EVAR Trends over the Past Decade and Their Impact on Aneurysm Mortality: National Health Insurance Data Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
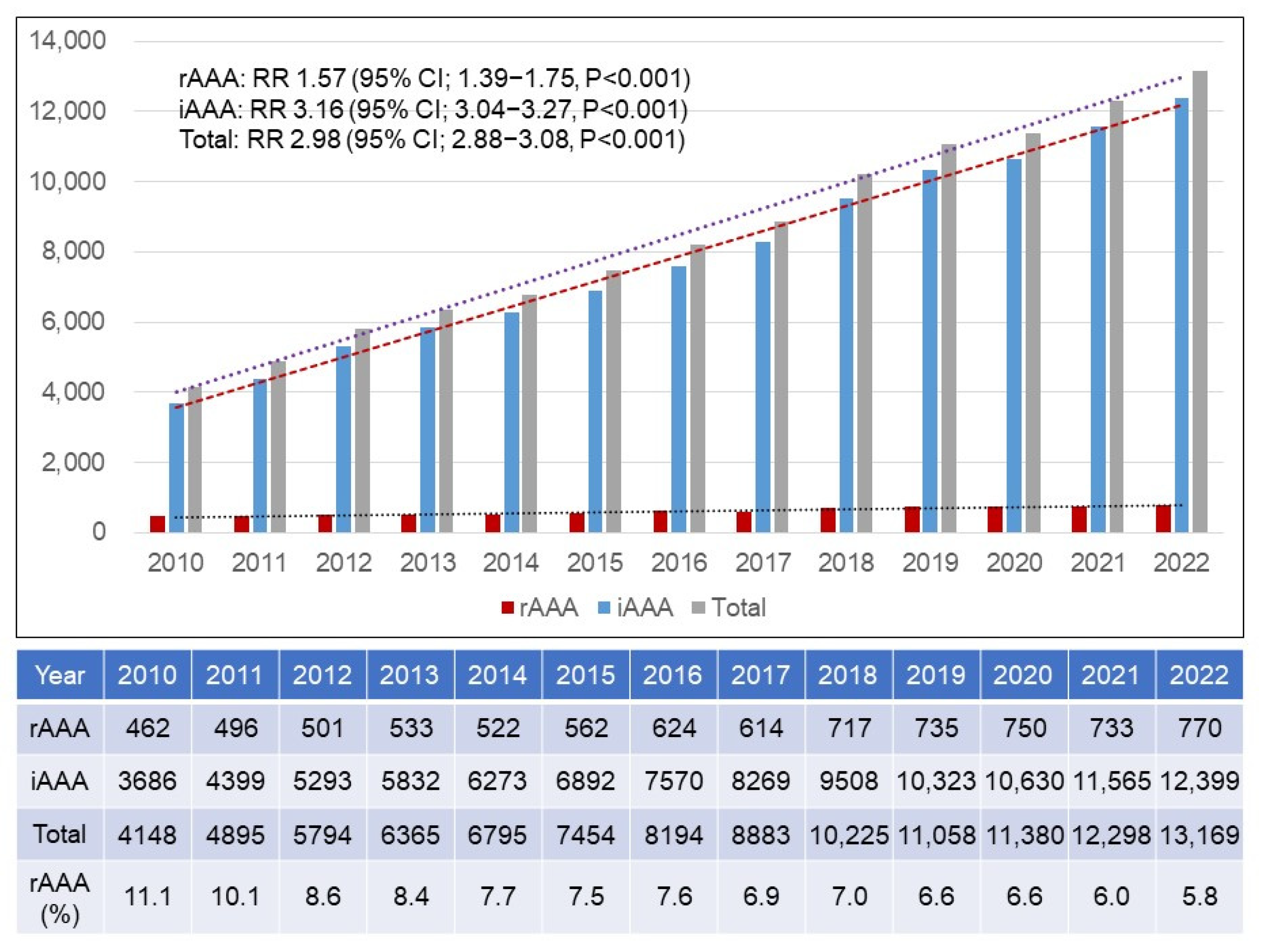
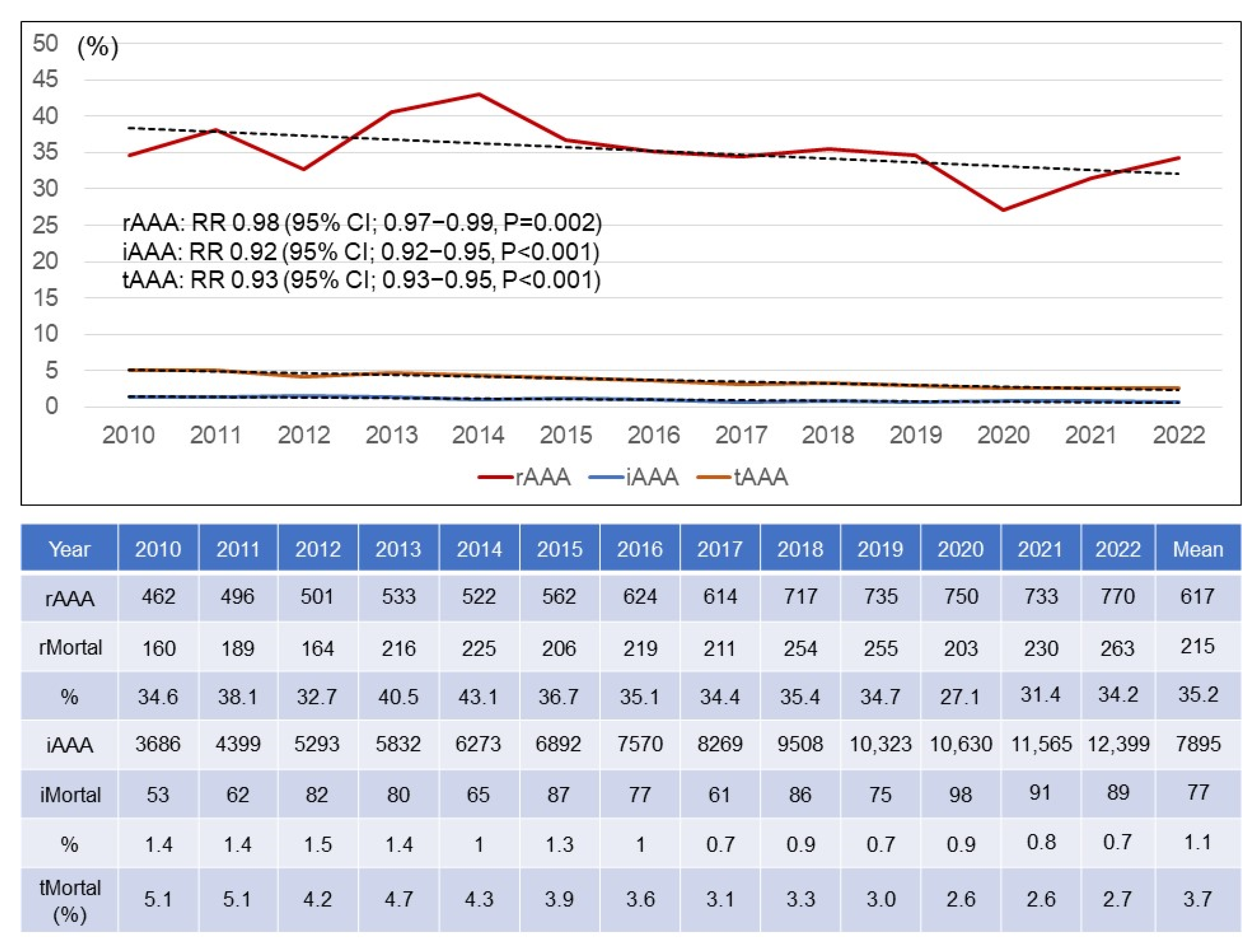
3.1. Annual Numbers of rAAAs and iAAAs
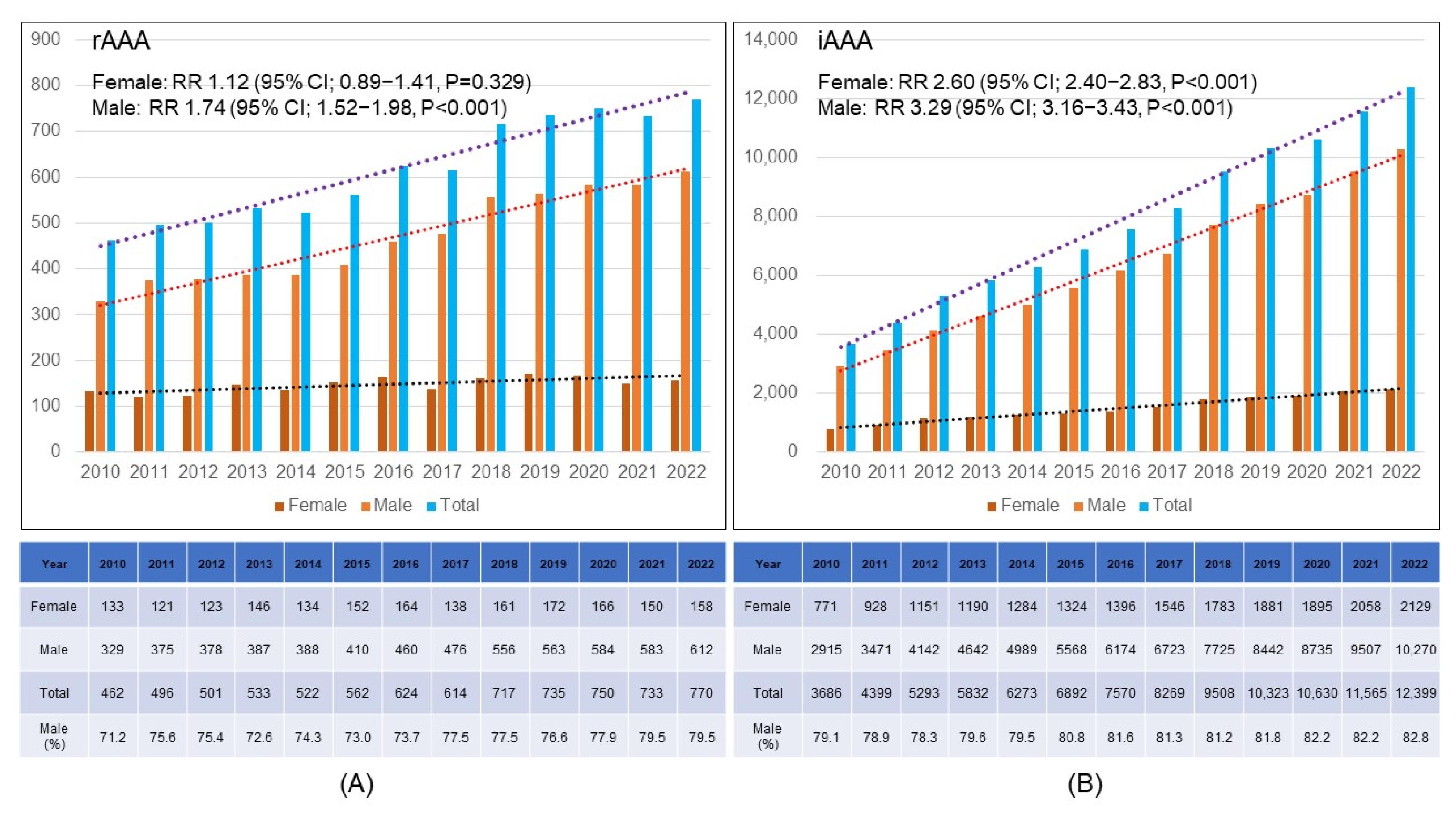
3.2. Gender Distribution
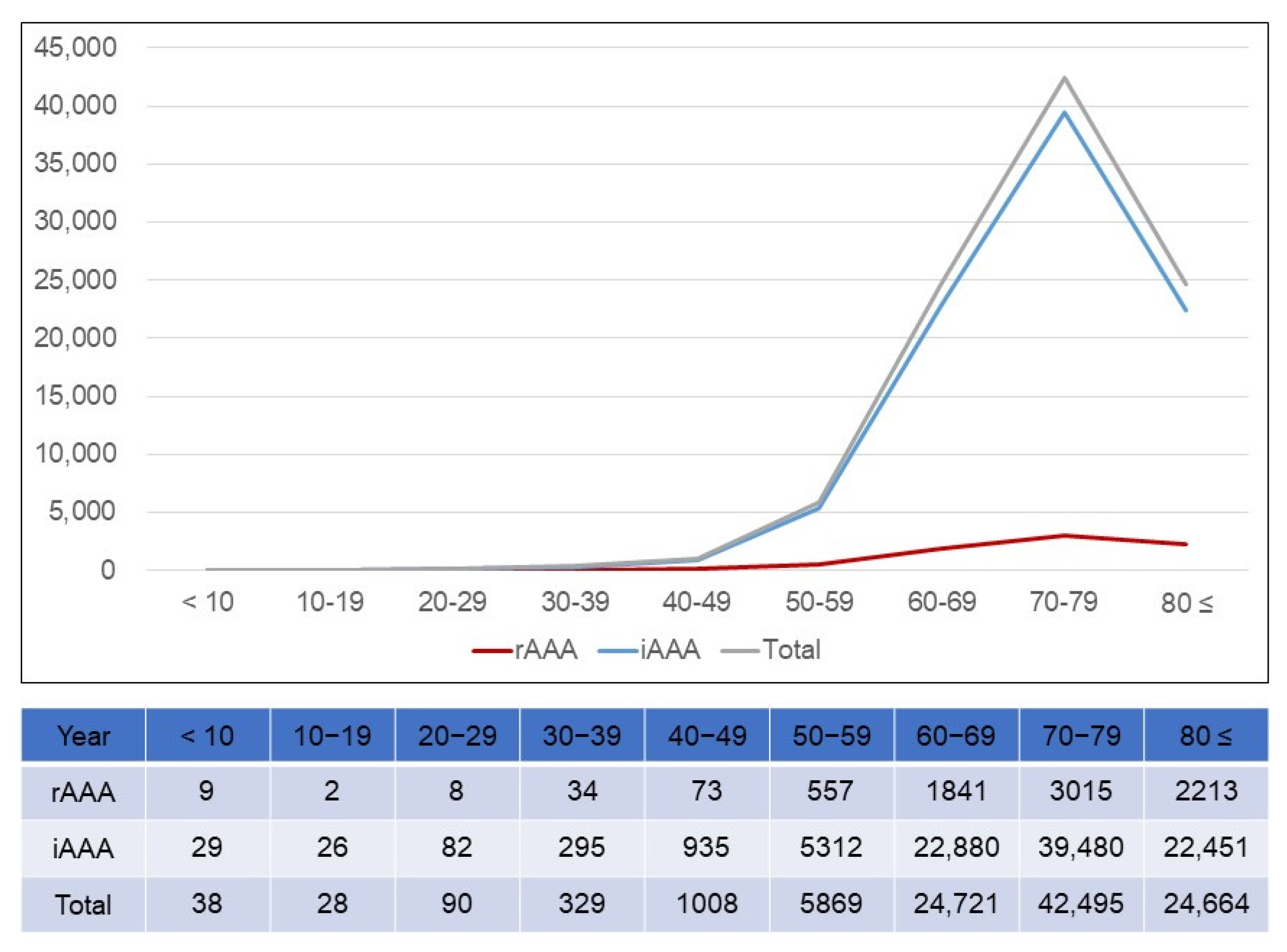
3.3. Age Distribution
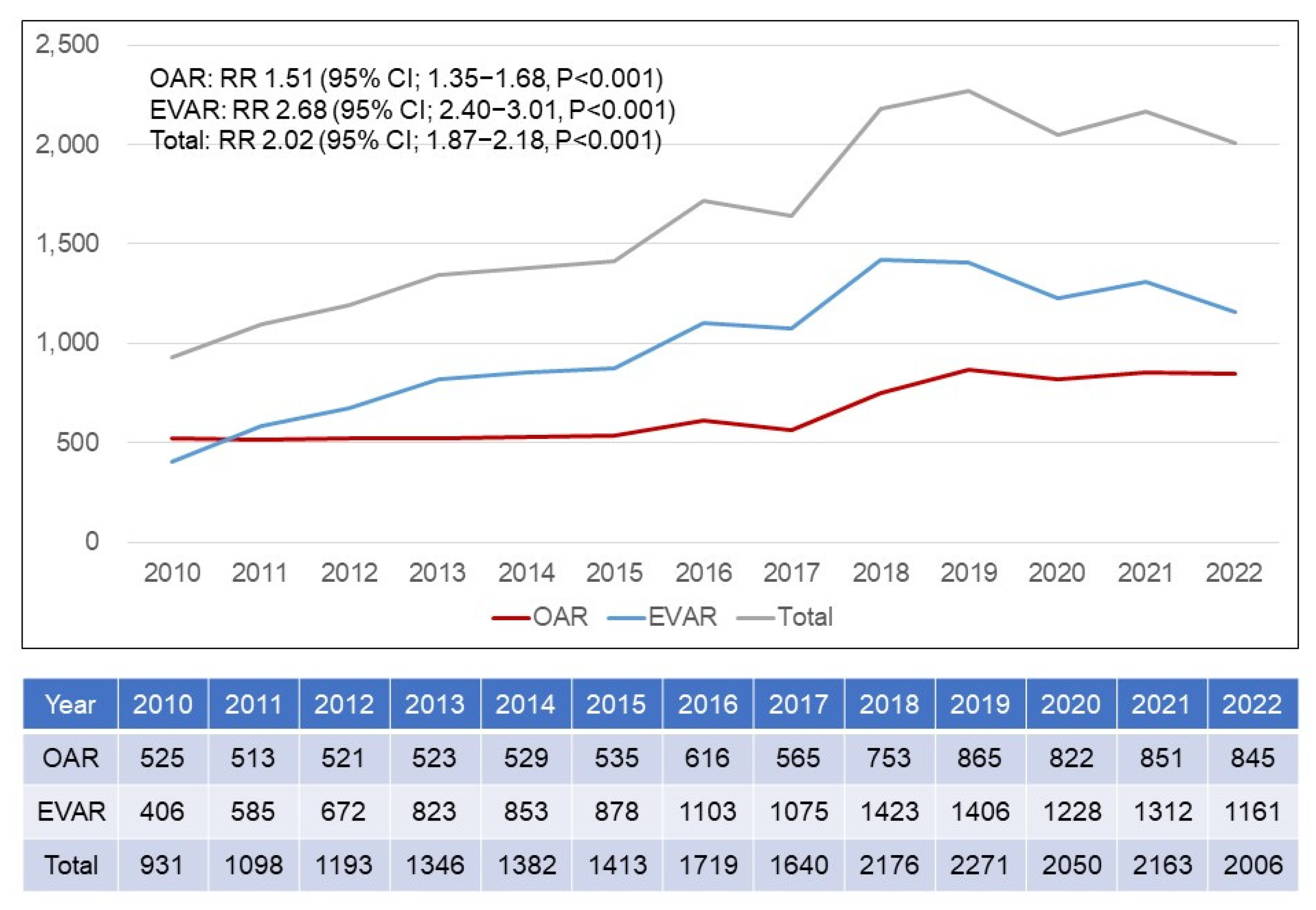
3.4. Annual Trends in EVAR Versus OAR
3.5. Mortality Rate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parodi, J.C.; Palmaz, J.C.; Barone, H.D. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann. Vasc. Surg. 1991, 5, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, R.M.; Brown, L.C.; Kwong, G.P.; Powell, J.T.; Thompson, S.G.; EVAR trial participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: Randomised controlled trial. Lancet 2004, 364, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Prinssen, M.; Verhoeven, E.L.; Buth, J.; Cuypers, P.W.; van Sambeek, M.R.; Balm, R.; Buskens, E.; Grobbee, D.E.; Blankensteijn, J.D.; Dutch Randomized Endovascular Aneurysm Management (DREAM) Trial Group. