Clinical Instability at Discharge and Post-Discharge Outcomes in Patients with Community-Acquired Pneumonia: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Sources
2.2. Definition of Clinical Instability
2.3. Outcomes
2.4. Statistical Analysis
2.5. Sensitivity Analysis
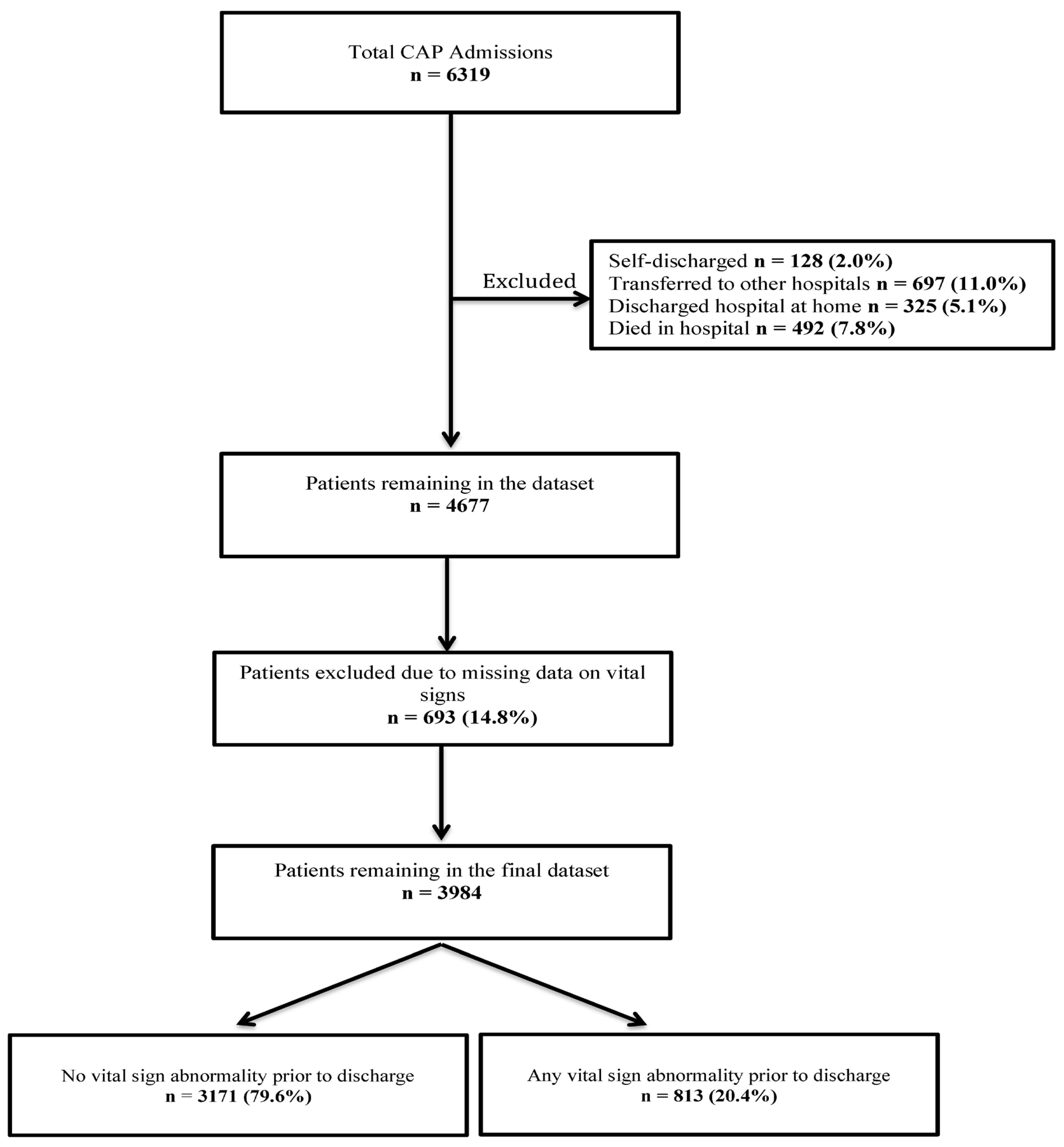
3. Results
4. Discussion
4.1. Key Findings and Comparison with Literature
4.2. Vital Sign Abnormalities and Individual Outcomes
4.3. Temporal Trends and COVID-19 Context
4.4. Implications for Discharge Planning and Post-Discharge Care
4.5. Strengths
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAP | Community-Acquired Pneumonia |
| LOS | Length of Stay |
| aOR | Adjusted Odds Ratio |
| CI | Confidence Interval |
| ICD-10-AM | International Classification of Diseases, 10th Revision, Australian Modification |
| EMR | Electronic Medical Records |
| CURB-65 | Confusion, Urea, Respiratory rate, Blood pressure, Age ≥65 (Pneumonia severity score) |
| HFRS | Hospital Frailty Risk Score |
| ED | Emergency Department |
| SHR | Subdistribution Hazard Ratio |
| SBP | Systolic Blood Pressure |
| RR | Respiratory Rate |
| CCI | Charlson Comorbidity Index |
References
- Del Rosal, T.; Caminoa, M.B.; González-Guerrero, A.; Falces-Romero, I.; Romero-Gómez, M.P.; Baquero-Artigao, F.; Sainz, T.; Méndez-Echevarría, A.; Escosa-García, L.; Aracil, F.J.; et al. Outcome of Severe Bacterial Pneumonia in the Era of Pneumococcal Vaccination. Front. Pediatr. 2020, 8, 576519. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Miller, M.; Kaambwa, B.; Shahi, R.; Hakendorf, P.; Horwood, C.; Thompson, C. Malnutrition and its association with readmission and death within 7 days and 8–180 days postdischarge in older patients: A prospective observational study. BMJ Open 2017, 7, e018443. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.J.; Barrett, M.L.; Steiner, C.A. Trends and Projections in Inpatient Hospital Costs and Utilization, 2003–2013. In Healthcare Cost and Utilization Project (HCUP) Statistical Briefs; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2006. [Google Scholar]
