Biochemical Profile Variations Among Type 2 Diabetic Patients Stratified by Hemoglobin A1c Levels in a Saudi Cohort: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Data
2.3. Statistical Analysis
3. Results
3.1. Study Population
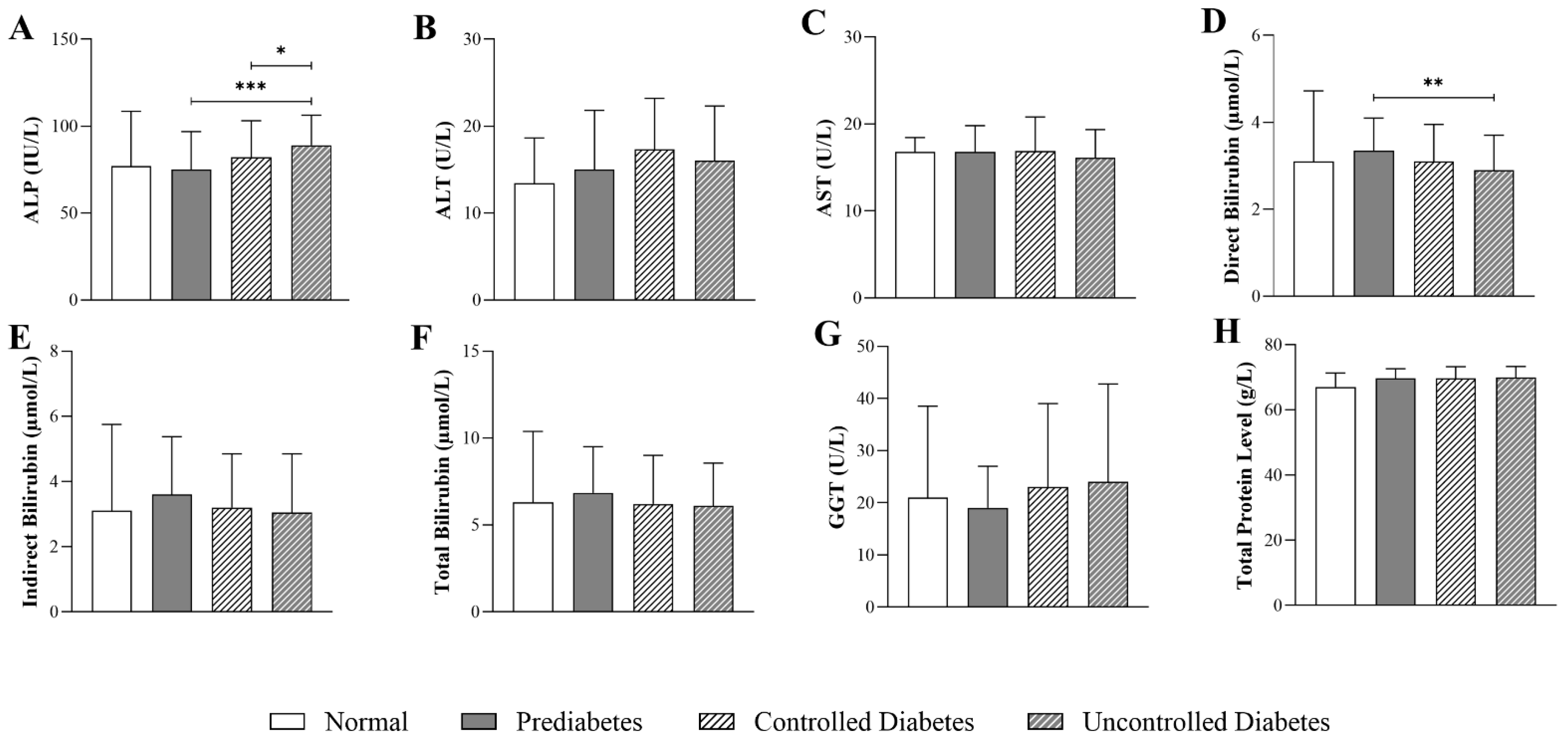
3.2. Impact of HbA1c Levels on Liver-Function Parameters in Patients with T2DM
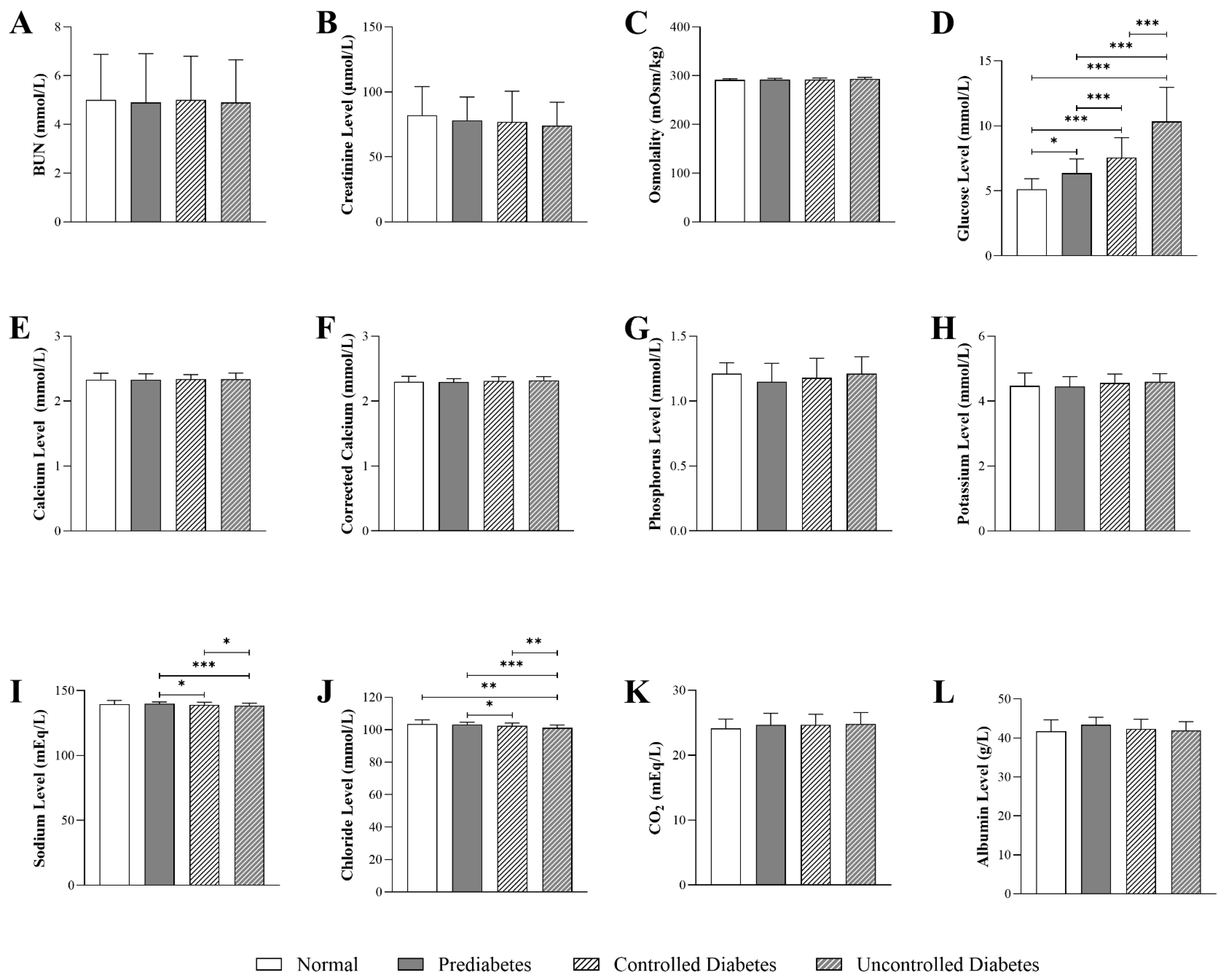
3.3. Impact of HbA1c Levels on Kidney-Function Parameters in Patients with T2DM
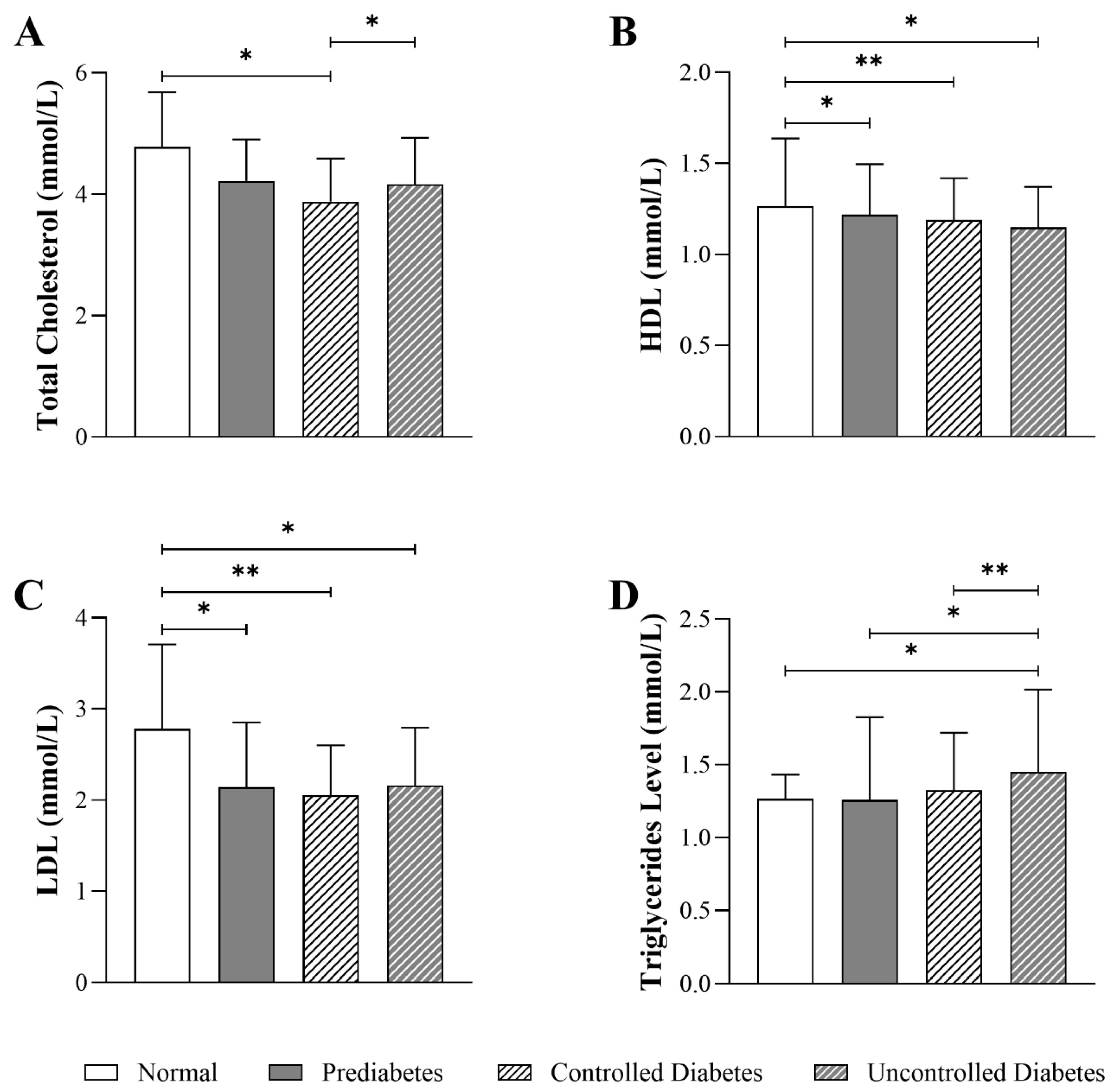
3.4. Impact of HbA1c Levels on Lipid Parameters in Patients with T2DM
3.5. Relationship Between Demographic, Clinical Factors, and Biochemical Parameters in Diabetic Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Association |
| ALP | Alkaline Phosphatase |
| ALT | Alanine Aminotransaminase |
| AST | Aspartate Aminotransferase |
| BMI | Body Mass Index |