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N. Engl. J. Med. 2004, 351, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Lederle, F.A.; Freischlag, J.A.; Kyriakides, T.C.; Padberg, F.T., Jr.; Matsumura, J.S.; Kohler, T.R.; Lin, P.H.; Jean-Claude, J.M.; Cikrit, D.F.; Swanson, K.M.; et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: A randomized trial. JAMA 2009, 302, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77. [Google Scholar] [CrossRef] [PubMed]
- Wanhainen, A.; Van Herzeele, I.; Bastos Goncalves, F.; Bellmunt Montoya, S.; Berard, X.; Boyle, J.R.; D’Oria, M.; Prendes, C.F.; Karkos, C.D.; Kazimierczak, A.; et al. European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 192–331. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Kim, J.A.; Kim, S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol. Health 2014, 36, e2014008. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, G.A.; Giannoukas, A.D.; Georgiadis, G.S.; Antoniou, S.A.; Simopoulos, C.; Prassopoulos, P.; Lazarides, M.K. Increased prevalence of abdominal aortic aneurysm in patients undergoing inguinal hernia repair compared with patients without hernia receiving aneurysm screening. J. Vasc. Surg. 2011, 53, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.G.; Ashton, H.A.; Gao, L.; Buxton, M.J.; Scott, R.A.; Multicentre Aneurysm Screening Study (MASS) Group. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br. J. Surg. 2012, 99, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Guirguis-Blake, J.M.; Beil, T.L.; Senger, C.A.; Whitlock, E.P. Ultrasonography screening for abdominal aortic aneurysms: A systematic evidence review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2014, 160, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.U.; Fitzpatrick-Lewis, D.; Kenny, M.; Miller, J.; Raina, P.; Sherifali, D. A systematic review of short-term vs long-term effectiveness of one-time abdominal aortic aneurysm screening in men with ultrasound. J. Vasc. Surg. 2018, 68, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Hurks, R.; Ultee, K.H.J.; Buck, D.B.; DaSilva, G.S.; Soden, P.A.; van Herwaarden, J.A.; Verhagen, H.J.M.; Schermerhorn, M.L. The impact of endovascular repair on specialties performing abdominal aortic aneurysm repair. J. Vasc. Surg. 2015, 62, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Tilson, M.D. Public awareness of aortic aneurysm. Cardiovasc. Surg. 2001, 9, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Malayala, S.V.; Raza, A.; Vanaparthy, R. Gender-Based Differences in Abdominal Aortic Aneurysm Rupture: A Retrospective Study. J. Clin. Med. Res. 2020, 12, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Stuntz, M.; Audibert, C.; Su, Z. Persisting disparities between sexes in outcomes of ruptured abdominal aortic aneurysm hospitalizations. Sci. Rep. 2017, 7, 17994. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Bønaa, K.H.; Jacobsen, B.K.; Bjørk, L.; Solberg, S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: The Tromsø Study. Am. J. Epidemiol. 2001, 154, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.P.; Banerjee, A.; Fairhead, J.F.; Handa, A.; Silver, L.E.; Rothwell, P.M.; Oxford Vascular Study. Age-specific incidence, risk factors and outcome of acute abdominal aortic aneurysms in a defined population. Br. J. Surg. 2015, 102, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Gormley, S.; Bernau, O.; Xu, W.; Sandiford, P.; Khashram, M. Incidence and Outcomes of Abdominal Aortic Aneurysm Repair in New Zealand from 2001 to 2021. J. Clin. Med. 2023, 12, 2331. [Google Scholar] [CrossRef] [PubMed]
