Obesity Is a Thrombotic Risk Factor in Pregnant Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Cohorts
2.2. Materials
2.3. Blood Plasma Sampling
2.4. Preparation of Platelet-Rich Blood Plasma (PRP)
2.5. Fibrinogen
2.6. Soluble Fibrin
2.7. D-dimer
2.8. Platelet Aggregation
2.9. Statistics
3. Results
3.1. Characterization of Selected Cohorts
3.2. Fibrinogen
3.3. D-dimer
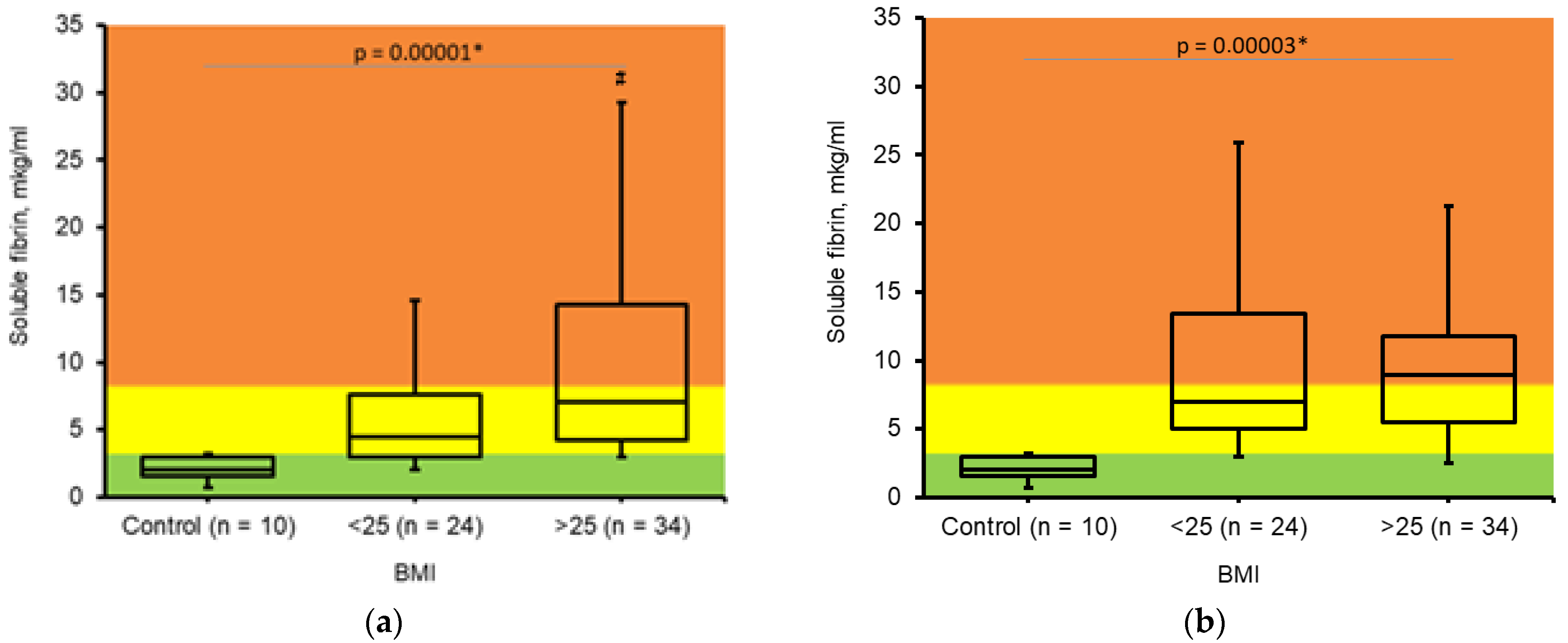
3.4. Soluble Fibrin
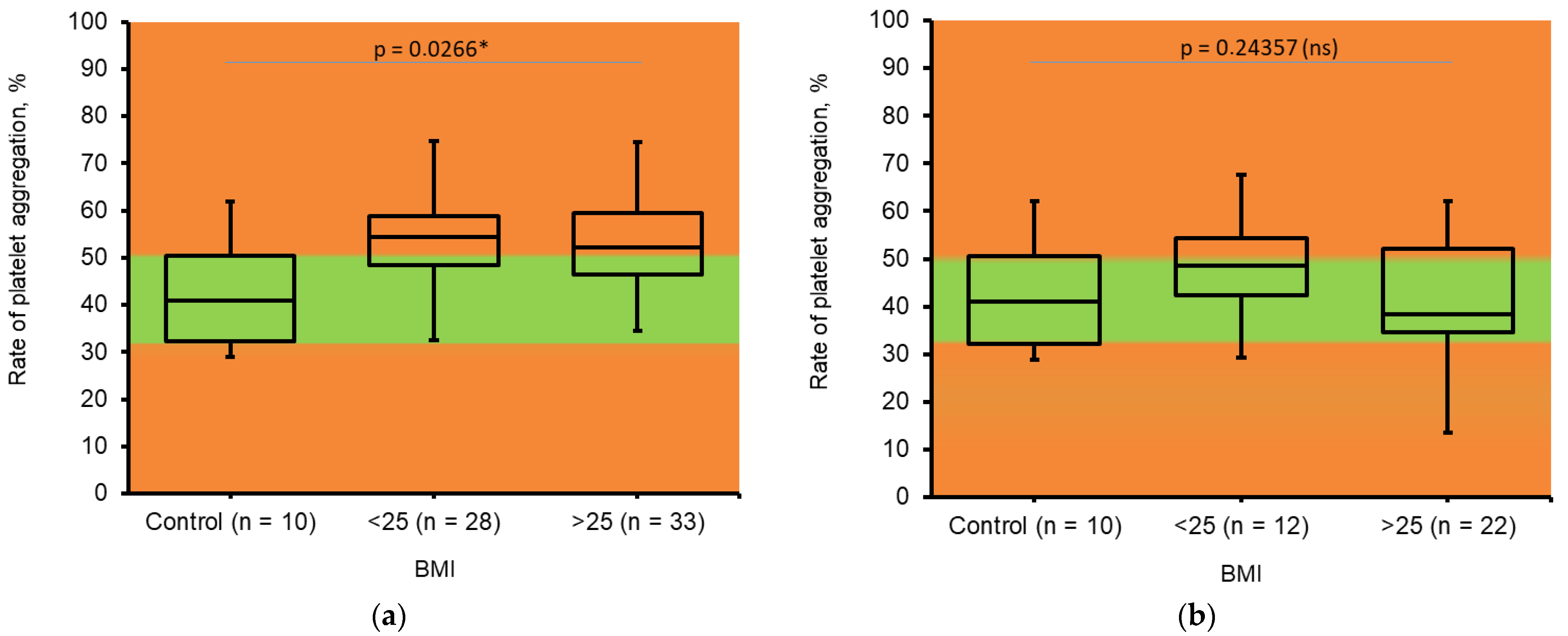
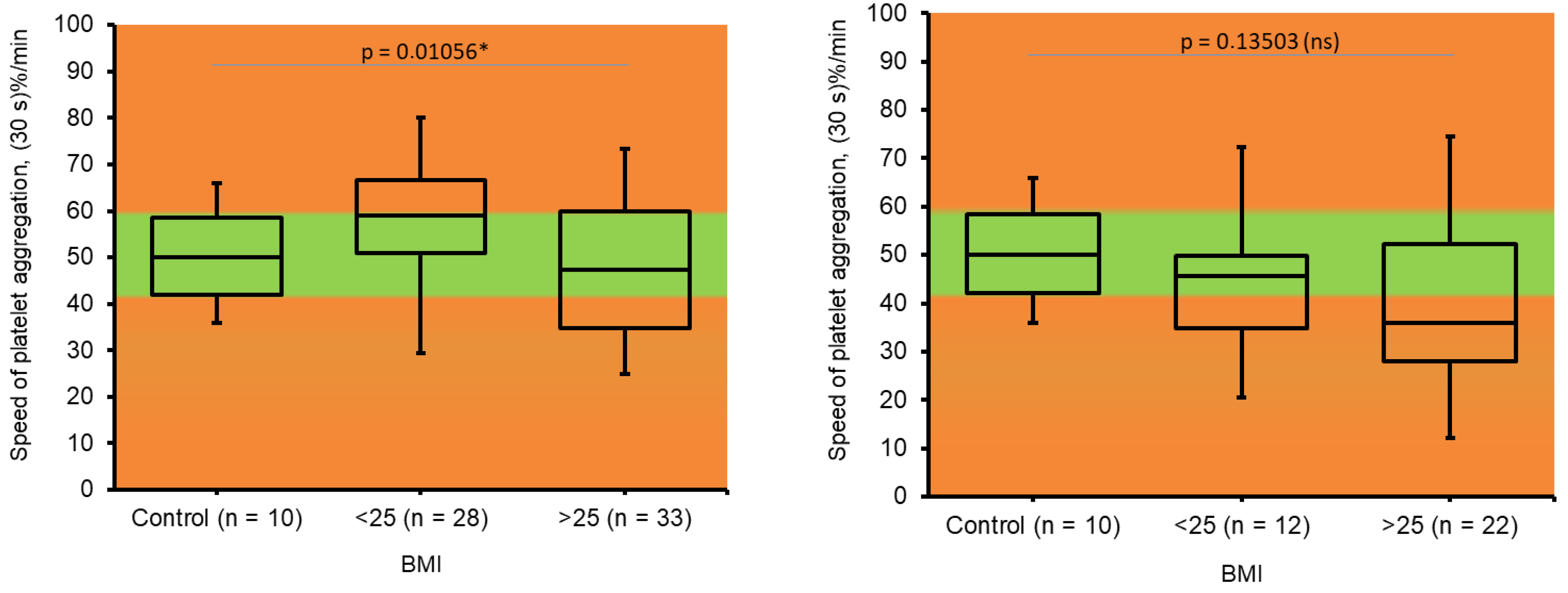
3.5. Platelet Aggregation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VTE | Venous thromboembolism |
| BMI | Body mass index |
| PRP | Platelet rich blood plasma |
References