| BUN | Blood Urea Nitrogen |
| CAP | College of American Pathologists |
| CKD | Chronic Kidney Disease |
| CO2 | Carbon Dioxide |
| CVD | Cardiovascular Disease |
| DM | Diabetes Mellitus |
| GGT | Gamma-Glutamyl Transferase |
| HbA1c | Hemoglobin A1c |
| HDL | High-Density Lipoprotein |
| IDF | International Diabetes Federation |
| IRB | Institutional Review Board |
| KKUH | King Khalid University Hospital |
| LDL | Low-Density Lipoprotein |
| NAFLD | Nonalcoholic Fatty Fiver Disease |
| Q1–Q3 | First and third quartiles |
| T2DM | Type 2 Diabetes Mellitus |
Appendix A
| Independent Variables | Dependent Variables | |||||||
|---|---|---|---|---|---|---|---|---|
| ALP | Direct Bilirubin | Chloride | Glucose Level | Sodium Level | Total Cholesterol | LDL | Triglycerides Level | |
| Sex | 0.052 (p = 0.221) | −0.040 (p = 0.353) | 0.026 (p = 0.533) | 0.040 (p = 0.333) | 0.010 (p = 0.814) | 0.132 (p = 0.003) | 0.069 (p = 0.123) | −0.038 (p = 0.400) |