- Yei, K.; Mathlouthi, A.; Naazie, I.; Elsayed, N.; Clary, B.; Malas, M. Long-term Outcomes Associated with Open vs Endovascular Abdominal Aortic Aneurysm Repair in a Medicare-Matched Database. JAMA Netw. Open. 2022, 5, e2212081. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Powell, J.T.; Sweeting, M.J.; Epstein, D.M.; Barrett, J.K.; Greenhalgh, R.M. The UK EndoVascular Aneurysm Repair (EVAR) randomised controlled trials: Long-term follow-up and cost-effectiveness analysis. Health Technol. Assess. 2018, 22, 1–132. [Google Scholar] [CrossRef] [PubMed]
- Lederle, F.A.; Kyriakides, T.C.; Stroupe, K.T.; Freischlag, J.A.; Padberg, F.T., Jr.; Matsumura, J.S.; Huo, Z.; Johnson, G.R.; OVER Veterans Affairs Cooperative Study Group. Open versus Endovascular Repair of Abdominal Aortic Aneurysm. N. Engl. J. Med. 2019, 380, 2126–2135. [Google Scholar] [CrossRef] [PubMed]
- Karkos, C.D.; Harkin, D.W.; Giannakou, A.; Gerassimidis, T.S. Mortality after endovascular repair of ruptured abdominal aortic aneurysms: A systematic review and meta-analysis. Arch. Surg. 2009, 144, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Hoornweg, L.L.; Storm-Versloot, M.N.; Ubbink, D.T.; Koelemay, M.J.; Legemate, D.A.; Balm, R. Meta analysis on mortality of ruptured abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 558–570. [Google Scholar] [CrossRef] [PubMed]

