; Bax, J.J.; Risum, N.; Olsen, N.T.; Singh, J.P.; Abraham, W.T.; Borer, J.S.; Dickstein, K.; Gras, D.; et al. Cardiac Resynchronization Therapy in Patients with Heart Failure and Narrow QRS Complexes. J. Am. Coll. Cardiol. 2018, 71, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.S.; Leon, A.R.; Tavazzi, L.; Sun, J.-P.; Nihoyannopoulos, P.; Merlino, J.; Abraham, W.T.; Ghio, S.; Leclercq, C.; Bax, J.J.; et al. Results of the Predictors of Response to CRT (PROSPECT) Trial. Circulation 2008, 117, 2608–2616. [Google Scholar] [CrossRef] [PubMed]
- Risum, N.; Tayal, B.; Hansen, T.F.; Bruun, N.E.; Jensen, M.T.; Lauridsen, T.K.; Saba, S.; Kisslo, J.; Gorcsan, J.; Sogaard, P. Identification of Typical Left Bundle Branch Block Contraction by Strain Echocardiography Is Additive to Electrocardiography in Prediction of Long-Term Outcome After Cardiac Resynchronization Therapy. J. Am. Coll. Cardiol. 2015, 66, 631–641. [Google Scholar] [CrossRef]
- Tayal, B.; Gorcsan, J.; Delgado-Montero, A.; Goda, A.; Ryo, K.; Saba, S.; Risum, N.; Sogaard, P. Comparative Long-Term Outcomes after Cardiac Resynchronization Therapy in Right Ventricular Paced Patients versus Native Wide Left Bundle Branch Block Patients. Heart Rhythm 2016, 13, 511–518. [Google Scholar] [CrossRef]
- Calle, S.; Duchenne, J.; Beela, A.S.; Stankovic, I.; Puvrez, A.; Winter, S.; Fehske, W.; Aarones, M.; De Buyzere, M.; De Pooter, J.; et al. Clinical and Experimental Evidence for a Strain-Based Classification of Left Bundle Branch Block-Induced Cardiac Remodeling. Circ. Cardiovasc. Imaging 2022, 15, e014296. [Google Scholar] [CrossRef]
- Cleland, J.G.F.; Daubert, J.-C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L.; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The Effect of Cardiac Resynchronization on Morbidity and Mortality in Heart Failure. N. Engl. J. Med. 2005, 352, 1539–1549. [Google Scholar] [CrossRef]
- Abraham, W.T.; Fisher, W.G.; Smith, A.L.; Delurgio, D.B.; Leon, A.R.; Loh, E.; Kocovic, D.Z.; Packer, M.; Clavell, A.L.; Hayes, D.L.; et al. Cardiac Resynchronization in Chronic Heart Failure. N. Engl. J. Med. 2002, 346, 1845–1853. [Google Scholar] [CrossRef]
- Bristow, M.R.; Saxon, L.A.; Boehmer, J.; Krueger, S.; Kass, D.A.; De Marco, T.; Carson, P.; DiCarlo, L.; DeMets, D.; White, B.G.; et al. Cardiac-Resynchronization Therapy with or without an Implantable Defibrillator in Advanced Chronic Heart Failure. N. Engl. J. Med. 2004, 350, 2140–2150. [Google Scholar] [CrossRef]
- Linde, C.; Abraham, W.T.; Gold, M.R.; St John Sutton, M.; Ghio, S.; Daubert, C.; REVERSE (REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction) Study Group. Randomized Trial of Cardiac Resynchronization in Mildly Symptomatic Heart Failure Patients and in Asymptomatic Patients with Left Ventricular Dysfunction and Previous Heart Failure Symptoms. J. Am. Coll. Cardiol. 2008, 52, 1834–1843. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Klein, H.; Brown, M.W.; Daubert, J.P.; Estes, N.A.M.; Foster, E.; Greenberg, H.; Higgins, S.L.; et al. Cardiac-Resynchronization Therapy for the Prevention of Heart-Failure Events. N. Engl. J. Med. 2009, 361, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.S.L.; Wells, G.A.; Talajic, M.; Arnold, M.O.; Sheldon, R.; Connolly, S.; Hohnloser, S.H.; Nichol, G.; Birnie, D.H.; Sapp, J.L.; et al. Cardiac-Resynchronization Therapy for Mild-to-Moderate Heart Failure. N. Engl. J. Med. 2010, 363, 2385–2395. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-M.; Chan, J.Y.-S.; Zhang, Q.; Omar, R.; Yip, G.W.-K.; Hussin, A.; Fang, F.; Lam, K.H.; Chan, H.C.-K.; Fung, J.W.-H. Biventricular Pacing in Patients with Bradycardia and Normal Ejection Fraction. N. Engl. J. Med. 2009, 361, 2123–2134. [Google Scholar] [CrossRef]
- Stockburger, M.; Gómez-Doblas, J.J.; Lamas, G.; Alzueta, J.; Fernández-Lozano, I.; Cobo, E.; Wiegand, U.; de la Concha, J.F.; Navarro, X.; Navarro-López, F.; et al. Preventing Ventricular Dysfunction in Pacemaker Patients without Advanced Heart Failure: Results from a Multicentre International Randomized Trial (PREVENT-HF). Eur. J. Heart Fail. 2011, 13, 633–641. [Google Scholar] [CrossRef]
- Curtis, A.B.; Worley, S.J.; Adamson, P.B.; Chung, E.S.; Niazi, I.; Sherfesee, L.; Shinn, T.; Sutton, M.S.J. Biventricular Pacing for Atrioventricular Block and Systolic Dysfunction. N. Engl. J. Med. 2013, 368, 1585–1593. [Google Scholar] [CrossRef]