- Kent, L.; McGirr, M.; Eastwood, K.A. Global trends in prevalence of maternal overweight and obesity: A systematic review and meta-analysis of routinely collected data retrospective cohorts. Int. J. Popul. Data Sci. 2024, 9, 2401. [Google Scholar] [CrossRef] [PubMed]
- Fatokun, T.B.; Swartz, S.E.; Ebeid, A.; Cordes, S.A.; Gimovsky, A.C.; Sparks, A.D.; Amdur, R.L.; Ahmadzia, H.K. Venous Thromboembolism Risk Factors in Women with Obesity Who Undergo Cesarean Delivery. Clin. Appl. Thromb. Hemost. 2024, 30, 10760296241247203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fitzsimons, K.J.; Modder, J.; Greer, I.A. Obesity in pregnancy: Risks and management. Obstet. Med. 2009, 2, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef] [PubMed]
- Hotoleanu, C. Association between obesity and venous thromboembolism. Med. Pharm. Rep. 2020, 93, 162–168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, G.; De Staercke, C.; Hooper, W.C. The effects of obesity on venous thromboembolism: A review. Open J. Prev. Med. 2012, 2, 499–509. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reducing the Risk of Thrombosis and Embolism During Pregnancy and the Puerperium (Green-Top Guideline No. 37a). Available online: https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/reducing-the-risk-of-thrombosis-and-embolism-during-pregnancy-and-the-puerperium-green-top-guideline-no-37a/ (accessed on 21 April 2025).