| Age | −0.057 (p = 0.180) | 0.065 (p = 0.132) | −0.045 (p = 0.274) | −0.044 (p =0.288) | −0.038 (p =0.364) | −0.201 (p ≤0.001) | −0.211 (p ≤0.001) | 0.023 (p =0.608) |
| BMI | 0.037 (p = 0.458) | −0.019 (p = 0.700) | 0.005 (p = 0.914) | 0.060 (p = 0.214) | −0.045 (p = 0.356) | 0.039 (p = 0.438) | 0.053 (p = 0.292) | 0.034 (p = 0.499) |
| Height | 0.024 (p = 0.615) | 0.021 (p = 0.662) | −0.001 (p = 0.984) | −0.050 (p = 0.288) | 0.055 (p = 0.240) | −0.053 (p = 0.285) | −0.055 (p = 0.266) | 0.070 (p = 0.160) |
| Weight | 0.100 (p = 0.028) | −0.010 (p = 0.829) | 0.031 (p = 0.483) | 0.049 (p = 0.262) | −0.005 (p = 0.912) | 0.039 (p = 0.410) | 0.019 (p = 0.678) | 0.146 (p = 0.002) |
| Smoking Status | −0.019 (p = 0.657) | 0.010 (P = 0.819) | 0.005 (p = 0.907) | −0.011 (p = 0.795) | 0.024 (p = 0.558) | −0.054 (p = 0.226) | −0.044 (p = 0.322) | −0.017 (p = 0.697) |
| Marital Status | −0.038 (p = 0.368) | 0.040 (p = 0.346) | 0.071 (p = 0.085) | −0.046 (p = 0.268) | 0.018 (p = 0.667) | −0.030 (p = 0.495) | −0.058 (p = 0.195) | −0.019 (p = 0.674) |
| Comorbidity | 0.103 (p = 0.016) | 0.000 (p = 0.994) | 0.059 (p = 0.155) | 0.010 (p = 0.802) | −0.037 (p = 0.375) | −0.066 (p = 0.142) | −0.044 (p = 0.325) | −0.033 (p = 0.456) |
| Metformin | 0.078 (p = 0.068) | −0.004 (p = 0.932) | −0.009 (p = 0.826) | 0.014 (p = 0.729) | −0.110 (p = 0.008) | −0.007 (p = 0.874) | 0.016 (p = 0.722) | −0.018 (p = 0.687) |
References
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2024, 48, S27–S49. [Google Scholar] [CrossRef] [PubMed]
- CDC. National Diabetes Statistics Report. Available online: https://www.cdc.gov/diabetes/php/data-research/index.html (accessed on 15 May 2024).