- Yu, C.-M.; Fang, F.; Luo, X.-X.; Zhang, Q.; Azlan, H.; Razali, O. Long-Term Follow-up Results of the Pacing to Avoid Cardiac Enlargement (PACE) Trial. Eur. J. Heart Fail. 2014, 16, 1016–1025. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy: Developed by the Task Force on Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology (ESC) with the Special Contribution of the European Heart Rhythm Association (EHRA). EP Eur. 2022, 24, 71–164. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Chung, M.K.; Patton, K.K.; Lau, C.-P.; Forno, A.R.J.D.; Al-Khatib, S.M.; Arora, V.; Birgersdotter-Green, U.M.; Cha, Y.-M.; Chung, E.H.; Cronin, E.M.; et al. 2023 HRS/APHRS/LAHRS Guideline on Cardiac Physiologic Pacing for the Avoidance and Mitigation of Heart Failure. Heart Rhythm 2023, 20, e17–e91. [Google Scholar] [CrossRef] [PubMed]
- Beshai, J.F.; Grimm, R.A.; Nagueh, S.F.; Baker, J.H.; Beau, S.L.; Greenberg, S.M.; Pires, L.A.; Tchou, P.J. Cardiac-Resynchronization Therapy in Heart Failure with Narrow QRS Complexes. N. Engl. J. Med. 2007, 357, 2461–2471. [Google Scholar] [CrossRef] [PubMed]
- Thibault, B.; Harel, F.; Ducharme, A.; White, M.; Ellenbogen, K.A.; Frasure-Smith, N.; Roy, D.; Philippon, F.; Dorian, P.; Talajic, M.; et al. Cardiac Resynchronization Therapy in Patients with Heart Failure and a QRS Complex <120 Milliseconds. Circulation 2013, 127, 873–881. [Google Scholar] [CrossRef]
- Ruschitzka, F.; Abraham, W.T.; Singh, J.P.; Bax, J.J.; Borer, J.S.; Brugada, J.; Dickstein, K.; Ford, I.; Gorcsan, J.; Gras, D.; et al. Cardiac-Resynchronization Therapy in Heart Failure with a Narrow QRS Complex. N. Engl. J. Med. 2013, 369, 1395–1405. [Google Scholar] [CrossRef]
- Sipahi, I.; Chou, J.C.; Hyden, M.; Rowland, D.Y.; Simon, D.I.; Fang, J.C. Effect of QRS Morphology on Clinical Event Reduction with Cardiac Resynchronization Therapy: Meta-Analysis of Randomized Controlled Trials. Am. Heart J. 2012, 163, 260-7.e3. [Google Scholar] [CrossRef]
- Cunnington, C.; Kwok, C.S.; Satchithananda, D.K.; Patwala, A.; Khan, M.A.; Zaidi, A.; Ahmed, F.Z.; Mamas, M.A. Cardiac Resynchronisation Therapy Is Not Associated with a Reduction in Mortality or Heart Failure Hospitalisation in Patients with Non-Left Bundle Branch Block QRS Morphology: Meta-Analysis of Randomised Controlled Trials. Heart 2015, 101, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, I.; Aktas, M.K.; Zareba, W.; Tsu-Chau Huang, D.; Rosero, S.Z.; Younis, A.; McNitt, S.; Stockburger, M.; Steinberg, J.S.; Buttar, R.S.; et al. QRS Morphology and the Risk of Ventricular Tachyarrhythmia in Cardiac Resynchronization Therapy Recipients. JACC Clin. Electrophysiol. 2024, 10, 16–26. [Google Scholar] [CrossRef]
- Nguyên, U.C.; Rijks, J.H.J.; Plesinger, F.; Rademakers, L.M.; Luermans, J.; Smits, K.C.; van Stipdonk, A.M.W.; Prinzen, F.W.; Vernooy, K.; Halamek, J.; et al. Ultra-High-Frequency ECG in Cardiac Pacing and Cardiac Resynchronization Therapy: From Technical Concept to Clinical Application. J. Cardiovasc. Dev. Dis. 2024, 11, 76. [Google Scholar] [CrossRef]
- Gorcsan, J. Finding Pieces of the Puzzle of Nonresponse to Cardiac Resynchronization Therapy. Circulation 2011, 123, 10–12. [Google Scholar] [CrossRef]
- Jafferani, A.; Leal, M.A. Advances in Cardiac Resynchronization Therapy. J. Innov. Card. Rhythm Manag. 2019, 10, 3681–3693. [Google Scholar] [CrossRef]
- Hsu, J.C.; Solomon, S.D.; Bourgoun, M.; McNitt, S.; Goldenberg, I.; Klein, H.; Moss, A.J.; Foster, E.; MADIT-CRT Executive Committee. Predictors of Super-Response to Cardiac Resynchronization Therapy and Associated Improvement in Clinical Outcome: The MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy) Study. J. Am. Coll. Cardiol. 2012, 59, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.; Qiu, Y.; Tang, H.; Qian, Z.; Wu, H.; Zhu, R.; Wang, Y.; Hou, X.; Zhou, W.; Zou, J. Assessment of Left Ventricular Contraction Patterns Using Gated SPECT MPI to Predict Cardiac Resynchronization Therapy Response. J. Nucl. Cardiol. 2018, 25, 2029–2038. [Google Scholar] [CrossRef]
- Leyva, F. The Role of Cardiovascular Magnetic Resonance in Cardiac Resynchronization Therapy. Heart Fail. Clin. 2017, 13, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Derndorfer, M.; Kollias, G.; Martinek, M.; Pürerfellner, H. Is Conduction System Pacing Going to Be the New Gold Standard for Cardiac Resynchronization Therapy? J. Clin. Med. 2024, 13, 4320. [Google Scholar] [CrossRef]
- Deshmukh, P.; Casavant, D.A.; Romanyshyn, M.; Anderson, K. Permanent, Direct His-Bundle Pacing. Circulation 2000, 101, 869–877. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Sha, Y.-Q.; Sun, Q.-Y.; Qiu, Y.; Shao, B.; Ni, Y.-H.; Mei, Y.-K.; Zhang, C.-Y.; Wang, R.-X. The Long-Term Therapeutic Effects of His-Purkinje System Pacing on Bradycardia and Cardiac Conduction Dysfunction Compared with Right Ventricular Pacing: A Systematic Review and Meta-Analysis. J. Cardiovasc. Electrophysiol. 2020, 31, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Naperkowski, A.; Ellenbogen, K.A.; Dandamudi, G. Electrophysiologic Insights into Site of Atrioventricular Block: Lessons from Permanent His Bundle Pacing. JACC Clin. Electrophysiol. 2015, 1, 571–581. [Google Scholar] [CrossRef]