- Buie, V.C.; Owings, M.F.; DeFrances, C.J.; Golosinskiy, A. National hospital discharge survey: 2006 annual summary. Vital Health Stat. 2010, 13, 1–79. [Google Scholar]
- Auerbach, A.D.; Kripalani, S.; Vasilevskis, E.E.; Sehgal, N.; Lindenauer, P.K.; Metlay, J.P.; Fletcher, G.; Ruhnke, G.W.; Flanders, S.A.; Kim, C.; et al. Preventability and Causes of Readmissions in a National Cohort of General Medicine Patients. JAMA Intern. Med. 2016, 176, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Boussat, B.; Cazzorla, F.; Le Marechal, M.; Pavese, P.; Mounayar, A.L.; Sellier, E.; Gaillat, J.; Camara, B.; Degano, B.; Maillet, M.; et al. Incidence of Avoidable 30-Day Readmissions Following Hospitalization for Community-Acquired Pneumonia in France. JAMA Netw. Open 2022, 5, e226574. [Google Scholar] [CrossRef] [PubMed]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef] [PubMed]
- File, T.M., Jr.; Ramirez, J.A. Community-Acquired Pneumonia. N. Engl. J. Med. 2023, 389, 632–641. [Google Scholar] [CrossRef] [PubMed]
- The Dutch Working Party on Antibiotic Policy (SWAB) and Dutch Association of Chest Physicians (NVALT). Management of Community-Acquired Pneumonia in Adults: The 2024 Practice Guideline; SWAB/NVALT: Bergen, Norway, 2024; Available online: https://swab.nl/nl/cap (accessed on 10 July 2025).
- National Institute for Health and Care Excellence (NICE). Pneumonia in Adults: Diagnosis and Management. In NICE Guideline NG138; NICE: London, UK, 2023. [Google Scholar]
- Intermountain Health. Diagnosis and Management of Community-Acquired Pneumonia in Adults; Intermountain Clinical Guidelines: Salt Lake City, UT, USA, 2025. [Google Scholar]
- Medscape. Community-Acquired Pneumonia (CAP). 2025. Available online: https://emedicine.medscape.com/article/234240-overview (accessed on 10 July 2025).