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Magliano, D.J.; Boyko, E.J.; IDF Diabetes Atlas 10th Edition Scientific Committee. IDF DIABETES ATLAS. Available online: https://www.ncbi.nlm.nih.gov/books/NBK581934/ (accessed on 20 June 2025).
- Alwadeai, K.S.; Alhammad, S.A. Prevalence of type 2 diabetes mellitus and related factors among the general adult population in Saudi Arabia between 2016–2022: A systematic review and meta-analysis of the cross-sectional studies. Medicine 2023, 102, e34021. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.A.; Al Dawish, M.A. The Worrying Trend of Diabetes Mellitus in Saudi Arabia: An Urgent Call to Action. Curr. Diabetes Rev. 2020, 16, 204–210. [Google Scholar] [CrossRef]
- Alhowaish, A.K. Economic costs of diabetes in Saudi Arabia. J. Fam. Community Med. 2013, 20, 1–7. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights 2016, 11, 95–104. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Chwalba, A.; Basek, A.; Cieślar, G.; Pawlas, N. Glycated Hemoglobin and Cardiovascular Disease in Patients Without Diabetes. J. Clin. Med. 2024, 14, 53. [Google Scholar] [CrossRef]
- Sacks, D.B.; Arnold, M.; Bakris, G.L.; Bruns, D.E.; Horvath, A.R.; Lernmark, Å.; Metzger, B.E.; Nathan, D.M.; Kirkman, M.S. Guidelines and Recommendations for Laboratory Analysis in the Diagnosis and Management of Diabetes Mellitus. Diabetes Care 2023, 46, e151–e199. [Google Scholar] [CrossRef]
- Tabit, C.E.; Chung, W.B.; Hamburg, N.M.; Vita, J.A. Endothelial dysfunction in diabetes mellitus: Molecular mechanisms and clinical implications. Rev. Endocr. Metab. Disord. 2010, 11, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef]
- London, A.; Lundsgaard, A.M.; Kiens, B.; Bojsen-Møller, K.N. The Role of Hepatic Fat Accumulation in Glucose and Insulin Homeostasis-Dysregulation by the Liver. J. Clin. Med. 2021, 10, 390. [Google Scholar] [CrossRef]
- Ghouri, N.; Preiss, D.; Sattar, N. Liver enzymes, nonalcoholic fatty liver disease, and incident cardiovascular disease: A narrative review and clinical perspective of prospective data. Hepatology 2010, 52, 1156–1161. [Google Scholar] [CrossRef]
- Targher, G.; Corey, K.E.; Byrne, C.D.; Roden, M. The complex link between NAFLD and type 2 diabetes mellitus—Mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 599–612. [Google Scholar] [CrossRef]
- Naser, A.Y.; Alwafi, H.; Alotaibi, B.; Salawati, E.; Samannodi, M.; Alsairafi, Z.; Alanazi, A.F.R.; Dairi, M.S. Prevalence of Chronic Kidney Diseases in Patients with Diabetes Mellitus in the Middle East: A Systematic Review and Meta-Analysis. Int. J. Endocrinol. 2021, 2021, 4572743. [Google Scholar] [CrossRef] [PubMed]
- Liamis, G.; Liberopoulos, E.; Barkas, F.; Elisaf, M. Diabetes mellitus and electrolyte disorders. World J. Clin. Cases 2014, 2, 488–496. [Google Scholar] [CrossRef]