- Lustgarten, D.L.; Crespo, E.M.; Arkhipova-Jenkins, I.; Lobel, R.; Winget, J.; Koehler, J.; Liberman, E.; Sheldon, T. His-Bundle Pacing versus Biventricular Pacing in Cardiac Resynchronization Therapy Patients: A Crossover Design Comparison. Heart Rhythm 2015, 12, 1548–1557. [Google Scholar] [CrossRef]
- Upadhyay, G.A.; Vijayaraman, P.; Nayak, H.M.; Verma, N.; Dandamudi, G.; Sharma, P.S.; Saleem, M.; Mandrola, J.; Genovese, D.; Tung, R.; et al. His Corrective Pacing or Biventricular Pacing for Cardiac Resynchronization in Heart Failure. J. Am. Coll. Cardiol. 2019, 74, 157–159. [Google Scholar] [CrossRef]
- Vinther, M.; Risum, N.; Svendsen, J.H.; Møgelvang, R.; Philbert, B.T. A Randomized Trial of His Pacing Versus Biventricular Pacing in Symptomatic HF Patients with Left Bundle Branch Block (His-Alternative). JACC Clin. Electrophysiol. 2021, 7, 1422–1432. [Google Scholar] [CrossRef]
- Huang, W.; Wang, S.; Su, L.; Fu, G.; Su, Y.; Chen, K.; Zou, J.; Han, H.; Wu, S.; Sheng, X.; et al. His-Bundle Pacing vs Biventricular Pacing Following Atrioventricular Nodal Ablation in Patients with Atrial Fibrillation and Reduced Ejection Fraction: A Multicenter, Randomized, Crossover Study-The ALTERNATIVE-AF Trial. Heart Rhythm 2022, 19, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Herweg, B.; Ellenbogen, K.A.; Gajek, J. His-Optimized Cardiac Resynchronization Therapy to Maximize Electrical Resynchronization: A Feasibility Study. Circ. Arrhythm. Electrophysiol. 2019, 12, e006934. [Google Scholar] [CrossRef] [PubMed]
- Zweerink, A.; Zubarev, S.; Bakelants, E.; Potyagaylo, D.; Stettler, C.; Chmelevsky, M.; Lozeron, E.D.; Hachulla, A.-L.; Vallée, J.-P.; Burri, H. His-Optimized Cardiac Resynchronization Therapy with Ventricular Fusion Pacing for Electrical Resynchronization in Heart Failure. JACC Clin. Electrophysiol. 2021, 7, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.; Sattur, S.; Bechtol, T.; Heckman, L.I.B.; Prinzen, F.W.; Deshmukh, P. Sequential His Bundle and Left Ventricular Pacing for Cardiac Resynchronization. J. Cardiovasc. Electrophysiol. 2020, 31, 2448–2454. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Pokharel, P.; Subzposh, F.A.; Oren, J.W.; Storm, R.H.; Batul, S.A.; Beer, D.A.; Hughes, G.; Leri, G.; Manganiello, M.; et al. His-Purkinje Conduction System Pacing Optimized Trial of Cardiac Resynchronization Therapy vs Biventricular Pacing: HOT-CRT Clinical Trial. JACC Clin. Electrophysiol. 2023, 9, 2628–2638. [Google Scholar] [CrossRef]
- Huang, W.; Su, L.; Wu, S.; Xu, L.; Xiao, F.; Zhou, X.; Ellenbogen, K.A. A Novel Pacing Strategy with Low and Stable Output: Pacing the Left Bundle Branch Immediately Beyond the Conduction Block. Can. J. Cardiol. 2017, 33, e1–e1736. [Google Scholar] [CrossRef]
- Sharma, P.S.; Patel, N.R.; Ravi, V.; Zalavadia, D.V.; Dommaraju, S.; Garg, V.; Larsen, T.R.; Naperkowski, A.M.; Wasserlauf, J.; Krishnan, K.; et al. Clinical Outcomes of Left Bundle Branch Area Pacing Compared to Right Ventricular Pacing: Results from the Geisinger-Rush Conduction System Pacing Registry. Heart Rhythm 2022, 19, 3–11. [Google Scholar] [CrossRef]
- Leventopoulos, G.; Travlos, C.K.; Aronis, K.N.; Anagnostopoulou, V.; Patrinos, P.; Papageorgiou, A.; Perperis, A.; Gale, C.P.; Davlouros, P. Safety and Efficacy of Left Bundle Branch Area Pacing Compared with Right Ventricular Pacing in Patients with Bradyarrhythmia and Conduction System Disorders: Systematic Review and Meta-Analysis. Int. J. Cardiol. 2023, 390, 131230. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, J.; Gong, X.; Lu, H.; Yu, Z.; Zhang, L.; Li, M.; Pan, L.; Chen, X.; Cui, J.; et al. Left Bundle Branch Pacing Versus Biventricular Pacing for Acute Cardiac Resynchronization in Patients with Heart Failure. Circ. Arrhythm. Electrophysiol. 2022, 15, e011181. [Google Scholar] [CrossRef]
- Pujol-Lopez, M.; Jiménez-Arjona, R.; Garre, P.; Guasch, E.; Borràs, R.; Doltra, A.; Ferró, E.; García-Ribas, C.; Niebla, M.; Carro, E.; et al. Conduction System Pacing vs Biventricular Pacing in Heart Failure and Wide QRS Patients: LEVEL-AT Trial. JACC Clin. Electrophysiol. 2022, 8, 1431–1445. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Sharma, P.S.; Cano, Ó.; Ponnusamy, S.S.; Herweg, B.; Zanon, F.; Jastrzebski, M.; Zou, J.; Chelu, M.G.; Vernooy, K.; et al. Comparison of Left Bundle Branch Area Pacing and Biventricular Pacing in Candidates for Resynchronization Therapy. J. Am. Coll. Cardiol. 