| KCD codes 1 | Diseases |
| I713 | Abdominal aortic aneurysm, ruptured |
| I714 | Abdominal aortic aneurysm, without mention of rupture |
| EDI codes 2 | Open repair |
| O0223 | Abdominal aorta, suprarenal |
| O0224 | Abdominal aorta, infrarenal |
| O2034 | Abdominal aorta involving iliac artery |
| Endovascular repair | |
| M6612 | Placement of stent graft, abdominal aorta involving iliac artery |
| Year | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total number | 525 | 513 | 521 | 523 | 529 | 535 | 616 | 565 | 753 | 865 | 822 | 851 | 845 |
| Suprarenal | 454 | 422 | 454 | 437 | 442 | 461 | 521 | 456 | 631 | 702 | 697 | 713 | 721 |
| Infrarenal | 71 | 91 | 67 | 86 | 87 | 74 | 95 | 109 | 122 | 163 | 125 | 138 | 124 |
| Suprarenal % | 13.5 | 17.7 | 12.9 | 16.4 | 16.4 | 13.8 | 15.4 | 19.3 | 16.2 | 18.8 | 15.2 | 16.2 | 14.7 |
| Male | 426 | 423 | 429 | 421 | 437 | 421 | 492 | 466 | 627 | 686 | 668 | 703 | 728 |
| Female | 99 | 90 | 92 | 102 | 92 | 114 | 124 | 99 | 126 | 179 | 154 | 148 | 117 |
| Female % | 18.9 | 17.5 | 17.7 | 19.5 | 17.4 | 21.3 | 20.1 | 17.5 | 16.7 | 20.7 | 18.7 | 17.4 | 13.8 |