- Bursac, Z.; Gauss, C.H.; Williams, D.K.; Hosmer, D.W. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 2008, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Jeppsson, J.O.; Kobold, U.; Barr, J.; Finke, A.; Hoelzel, W.; Hoshino, T.; Miedema, K.; Mosca, A.; Mauri, P.; Paroni, R.; et al. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin. Chem. Lab. Med. 2002, 40, 78–89. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, C.; Li, J.; Zhang, Y.; Xie, D.; Liang, M.; Wang, B.; Song, Y.; Wang, X.; Huo, Y.; et al. Serum alkaline phosphatase levels and the risk of new-onset diabetes in hypertensive adults. Cardiovasc. Diabetol. 2020, 19, 186. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, H.S.; Park, H.M.; Lee, Y.J. Serum alkaline phosphatase level is positively associated with metabolic syndrome: A nationwide population-based study. Clin. Chim. Acta 2020, 500, 189–194. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Yue, R.; Ren, X.; Zhang, X.; Ni, A. The effects of diabetes mellitus and diabetic nephropathy on bone and mineral metabolism in T2DM patients. Diabetes Res. Clin. Pract. 2013, 100, 272–276. [Google Scholar] [CrossRef]
- Nannipieri, M.; Gonzales, C.; Baldi, S.; Posadas, R.; Williams, K.; Haffner, S.M.; Stern, M.P.; Ferrannini, E. Liver enzymes, the metabolic syndrome, and incident diabetes: The Mexico City diabetes study. Diabetes Care 2005, 28, 1757–1762. [Google Scholar] [CrossRef]
- Hanley, A.J.; Williams, K.; Festa, A.; Wagenknecht, L.E.; D’Agostino, R.B., Jr.; Kempf, J.; Zinman, B.; Haffner, S.M. Elevations in markers of liver injury and risk of type 2 diabetes: The insulin resistance atherosclerosis study. Diabetes 2004, 53, 2623–2632. [Google Scholar] [CrossRef]
- Kolivas, D.; Fraser, L.; Schweitzer, R.; Brukner, P.; Moschonis, G. A 6-Month mHealth Low-Carbohydrate Dietary Intervention Ameliorates Glycaemic and Cardiometabolic Risk Profile in People with Type 2 Diabetes. Nutrients 2025, 17, 937. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.; Luscombe-Marsh, N.D.; Thompson, C.H.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S., Jr.; Brinkworth, G.D. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: A randomized trial. Am. J. Clin. Nutr. 2015, 102, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Shahwan, M.J.; Jairoun, A.A.; Farajallah, A.; Shanabli, S. Prevalence of dyslipidemia and factors affecting lipid profile in patients with type 2 diabetes. Diabetes Metab. Syndr. 2019, 13, 2387–2392. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, I.; Ijaz, M.; Rahim, A. Correlation between hemoglobin A1c and serum lipid profile in Afghani patients with type 2 diabetes: Hemoglobin A1c prognosticates dyslipidemia. Ther. Adv. Endocrinol. Metab. 2017, 8, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.J.; Han, K.; Ko, S.H.; Ko, K.S.; Lee, W.Y. Increased risk for diabetes development in subjects with large variation in total cholesterol levels in 2,827,950 Koreans: A nationwide population-based study. PLoS ONE 2017, 12, e0176615. [Google Scholar] [CrossRef]
- Artha, I.; Bhargah, A.; Dharmawan, N.K.; Pande, U.W.; Triyana, K.A.; Mahariski, P.A.; Yuwono, J.; Bhargah, V.; Prabawa, I.P.Y.; Manuaba, I.; et al. High level of individual lipid profile and lipid ratio as a predictive marker of poor glycemic control in type-2 diabetes mellitus. Vasc. Health Risk Manag. 2019, 15, 149–157. [Google Scholar] [CrossRef]
- Taskinen, M.R. Diabetic dyslipidaemia: From basic research to clinical practice. Diabetologia 2003, 46, 733–749. [Google Scholar] [CrossRef]