2023, 82, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.C.; Sauer, W.H.; Duque, M.; Koplan, B.A.; Braunstein, E.D.; Marín, J.E.; Aristizabal, J.; Niño, C.D.; Bastidas, O.; Martinez, J.M.; et al. Left Bundle Branch Area Pacing Versus Biventricular Pacing as Initial Strategy for Cardiac Resynchronization. JACC Clin. Electrophysiol. 2023, 9, 1568–1581. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.C.; Tedrow, U.B.; Duque, M.; Aristizabal, J.; Braunstein, E.D.; Marin, J.; Niño, C.; Bastidas, O.; Lopez Cabanillas, N.; Koplan, B.A.; et al. Left Bundle Branch Pacing vs Left Ventricular Septal Pacing vs Biventricular Pacing for Cardiac Resynchronization Therapy. JACC Clin. Electrophysiol. 2024, 10, 295–305. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Zanon, F.; Ponnusamy, S.S.; Herweg, B.; Sharma, P.; Molina-Lerma, M.; Jastrzębski, M.; Whinnett, Z.; Vernooy, K.; Pathak, R.K.; et al. Conduction System Pacing Compared with Biventricular Pacing for Cardiac Resynchronization Therapy in Patients with Heart Failure and Mildly Reduced Left Ventricular Ejection Fraction: Results from International Collaborative LBBAP Study (I-CLAS) Group. Heart Rhythm 2024. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębski, M.; Moskal, P.; Huybrechts, W.; Curila, K.; Sreekumar, P.; Rademakers, L.M.; Ponnusamy, S.S.; Herweg, B.; Sharma, P.S.; Bednarek, A.; et al. Left Bundle Branch-Optimized Cardiac Resynchronization Therapy (LOT-CRT): Results from an International LBBAP Collaborative Study Group. Heart Rhythm 2022, 19, 13–21. [Google Scholar] [CrossRef]
- Feng, X.-F.; Yang, L.-C.; Zhao, Y.; Yu, Y.-C.; Liu, B.; Li, Y.-G. Effects of Adaptive Left Bundle Branch–Optimized Cardiac Resynchronization Therapy: A Single Centre Experience. BMC Cardiovasc. Disord. 2022, 22, 360. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Hou, X.; Wang, Z.; Zou, F.; Qian, Z.; Wei, Y.; Wang, X.; Zhang, L.; Li, X.; et al. Randomized Trial of Left Bundle Branch vs Biventricular Pacing for Cardiac Resynchronization Therapy. J. Am. Coll. Cardiol. 2022, 80, 1205–1216. [Google Scholar] [CrossRef]
- Jastrzębski, M.; Foley, P.; Chandrasekaran, B.; Whinnett, Z.; Vijayaraman, P.; Upadhyay, G.A.; Schaller, R.D.; Gardas, R.; Richardson, T.; Kudlik, D.; et al. Multicenter Hemodynamic Assessment of the LOT-CRT Strategy: When Does Combining Left Bundle Branch Pacing and Coronary Venous Pacing Enhance Resynchronization? Primary Results of the CSPOT Study. Circ. Arrhythmia Electrophysiol. 2024, 17, e013059. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, S.E.; Ellenbogen, K.A.; Vijayaraman, P.; Chelu, M.G. Clinical Outcomes of Conduction System Pacing versus Biventricular Pacing for Cardiac Resynchronization Therapy: A Systematic Review and Meta-Analysis. J. Cardiovasc. Electrophysiol. 2023, 34, 1718–1729. [Google Scholar] [CrossRef]
| ECG Findings | AHA/ACC/HRS Definition | Strauss et al. [1] |
|---|---|---|
| QRS duration | Complete: ≥120 ms Incomplete: <120 ms | ≥140 ms (men), ≥130 ms (women) |
| Lateral leads (I, aVL, V5, V6) | Broad notched or slurred R waves Absent Q waves Occasional RS pattern in V5 and V6 | Broad notched or slurred R waves |
| Precordial leads (V1, V2, V3) | Small initial r waves in V1, V2, and V3 | Broad notched or slurred mid-QRS QS or rS in leads V1 and V2 |
| R peak time in lateral leads | >60 ms in V5 and V6 | Not applicable |
| ST and T waves | Usually opposite in direction to QRS Positive concordance | Not applicable |
| Patients with Cardiomyopathy | |||||
|---|---|---|---|---|---|
| Study Author | Study Design | Size | Population | Primary Endpoints | Outcomes |
| Abraham et al. NEJM 2002 [19] | Multicenter Prospective RCT | 453 GDMT: 225 CRT:228 | ICM or NICM LVEF < 35% QRS > 130 ms | NYHA status QOL score 6MWT | BiV-CRT improved patient functional status and ΔLVEF significantly. |
| Bristow et al. NEJM 2004 [20] | Multicenter Prospective RCT | 1520 GDMT: 308 CRT-P: 617 CRT-D: 595 | ICM or NICM LVEF < 35% NYHA III or IV QRS > 120 ms | Composite of - Overall death - Admission for any cause | BiV-CRT had 20% reduction in the primary endpoint. |
| Cleland et al. NEJM 2005 [18] | Multicenter Prospective RCT | 813 GDMT: 404 CRT: 409 | ICM or NICM LVEF < 35% NYHA III or IV QRS > 120 ms | Composite of - Overall death - Admission for MACE | BiV-CRT had 37% reduction in the primary endpoint. |
| Linde et al. JACC 2008 [21] | Multicenter Prospective RCT | 610 CRT-ON: 419 CRT-OFF: 191 | ICM or NICM LVEF < 40% NYHA I or II QRS > 120 ms | HF clinical composite response | BiV-CRT improved the primary endpoint. |
| Moss et al. NEJM 2009 [22] | Multicenter Prospective RCT | 1820 CRT-D: 1089 ICD: 731 | ICM or NICM LVEF < 30% NYHA I or II QRS > 130 ms | Composite of - Overall death - Nonfatal HF event | CRT-D had 34% reduction in the primary endpoint. |