- Obesity in Early Pregnancy Increases Long-Term Risk of Venous Thromboembolism. Available online: https://www.news-medical.net/news/20230907/Obesity-in-early-pregnancy-increases-long-term-risk-of-venous-thromboembolism.aspx (accessed on 21 April 2025).
- Campello, E.; Zabeo, E.; Radu, C.M.; Spiezia, L.; Gavasso, S.; Fadin, M.; Woodhams, B.; Vettor, R.; Simioni, P. Hypercoagulability in overweight and obese subjects who are asymptomatic for thrombotic events. Thromb. Haemost. 2015, 113, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Hunt, B.J. The effect of BMI on haemostasis: Implications for thrombosis in women’s health. Thromb. Res. 2017, 151, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Dai, L.; Chen, H.-Q.; Xia, W.; Wang, Q.-L.; Zhu, C.-R.; Zhou, R. Specific changes and clinical significance of plasma D-dimer during pregnancy and puerperium: A prospective study. BMC Pregnancy Childbirth 2023, 23, 248. [Google Scholar] [CrossRef] [PubMed]
- Arachchillage, D.J.; Mackillop, L.; Chandratheva, A.; Motawani, J.; MacCallum, P.; Laffan, M. Thrombophilia testing: A British Society for Haematology guideline. Br. J. Haematol. 2022, 198, 443–458. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montagnana, M.; Lippi, G.; Danese, E. An Overview of Thrombophilia and Associated Laboratory Testing. Methods Mol. Biol. 2017, 1646, 113–135. [Google Scholar] [CrossRef] [PubMed]
- Korolova, D.S.; Syrko, M.; Stohnii, Y.; Chernyshenko, T.; Gogolinska, G. Standardization of the protein calibrators isolation methodology for thrombophilia markers detecting immunodiagnostic test systems. Biotechnol. Acta 2021, 14, 61–69. [Google Scholar] [CrossRef]
- Chernyshenko, V.; Shteinberg, K.; Lugovska, N.; Ryzhykova, M.; Platonova, T.; Korolova, D.; Lugovskoy, E. Preparation of highly-concentrated autologous platelet-rich plasma for biomedical use. Ukr. Biochem. J. 2019, 91, 19–27. [Google Scholar] [CrossRef]
- Sokolovska, A.S.; Chernyshenko, T.M.; Ivanenko, T.I. Comparative characteristic of fibrinogen level determination methods in blood plasma. Exper. Clin. Phisiol. Biochem. 2002, 3, 82–86. [Google Scholar]
- Lugovskoy, E.V.; Kolesnikova, I.N.; Komisarenko, S.V. Usage of monoclonal antibodies for determination of localization of antigenic determinants and fibrin polymerization sites within fibrinogen and fibrin molecules and their application in test-systems for diagnostics and the threat of thrombus formation. Biotechnol. Acta 2013, 6, 33–42. [Google Scholar] [CrossRef]
- Lugovskoi, E.V.; Kolesnikova, I.N.; Lugovskaia, N.E.; Litvinova, L.M.; Gritsenko, P.G.; Gogolinskaia, G.K.; Liashko, E.D.; Kostiuchenko, E.P.; Remizovskiĭ, G.A.; Pedchenko, V.N.; et al. Quantification of D-dimer and soluble fibrin in blood plasma of people with ischemic heart disease and hypertension. Ukr. Biokhim Zh. 1999, 76, 136–141. [Google Scholar] [PubMed]