- Erion, D.M.; Park, H.J.; Lee, H.Y. The role of lipids in the pathogenesis and treatment of type 2 diabetes and associated co-morbidities. BMB Rep. 2016, 49, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, S.H.; Baig, M.; Aashi, M.M.; Al-Shaibi, F.K.; Alqarni, D.A.; Bakhamees, W.H. Association between glycated hemoglobin (HbA1c) and the lipid profile in patients with type 2 diabetes mellitus at a tertiary care hospital: A retrospective study. Diabetes Metab. Syndr. Obes. 2019, 12, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Shahwan, M.J.; Khattab, A.H.; Khattab, M.H.; Jairoun, A.A. Association between abnormal serum hepatic enzymes, lipid levels and glycemic control in patients with type 2 diabetes mellitus. Obes. Med. 2019, 16, 100137. [Google Scholar] [CrossRef]
- Lin, D.; Qi, Y.; Huang, C.; Wu, M.; Wang, C.; Li, F.; Yang, C.; Yan, L.; Ren, M.; Sun, K. Associations of lipid parameters with insulin resistance and diabetes: A population-based study. Clin. Nutr. 2018, 37, 1423–1429. [Google Scholar] [CrossRef]
- Alam, S.; Raghav, A.; Reyaz, A.; Ahsan, A.; Ahirwar, A.K.; Jain, V.; Agarwal, S.; Tripathi, P. Prevalence of elevated liver enzymes and its relationship with type 2 diabetes mellitus in North Indian adults. Metab. Open 2021, 12, 100130. [Google Scholar] [CrossRef]
- Visaria, A.; Kanaya, A.; Setoguchi, S.; Gadgil, M.; Satagopan, J. Inverse association between total bilirubin and type 2 diabetes in U.S. South Asian males but not females. PLoS ONE 2024, 19, e0297685. [Google Scholar] [CrossRef]
- Abbasi, A.; Deetman, P.E.; Corpeleijn, E.; Gansevoort, R.T.; Gans, R.O.; Hillege, H.L.; van der Harst, P.; Stolk, R.P.; Navis, G.; Alizadeh, B.Z.; et al. Bilirubin as a potential causal factor in type 2 diabetes risk: A Mendelian randomization study. Diabetes 2015, 64, 1459–1469. [Google Scholar] [CrossRef]
- Jang, B.K. Elevated serum bilirubin levels are inversely associated with nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2012, 18, 357–359. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Electrolyte and Acid–Base Disturbances in Patients with Diabetes Mellitus. N. Engl. J. Med. 2015, 373, 548–559. [Google Scholar] [CrossRef]
- Matyus, S.P.; Braun, P.J.; Wolak-Dinsmore, J.; Jeyarajah, E.J.; Shalaurova, I.; Xu, Y.; Warner, S.M.; Clement, T.S.; Connelly, M.A.; Fischer, T.J. NMR measurement of LDL particle number using the Vantera Clinical Analyzer. Clin. Biochem. 2014, 47, 203–210. [Google Scholar] [CrossRef] [PubMed]
| Variable | Groups | |||
|---|---|---|---|---|
| Normal (<5.7%) (23 Patients) | Prediabetes (5.7–6.4%) (98 Patients) | Controlled Diabetes (6.5–7.9%) (228 Patients) | Uncontrolled Diabetes (≥8.0%) (272 Patients) | |
| Sex, n (%) | ||||
| Male | 11 (47.8) | 46 (46.9) | 110 (48.2) | 113 (41.5) |
| Female | 12 (52.2) | 52 (53.1) | 128 (51.8) | 159 (58.5) |
| Age (years), n (%) | ||||
| 18–30 | 0 (0) | 0 (0) | 4 (1.8) | 12 (4.4) |
| 31–40 | 1 (4.3) | 1 (1.0) | 7 (3.1) | 4 (1.5) |
| 41–50 | 2 (8.7) | 11 (11.2) | 29 (12.7) | 35 (12.9) |
| 51–60 | 8 (34.8) | 26 (26.5) | 51 (22.4) | 64 (23.5) |
| >60 | 12 (52.2) | 60 (61.2) | 137 (60.1) | 156 (57.4) |