| Tang et al. NEJM 2010 [23] | Multicenter Prospective RCT | 1798 ICD: 904 CRT-D: 894 | ICM or NICM LVEF < 30% NYHA II or III QRS > 120 ms | Composite of - Overall death - Admission for HF | CRT-D had 25% reduction in the primary endpoint. |
| Patients with Standard Bradycardia Pacing Indication and Preserved LVEF | |||||
| Study Name | Study Design | Size | Population | Primary Endpoints | Outcomes |
| Yu et al. Eur J Heart Fail. 2009 [24] | Multicenter Prospective RCT | 177 RV apex: 88 CRT: 89 | LVEF > 45% Pacing for AVB | 12-month changes in - LVESV - LVEF | BiV-CRT improved LVEF and decreased LVESV. |
| Stockburger et al. Eur J Heart Fail. 2011 [25] | Multicenter Prospective RCT | 108 RV apex: 58 CRT: 50 | Pacing for AVB Expected RV pacing burden > 80% | 12-month change in LVEDV | BiV-CRT did not show improvements in the primary endpoint. |
| Curtis et al. NEJM 2013 [26] | Multicenter Prospective RCT | 691 RV apex: 342 CRT: 349 | LVEF < 50% NYHA I, II, III Pacing for AVB | Composite of - Overall death - HF-related admission - Increase in LVESV index | BiV-CRT had 26% reduction in the primary endpoint. |
| Yu et al. Eur J Heart Fail. 2014 [27] | Multicenter Prospective RCT | 177 RV apex: 88 CRT: 89 | LVEF > 45% | LVESV LVEF | BiV-CRT improved LVEF and decreased LVESV. |
| Rhythm | QRS Morphology | QRS Width | NYHA Functional Status | Level of Recommendation |
|---|---|---|---|---|
| Sinus | LBBB | >150 ms | II, III, Ambulatory IV | Class I |
| >150 ms | I, LVEF < 30%, ICM | Class IIb | ||
| 120–149 ms | II, III, Ambulatory IV | Class IIa | ||
| 120–149 ms | I | Class III | ||
| <120 ms | I, II, III, Ambulatory IV | Class III | ||
| Non-LBBB | >150 ms | III, Ambulatory IV | Class IIa | |
| >150 ms | II | Class IIb | ||
| >150 ms | I | Class III | ||
| 120–149 ms | III, Ambulatory IV | Class IIb | ||
| 120–149 ms | I, II | Class III | ||
| Atrial Fibrillation with Any QRS | >120 ms | III, Ambulatory IV | Class IIa | |
| High RV Pacing Burden (>40%) | Any | I, II, III, Ambulatory IV | Class IIa | |
| HBP vs. RV Apical Pacing | |||||
|---|---|---|---|---|---|
| Study Author | Study Design | Size | Major Findings [95% Confidence Interval] | ||
| Sun et al. JCE 2020 [46] | Systematic Review Meta-Analysis | 2348 cases 13 studies | Improvement of LVEF (MD: +5.65 [4.38–6.92]) Narrower paced QRS (MD: −39.29 [−41.90–−36.68]) Lower risk of HF-related admission (OR: 0.65 [0.44–0.96]) Higher pacing threshold (MD: +0.8 [0.71–0.89]) Unchanged LVEDV (MD: −0.05 [−6.71–6.60]) Unchanged LVESV (MD: −1.37 [−5.75–3.01]) | ||
| HBP vs. Traditional BiV CRT | |||||
| Study Author | Study Design | Size | Population | Primary Endpoints | Outcomes |
| Lustgarten et al. Heart Rhythm 2015 [48] | Multicenter Prospective Crossover Randomized | 29 Both HBP and CS leads were implanted. | Standard indications for CRT | Feasibility Paced QRS width Quality of life NYHA status 6 min walk test LVEF | Primary endpoints were significantly improved by both HBP and Biv-CRT. |
| Upadhyay et al. JACC 2019 [49] | Multicenter Prospective RCT | 41 HBP: 21 BiV-CRT: 20 | Standard indications for CRT | Feasibility Paced QRS width ΔLVEF at 6 months CV death or admissions at 12 months | No significant differences between 2 groups |
| Vinther et al. JACC EP 2021 [50] | Single-Center Prospective RCT | 50 HBP: 25 BiV-CRT: 25 | Standard indications for CRT with LBBB | Successful HBP implantation | Successful implantation: HBP 72% vs. BiV-CRT: 96% |
| Huang et al. Heart Rhythm 2022 [51] | Multicenter Prospective RCT Crossover | 50: All with both HBP and BiV-CRT | Persistent AF and reduced LVEF (<40%) undergoing AVN ablation | Change in LVEF | Significant ΔLVEF (HBP: 21.3% vs. BiV-CRT: 16.7%) |
| HOT-CRT (His-Bundle Optimized CRT) vs. Traditional BiV CRT | |||||
| Study Author | Study Design | Size | Population | Primary Endpoints | Outcomes |
| Vijayaraman et al. Circ EP 2019 [52] | Multicenter Observational Retrospective | 27 HOT-CRT: 27 | CLBBB (n = 17) IVCD (n = 5) with QRS >140 ms RV pacing (n = 5) | QRS width Echocardiographic parameter NYHA status | HOT-CRT showed - narrower paced QRS - better LVEF and NYHA status |
| Zweerink et al. JACC EP 2021 [53] | Single-Center Observational Prospective | 19 HOT-CRT: 14 BiV CRT: 5 | Standard indications for CRT | Reduction in LVAT | ΔLVAT Change: HOT-CRT: 66 ± 17 ms BiV-CRT: 90 ± 20 ms |
| Deshmukh et al. JCE 2020 [54] | Single-Center Observational Retrospective | 21 HOT-CRT: 21 | Standard indications for CRT HBP only not correcting the QRS | Paced QRS: HOT-CRT: 110 ± 14 ms BiV-CRT: 141 ± 15 ms | |
| Vijayaraman et al. JACC EP 2023 [55] | Multicenter Prospective RCT | 100 BiV-CRT: 50 HOT-CRT: 50 | Standard indications for CRT LVEF < 50% | Change in LVEF at 6 months | ΔLVEF: HOT-CRT: 12.4 ±7.3% BiV-CRT: 8.0 ± 10.1% (p = 0.02) |