- Born, G.V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962, 194, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Kattula, S.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen and Fibrin in Hemostasis and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 13–21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siennicka, A.; Kłysz, M.; Chełstowski, K.; Tabaczniuk, A.; Marcinowska, Z.; Tarnowska, P.; Kulesza, J.; Torbe, A.; Jastrzębska, M. Reference Values of D-Dimers and Fibrinogen in the Course of Physiological Pregnancy: The Potential Impact of Selected Risk Factors-A Pilot Study. BioMed Res. Int. 2020, 2020, 3192350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Targher, G.; Zoppini, G.; Moghetti, P.; Day, C.P. Disorders of coagulation and hemostasis in abdominal obesity: Emerging role of fatty liver. Semin. Thromb. Hemost. 2010, 36, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wauthier, L.; Favresse, J.; Hardy, M.; Douxfils, J.; Le Gal, G.; Roy, P.-M.; van Es, N.; Ay, C.; ten Cate, H.; Vander Borght, T.; et al. D-dimer Testing in Pulmonary Embolism with a Focus on Potential Pitfalls: A Narrative Review. Diagnostics. 2022, 12, 2770. [Google Scholar] [CrossRef] [PubMed]
- Forstner, D.; Guettler, J.; Gauster, M. Changes in Maternal Platelet Physiology during Gestation and Their Interaction with Trophoblasts. Int. J. Mol. Sci. 2021, 22, 10732. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, C.; Ma, H.; Zhang, T.; Liu, Y.; Zhang, C.; Deng, S. A Microflow Chip Technique for Monitoring Platelets in Late Pregnancy: A Possible Risk Factor for Thrombosis. J. Blood Med. 2025, 8, 15–25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| BMI < 25 kg/m2 | BMI > 25 kg/m2 | |||
|---|---|---|---|---|
| Trimester | II (n = 28) | III (n = 16) | II (n = 37) | III (n = 25) |
| Gestational age at sampling | 21.04 ± 3.87 | 32.94 ± 2.11 | 21.16 ± 3.68 | 33 ± 3.71 |
| Gestational age at delivery (w) | 38.1 ± 3.79 | 38 ± 3 | 38.24 ± 1.05 | 38.84 ± 1.62 |
| Weight gain during pregnancy (kg) | 11.79 ± 4.48 | 12.69 ± 4.16 | 11.38 ± 7.3 | 12.44 ± 6.42 |
| BW (g) | 3076.43 ± 876.92 | 3401.88 ± 622.9 | 3317.06 ± 489.78 | 3365.56 ± 559.35 |
| OGTT 0 | 4.24 ± 0.24 | 4.16 ± 0.27 | 4.34 ± 0.43 | 4.39 ± 0.4 |
| OGTT 120 | 5.13 ± 1.3 | 4.63 ± 0.86 | 4.89 ± 1.3 | 4.88 ± 1.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korolova, D.; Suranyi, A.; Pavlenko, A.; Altorjay, A.T.; Zhuk, S.; Us, I.; Melnyk, Y.; Chernyshenko, V.; Vari, S.G. Obesity Is a Thrombotic Risk Factor in Pregnant Women. J. Clin. Med. 2025, 14, 5310. https://doi.org/10.3390/jcm14155310
Korolova D, Suranyi A, Pavlenko A, Altorjay AT, Zhuk S, Us I, Melnyk Y, Chernyshenko V, Vari SG. Obesity Is a Thrombotic Risk Factor in Pregnant Women. Journal of Clinical Medicine. 2025; 14(15):5310. https://doi.org/10.3390/jcm14155310
Chicago/Turabian StyleKorolova, Daria, Andrea Suranyi, Anastasiia Pavlenko, Abel T. Altorjay, Svitlana Zhuk, Iryna Us, Yurii Melnyk, Volodymyr Chernyshenko, and Sandor G. Vari. 2025. "Obesity Is a Thrombotic Risk Factor in Pregnant Women" Journal of Clinical Medicine 14, no. 15: 5310. https://doi.org/10.3390/jcm14155310
APA StyleKorolova, D., Suranyi, A., Pavlenko, A., Altorjay, A. T., Zhuk, S., Us, I., Melnyk, Y., Chernyshenko, V., & Vari, S. G. (2025). Obesity Is a Thrombotic Risk Factor in Pregnant Women. Journal of Clinical Medicine, 14(15), 5310. https://doi.org/10.3390/jcm14155310