| Glucose fasting (mmol/L), median (Q1–Q3) | 5.9 (5.6–6.0) | 6.7 (6.1–7.3) | 8.4 (7.1–9.2) | 9.4 (8.5–12.5) |
| BMI, median (Q1–Q3) | 32.8 (26.2–38.9) | 29.2 (26.1–32.0) | 30.1 (26.3–33.6) | 30.1 (26.8–33.4) |
| Height (cm), median (Q1–Q3) | 157.0 (152.3–165.5) | 161.0 (156.0–170.0) | 162.0 (152.0–169.0) | 159.0 (153.0–168.0) |
| Weight (kg), median (Q1–Q3) | 80.5 (64.5–94.3) | 77.0 (63.2–86.8) | 77.0 (64.0–89.0) | 78.0 (67.1–87.5) |
| Smoking Status, n (%) | ||||
| Smoker | 0 (0.0) | 2 (2.0) | 3 (1.3) | 0 (0.0) |
| Non-smoker | 23 (100.0) | 96 (98.0) | 225 (98.7) | 272 (100.0) |
| Marital Status, n (%) | ||||
| Single | 5 (21.7) | 19 (19.4) | 55 (24.1) | 63 (23.2) |
| Married | 18 (78.3) | 79 (80.6) | 173 (75.9) | 209 (76.8) |
| Comorbidity, n (%) | ||||
| Circulatory Complications | 0 (0.0) | 1 (1.0) | 0 (0.0) | 1 (0.4) |
| Cardiomyopathy | 0 (0.0) | 0 (0.0) | 1 (0.4) | 0 (0.0) |
| Neuropathy | 1 (4.3) | 3 (3.1) | 2 (0.9) | 4 (1.5) |
| Nephropathy | 0 (0.0) | 0 (0.0) | 1 (0.4) | 0 (0.0) |
| Ketoacidosis | 0 (0.0) | 0 (0.0) | 1 (0.4) | 0 (0.0) |
| Musculoskeletal | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) |
| Insulin resistance | 2 (8.7) | 0 (0.0) | 1 (0.4) | 0 (0.0) |
| No comorbidity | 20 (87.0) | 94 (95.9) | 221 (96.9) | 265 (97.4) |
| Taking Metformin, n (%) | ||||
| No | 22 (95.7) | 90 (91.8) | 214 (93.9) | 265 (97.4) |
| Yes | 1 (4.3) | 8 (8.2) | 14 (6.1) | 7 (2.6) |
| Independent Variables | Coefficient (B) | R Square |
|---|---|---|
| ALP | 0.021 (p = 0.042) | |
| Age | −2.093 (p = 0.486) | |
| Weight | 0.204 (p = 0.047) | |
| Comorbidity | 6.406 (p = 0.153) | |
| Metformin | 20.013 (p = 0.096) | |
| Cholesterol | ||
| Sex | 0.324 (p ≤0. 001) | |
| Age | −0.264 (p ≤ 0.001) | 0.073 (p ≤ 0.001) |
| Comorbidity | −0.240 (p = 0.136) | |
| LDL | ||
| Sex | 0.158 (p = 0.046) | |
| Age | −0.235 (p ≤ 0.001) | 0.060 (p ≤ 0.001) |
| Marital Status | 0.056 (p = 0.599) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshalani, A.; AlAhmari, N.; Amin, H.A.; Aljedai, A.; AlSudais, H. Biochemical Profile Variations Among Type 2 Diabetic Patients Stratified by Hemoglobin A1c Levels in a Saudi Cohort: A Retrospective Study. J. Clin. Med. 2025, 14, 5324. https://doi.org/10.3390/jcm14155324
Alshalani A, AlAhmari N, Amin HA, Aljedai A, AlSudais H. Biochemical Profile Variations Among Type 2 Diabetic Patients Stratified by Hemoglobin A1c Levels in a Saudi Cohort: A Retrospective Study. Journal of Clinical Medicine. 2025; 14(15):5324. https://doi.org/10.3390/jcm14155324
Chicago/Turabian StyleAlshalani, Abdulrahman, Nada AlAhmari, Hajar A. Amin, Abdullah Aljedai, and Hamood AlSudais. 2025. "Biochemical Profile Variations Among Type 2 Diabetic Patients Stratified by Hemoglobin A1c Levels in a Saudi Cohort: A Retrospective Study" Journal of Clinical Medicine 14, no. 15: 5324. https://doi.org/10.3390/jcm14155324
APA StyleAlshalani, A., AlAhmari, N., Amin, H. A., Aljedai, A., & AlSudais, H. (2025). Biochemical Profile Variations Among Type 2 Diabetic Patients Stratified by Hemoglobin A1c Levels in a Saudi Cohort: A Retrospective Study. Journal of Clinical Medicine, 14(15), 5324. https://doi.org/10.3390/jcm14155324