| LBAP vs. RV Apical Pacing | |||||
|---|---|---|---|---|---|
| Study Author | Study Design | Size | Major Findings [95% Confidence Interval] | ||
| Sharma et al. Heart Rhythm 2022 [57] | Multicenter Observational Retrospective | LBAP: 321 RVP: 382 | Composite outcome of overall death, HF admission, or upgrade to CRT: LBAP: 10.0% vs. RVP: 23.3% (HR: 0.46 [0.306–0.698]) Composite outcome, especially in patients with RV pacing > 20%: LBAP: 8.4% vs. RVP: 26.1% (HR: 0.32 [0.187–0.540]) | ||
| Leventopoulos et al. IJC 2023 [58] | Systematic Review Meta-Analysis | 4250 25 studies LBAP: 2127 RVP: 2123 | LBAP showed - HF admissions (OR: 0.33 [0.21–0.50]) - Overall death (OR: 0.52 [0.34–0.80]) - AF occurrence (OR: 0.43 [0.27–0.68]) | ||
| LBAP vs. Traditional BiV-CRT | |||||
| Study Author | Study Design | Size | Population | Primary Endpoints | Outcomes |
| Liang et al. Circ EP 2022 [59] | Multicenter Crossover Observational Prospective | 21 LBAP: 21 | ICM or NICM CLBBB Standard indications for CRT | Paced QRS width Paced QRS area Hemodynamic parameters | LBAP showed - greater reduction in paced QRS width and QRS area - hemodynamic improvements |
| “LEVEL-AT” Pujol-Lopez et al. JACC EP 2022 [60] | Multicenter Prospective RCT | 70 CSP: 35 - HBP: 4 - LBAP: 31 BiV CRT: 35 | Standard indications for CRT | Change in LVAT at 6-month follow-up | CSP (mainly LBAP) attained similar degrees of CRT. |
| Vijayaraman et al. JACC 2023 [61] | Multicenter Observational Retrospective | 1778 Biv-CRT: 981 LBAP: 797 | Standard indications for CRT | Composite of - Overall death - HF admission | LBAP had 26% reduction in the primary endpoint. |
| Diaz et al. JACC EP 2023 [62] | Multicenter Observational Prospective | 371 BiV-CRT: 243 LBAP: 128 | Standard indications for CRT | Composite of - Overall death - HF admission | LBAP had 38% reduction in the primary endpoint. |
| Diaz et al. JACC EP 2024 [63] | Multicenter Observational Prospective | 415 BiV-CRT: 243 LBAP: 141 LVSP: 31 | Standard indications for CRT | Composite of - Overall death - HF admission | LBAP reduced primary composite outcomes significantly. No significant differences in clinical outcomes between LVSP and BiV-CRT. |
| Vijayaraman et al. Heart Rhythm 2024 [64] | Multicenter Observational Retrospective | 1004 BiV-CRT: 178 HBP: 154 LBAP: 672 | Standard indications for CRT NYHA II-IV Baseline LVEF: 36–50% | Composite of - Death - HF admissions | CSP (predominantly LBAP) had 36% reduction in the primary composite endpoint. |
| LOT-CRT (Left Bundle Optimized CRT) vs. Traditional BiV CRT | |||||
| Study Author | Study Design | Size | Population | Primary Endpoints | Outcomes |
| Jastzebski et al. Heart Rhythm 2021 [65] | Multicenter Observational Prospective | 112 LOT-CRT: 91 | Standard indications for CRT BiV CRT non-responders | LOT-CRT was better than BiV-CRT for - narrower paced QRS - ΔLVEF > 3 month - improvement on NYHA status > 3 months | |
| Feng et al. BMC Cardiovasc Disord 2022 [66] | Single-Center Observational Prospective | 21 LOT-CRT: 10 BiV CRT: 11 | Standard indications for CRT | LOT-CRT was better than BiV-CRT for - narrower paced QRS - ΔLVEF > 9 month | |
| Wang et al. JACC 2022 [67] | Multicenter Prospective RCT | 40 LOT-CRT: 20 BiV-CRT: 20 | NICM NSR LBBB NYHA II-IV | LVEF at 6 months | Significant ΔLVEF at 6 months (MD: 5.6% [0.3–10.9]) |
| Jastrzebski et al. Circ EP 2024 [68] | Crossover Multicenter Prospective Observational Open-Label | 48 | Standard indications for CRT Cardiomyopathy NSR IVCD: 29 LBBB: 19 | LV dP/dt max from baseline to BiV-CRT, LBAP, or LOT-CRT | In patients with advanced conduction diseases, both LOT-CRT and BVP showed greater hemodynamic benefits than LBAP alone. |
| Study | Study Design | Size | Population | Endpoints |
|---|---|---|---|---|
| NCT05015660 Canada Recruiting | Single-center Prospective RCT Single-blinded | 1300 LBAP vs. RVP (1:1) | LVEF > 35% Standard indications for pacemakers Anticipated RV pacing >90% | Primary: at 36 months - Time to cardiovascular death - Time to HF admission - Worsening LVESV index Secondary: at 22 months - Overall and CV mortality - HF admissions - QOL improvement |
| Spain Recruiting | Single-center Prospective RCT Single-blinded | 200 HBP/LBAP vs. RVP (1:1) | LVEF > 50% Standard indications for pacemakers | Primary: at 12 months - Incidence of pacemaker-induced cardiomyopathy Secondary: at 12 months - Change in LVESV - New HF admissions |
| “LEAP” NCT04595487 Europe Recruiting | Multicenter Prospective RCT Single-blinded | 470 LVSP vs. RVP (1:1) | LVEF > 40% Standard indications for pacemakers Expected RV pacing > 20% | Primary: at 12 months - Composite of all-cause death, HF admissions, LVEF decline (> 10%) Secondary: at 12 months - New HF admissions - All-cause death - De novo AF - QOL analysis |