| Non-octogenarians | 477 | 473 | 468 | 470 | 474 | 463 | 532 | 488 | 648 | 725 | 677 | 704 | 699 |
| Octogenarians | 48 | 40 | 53 | 53 | 55 | 72 | 84 | 77 | 105 | 140 | 145 | 147 | 146 |
| Octogenarians % | 9.1 | 7.8 | 10.2 | 10.1 | 10.4 | 13.5 | 13.6 | 13.6 | 13.9 | 16.2 | 17.6 | 17.3 | 17.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.; Joh, J.H. EVAR Trends over the Past Decade and Their Impact on Aneurysm Mortality: National Health Insurance Data Analysis. J. Clin. Med. 2025, 14, 5277. https://doi.org/10.3390/jcm14155277
Cho S, Joh JH. EVAR Trends over the Past Decade and Their Impact on Aneurysm Mortality: National Health Insurance Data Analysis. Journal of Clinical Medicine. 2025; 14(15):5277. https://doi.org/10.3390/jcm14155277
Chicago/Turabian StyleCho, Sungsin, and Jin Hyun Joh. 2025. "EVAR Trends over the Past Decade and Their Impact on Aneurysm Mortality: National Health Insurance Data Analysis" Journal of Clinical Medicine 14, no. 15: 5277. https://doi.org/10.3390/jcm14155277
APA StyleCho, S., & Joh, J. H. (2025). EVAR Trends over the Past Decade and Their Impact on Aneurysm Mortality: National Health Insurance Data Analysis. Journal of Clinical Medicine, 14(15), 5277. https://doi.org/10.3390/jcm14155277