| “PROTECT-SYNC” NCT05585411 Korea Recruiting | Multicenter Prospective RCT Double-blinded | 450 LBAP vs. RVP (1:1) | Standard indications for pacemakers Expected RV pacing > 40% | Primary: at 24 months - Composite of all-cause death, HF admissions, occurrence of pacing cardiomyopathy Secondary: at 24 months - New HF admissions - All-cause and CV death - De novo AF - QOL analysis |
| “LEAP-Block” NCT04730921 China Recruiting | Multicenter Prospective RCT Double-blinded | 458 LBAP vs. RVP (1:1) | Standard indications for pacemakers Expected RV pacing > 40% | Primary: at 24 months - Composite of all-cause death, HF admissions, occurrence of pacing cardiomyopathy Secondary: at 24 months - Composite of new LVEF < 50% and/or increase in LVESV > 15% - QOL analysis |
| “OptimPacing” NCT04624763 China Recruiting | Multicenter Prospective RCT Double-blinded | 683 LBAP vs. RVP (1:1) | Standard indications for pacemakers LVEF > 35% NYHA I-III status | Primary: at 36 months - Composite of all-cause death, HF admissions, occurrence of pacing cardiomyopathy Secondary: at 36 months - LVEF, LVESV, and LVEDV - NYHA status - QOL analysis |
| “PROTECT-HF” NCT05815745 UK, Slovenia Recruiting | Multicenter Prospective RCT Double-blinded | 2600 HBP/LBAP vs. RVP (1:1) | Standard indications for pacemakers LVEF > 35% | Primary: at 78 months - All-cause death - HF admissions Secondary: at 78 months - Upgrade to BiV-CRT - NYHA status - QOL analysis |
| Study Name | Study Design | Size | Population | Endpoints |
|---|---|---|---|---|
| “LIT-HF” NCT05572957 China Recruiting | Multicenter Prospective RCT No blinding | 50 HBP/LBAP vs. GDMT | Symptomatic NICM with LVEF < 35% Complete LBBB NYHA II-III status Standard indications for pacemakers LVEF > 35% | Primary: at 6 months - Requirements of new ICD for SCD - New reduction in LVEF < 35% Secondary: at 18 months - LVEF, LVESV, and LVEDV - NYHA status - QOL analysis |
| “HIS-CRT” NCT05265520 USA Recruiting | Multicenter Prospective RCT Single-blinded | 120 HOT-CRT vs. BiV-CRT | LVEF < 35% with RBBB QRS >150 ms LVEF <35% with RBBB QRS 120–149 ms | Primary: at 6 months - Change in LVEF Secondary: at 6 months - Paced QRS width - LVESV and LVEDV |
| “REINVENT” NCT05652218 USA Completed recruiting/not published yet | Multicenter Crossover Prospective RCT Single-arm Double-blinded | 21 LOT-CRT or BiV-CRT | Strict LBBB NYHA I-IV status LVEF > 35% on stable GDMT | Primary: at 6 months - Change in myocardial performance index Secondary: - None |
| “HIS-alt_2” NCT04409119 Denmark Recruiting | Multicenter Prospective RCT Single-blinded | 125 HBP/LBAP vs. BiV-CRT | NICM LVEF < 35% Typical LBBB NYHA II-IV status Planned CRT-P/CRT-D upgrade following standard indications | Primary: at 6 months - Change in LVESV - Successful rate of narrowed paced QRS width with HBP or LBAP Secondary: at 6 months - LVEF and LVEDD - NYHA status - QOL |
| “LBBAP-AFHF” NCT05549544 China Recruiting | Multicenter Prospective RCT Double-blinded | 60 LBAP vs. BiV-CRT | LVEF <50% with 3-month GDMT Permanent AF with planned AVN ablation Permanent AF with slow ventricular rate (anticipated RV pacing > 40%) | Primary: at 6 months - Change in LVEF Secondary: at 6 months - ΔLVEDD and ΔLVEDV -LVEF increase > 5–15% - Composite of all-cause death and/or HF admissions |
| “CSP-SYNC” NCT05155865 Slovenia Completed recruiting completed/not published yet | Single-center Prospective RCT Open-label | 62 LBAP vs. BiV-CRT | LVEF < 35% with 3-month GDMT CLBBB NYHA II-III status | Primary: at 12 months - Change in LV volume and LVEF - 6 min walk test Secondary: at 12 months - Myocardial work redistribution - Paced QRS width - Arrhythmia occurrence |
| “CONSYST-CRT” NCT05187611 Spain Completed recruiting/not published yet | Multicenter Prospective RCT Non-inferiority | 130 HBP/LBAP vs. BiV-CRT | LVEF < 35% with 3-month GDMT LBBB QRS > 130 ms IVCD QRS > 150 ms NYHA III-IV status | Primary: at 12 months - Composite of all-cause death, heart transplant, HF admissions, and LVEF improvement (>5%) Secondary: at 12 months - LVEF and LVESV - Paced QRS width - NYHA status |
| “Safety and Effectiveness of Left Bundle Branch Pacing in Patients With Cardiac Dysfunction and AV Block” NCT05553626 China Not yet recruiting | Multicenter Open-label Prospective RCT | 160 LBAP vs. BiV-CRT | LVEF < 50% NYHA I-III Second or third AVB Anticipated RV pacing > 40% | Primary: at 12 months - Change in LVEF Secondary: at 12 months - Change in LVESV - Implant success - All-cause death and/or HF admissions - Paced QRS width |
| “LeCaRT” NCT05365568 Belgium Active/not recruiting | Multicenter Open-label Prospective RCT | 170 LBAP vs. BiV-CRT | Standard indications for CRT NYHA II-IV LBBB QRS > 130 ms IVCD QRS > 150 ms Wide paced QRS | Primary: at 12 months - Composite of all-cause death, HF admissions, implant failure, and CIED re-intervention Secondary: at 12 months - Procedure and fluoroscopy time - Paced QRS width - 6 min walk - ICD therapy |
| “LEFT-BUNDLE-CRT” NCT05434962 Spain Recruiting | Multicenter Non-inferiority Open-label Prospective RCT | 176 LBAP/LOT-CRT vs. BiV-CRT | Standard indications for CRT (class I or IIa) LBBB | Primary: at 12 months - CRT response Secondary: at 12 months - LVEF - Clinical outcomes - Death, HF admissions, heart transplantation, or VT/VF events |
| “PhysioSync-HF” NCT05572736 Brazil Active/not recruiting | Multicenter Prospective RCT Double-blinded | 304 HBP/LBAP vs. BiV-CRT | LVEF < 35% NYHA II-III status LBBB QRS > 130 ms Standard indications for CRT | Primary: at 12 months - Composite of all-cause death, HF admissions, and LVEF change Secondary: at 12 months - Cost-effectiveness - Composite of all-cause death, HF admissions, and urgent HF visit |
| “Left vs. Left Randomized Clinical Trial” NCT05650658 USA, Canada Recruiting | Multicenter Prospective RCT Triple-blinded | 2136 HBP/LBAP vs. BiV-CRT | LVEF < 50% QRS > 130 ms Upgrade to CRT due to RV pacing > 40% | Primary: at 66 months - Composite of all-cause death and HF admissions Secondary: at 66 months - QOL - Clinical outcomes - Changes in LVESV index - NYHA status - Appropriate ICD therapy |
| Study Name | Study Design | Size | Population | Endpoints |
|---|---|---|---|---|
| “LBBAP-AFHF” NCT05549544 China Recruiting | Multicenter Double-blinded Prospective RCT | 60 LBAP vs. BiV-CRT | LVEF < 50% NYHA II-IV status Permanent AF QRS < 130 ms Anticipated RV pacing > 40% due to planned AVN ablation or slow ventricular rate | Primary: at 6 months - ΔLVEF Secondary: at 6 months - Implant success rate - ΔLVEDD and ΔLVEDV - All-cause death and HF admissions |
| “CONDUCT-AF” NCT05467163 Europe Recruiting | Multicenter Open-Label Prospective RCT | 82 HBP/LBAP vs. BiV-CRT | LVEF < 50% QRS < 120 ms Permanent AF with anticipated AVN ablation after CIED implantation | Primary: at 24 months - ΔLVEF Secondary: at 24 months - HF admissions and/or cardiac death - ΔLVEDD and ΔLVEDV - NYHA status |
| “RAFT-P&A” NCT05428787 Canada Active/not recruiting | Multicenter Double-blinded Prospective RCT | 284 LBAP vs. BiV-CRT AVN ablation after 4 weeks of CIED implantation | LVEF < 50% QRS < 120 ms NYHA I-Iva status Permanent AF considered for AVN ablation | Primary: at 12 months - ΔNT-proBNP Secondary: at 12 months - Composite of HF admissions and/or all-cause death - Change in QOL - ΔLVEF, ΔLVESV index, and ΔLV global longitudinal strain - NYHA status |
| Study Name | Study Design | Size | Population | Endpoints |
|---|---|---|---|---|
| “PHYS-TAVI” NCT04482816 Spain Completed recruiting/not published yet | Single-center Double-blinded Prospective RCT | 24 HBP/LBAP vs. RVP | Post-TAVI AVB LVEF > 50% | Primary: at 12 months - Composite of overall survival, improvement in NYHA status, and/or 6 min walk Secondary: at 12 months - ΔLVEF - Correction of echocardiographic asynchrony - HF admissions - Paced QRS width |
| “PLANET” NCT05024279 Germany Active/not recruiting | Single-center Double-blinded Prospective RCT | 30 LBAP vs. RVP | Post-TAVI AVB Post-TAVI AF with anticipated RV pacing > 20% LVEF > 50% | Primary: at 24 months - Paced QRS width Secondary: at 24 months - Clinical outcomes - HF admissions - ΔLVEF and ΔLVEDD - NYHA status |
| “Left Bundle BRAVE” NCT05541679 USA Recruiting | Multicenter Crossover Double-blinded Prospective RCT | 46 LBAP vs. RVP | Post-TAVI AVB LVEF > 50% | Primary: at 18 months - Change in LVEF and global longitudinal strain - Composite of LV septal or coronary perforation, lead dislodgement Secondary: at 18 months - Clinical outcomes - Echocardiographic parameters |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Neill, T.G.; Tsushima, T.; Tayal, B. Cardiac Resynchronization Therapy and Conduction System Pacing. J. Clin. Med. 2025, 14, 3212. https://doi.org/10.3390/jcm14093212
O’Neill TG, Tsushima T, Tayal B. Cardiac Resynchronization Therapy and Conduction System Pacing. Journal of Clinical Medicine. 2025; 14(9):3212. https://doi.org/10.3390/jcm14093212
Chicago/Turabian StyleO’Neill, Thomas Garvey, Takahiro Tsushima, and Bhupendar Tayal. 2025. "Cardiac Resynchronization Therapy and Conduction System Pacing" Journal of Clinical Medicine 14, no. 9: 3212. https://doi.org/10.3390/jcm14093212
APA StyleO’Neill, T. G., Tsushima, T., & Tayal, B. (2025). Cardiac Resynchronization Therapy and Conduction System Pacing. Journal of Clinical Medicine, 14(9), 3212. https://doi.org/10.3390/jcm14093212