- Nguyen, O.K.; Makam, A.N.; Clark, C.; Zhang, S.; Xie, B.; Velasco, F.; Amarasingham, R.; Halm, E.A. Vital Signs Are Still Vital: Instability on Discharge and the Risk of Post-Discharge Adverse Outcomes. J. Gen. Intern. Med. 2017, 32, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.H.; Chen, J.; Zhu, R.X. The relationship between frailty and community-acquired pneumonia in older patients. Aging Clin. Exp. Res. 2023, 35, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Tani, T.; Imai, S.; Fushimi, K. Impact of the COVID-19 pandemic on emergency admission for patients with stroke: A time series study in Japan. Neurol. Res. Pract. 2021, 3, 64. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Komiya, K.; Fujita, N.; Okabe, E.; Hiramatsu, K.; Kadota, J.I. COVID-19 pandemic and the incidence of community-acquired pneumonia in elderly people. Respir. Investig. 2020, 58, 435–436. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Horwood, C.; Hakendorf, P.; Au, J.; Thompson, C. Characteristics and clinical outcomes of index versus non-index hospital readmissions in Australian hospitals: A cohort study. Aust. Health Rev. 2020, 44, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Iwai-Saito, K.; Shobugawa, Y.; Aida, J.; Kondo, K. Frailty is associated with susceptibility and severity of pneumonia in older adults (A JAGES multilevel cross-sectional study). Sci. Rep. 2021, 11, 7966. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.E.; Costa, L.L.; Yakusheva, O.; Bobay, K.L. Validation of patient and nurse short forms of the Readiness for Hospital Discharge Scale and their relationship to return to the hospital. Health Serv. Res. 2014, 49, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Ilg, A.; Moskowitz, A.; Konanki, V.; Patel, P.V.; Chase, M.; Grossestreuer, A.V.; Donnino, M.W. Performance of the CURB-65 Score in Predicting Critical Care Interventions in Patients Admitted with Community-Acquired Pneumonia. Ann. Emerg. Med. 2019, 74, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Horwood, C.; Hakendorf, P.; Shahi, R.; Thompson, C. External Validation of the Hospital Frailty-Risk Score in Predicting Clinical Outcomes in Older Heart-Failure Patients in Australia. J. Clin. Med. 2022, 11, 2193. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, T.; Neuburger, J.; Kraindler, J.; Keeble, E.; Smith, P.; Ariti, C.; Arora, S.; Street, A.; Parker, S.; Roberts, H.C.; et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: An observational study. Lancet 2018, 391, 1775–1782. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hung, W.W.; Paik, M.C.; Ross, J.S.; Zhao, Z.; Kim, G.S.; Boockvar, K. Predictors and outcomes of unplanned readmission to a different hospital. Int. J. Qual. Health Care 2015, 27, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Dagan, E.; Novack, V.; Porath, A. Adverse outcomes in patients with community acquired pneumonia discharged with clinical instability from Internal Medicine Department. Scand. J. Infect. Dis. 2006, 38, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Mangoni, A.A.; Shahi, R.; Horwood, C.; Thompson, C. Recent temporal trends, characteristics and outcomes of patients with non-COVID-19 community-acquired pneumonia at two tertiary hospitals in Australia: An observational study. Intern. Med. J. 2024, 54, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Cillóniz, C.; Torres, A.; Niederman, M.S. Management of pneumonia in critically ill patients. BMJ 2021, 375, e065871. [Google Scholar] [CrossRef] [PubMed]
- Jelizarow, M.; Mansmann, U.; Goeman, J.J. A Cochran-Armitage-type and a score-free global test for multivariate ordinal data. Stat. Med. 2016, 35, 2754–2769. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C.; Fine, J.P. Practical recommendations for reporting Fine-Gray model analyses for competing risk data. Stat. Med. 2017, 36, 4391–4400. [Google Scholar] [CrossRef] [PubMed]
- Capelastegui, A.; España, P.P.; Bilbao, A.; Martinez-Vazquez, M.; Gorordo, I.; Oribe, M.; Urrutia, I.; Quintana, J.M. Pneumonia: Criteria for patient instability on hospital discharge. Chest 2008, 134, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.; Mangoni, A.A.; Horwood, C.; Thompson, C. External validation and comparative analysis of the HOSPITAL score and LACE index for predicting readmissions among patients hospitalised with community-acquired pneumonia in Australia. Aust. Health Rev. 2024, 48, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Cooksley, T.; Nanayakkara, P.W.; Nickel, C.H.; Subbe, C.P.; Kellett, J.; Kidney, R.; Merten, H.; Van Galen, L.; Henriksen, D.P.; Lassen, A.T.; et al. Readmissions of medical patients: An external validation of two existing prediction scores. QJM Int. J. Med. 2016, 109, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Jasti, H.; Mortensen, E.M.; Obrosky, D.S.; Kapoor, W.N.; Fine, M.J. Causes and risk factors for rehospitalization of patients hospitalized with community-acquired pneumonia. Clin. Infect. Dis. 2008, 46, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Naylor, M.D.; Brooten, D.; Campbell, R.; Jacobsen, B.S.; Mezey, M.D.; Pauly, M.V.; Schwartz, J.S. Comprehensive discharge planning and home follow-up of hospitalized elders: A randomized clinical trial. JAMA 1999, 281, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Halm, E.A.; Fine, M.J.; Kapoor, W.N.; Singer, D.E.; Marrie, T.J.; Siu, A.L. Instability on hospital discharge and the risk of adverse outcomes in patients with pneumonia. Arch. Intern. Med. 2002, 162, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Ewig, S.; Schäfer, H.; Torres, A. Severity assessment in community-acquired pneumonia. Eur. Respir. J. 2000, 16, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Stotts, C.; Corrales-Medina, V.F.; Rayner, K.J. Pneumonia-Induced Inflammation, Resolution and Cardiovascular Disease: Causes, Consequences and Clinical Opportunities. Circ. Res. 2023, 132, 751–774. [Google Scholar] [CrossRef] [PubMed]
- Ukai, T.; Maruyama, T.; Tomioka, S.; Fukui, T.; Matsuda, S.; Fushimi, K.; Iso, H. Predictors of hospital mortality and multidrug-resistant pathogens in hospitalized pneumonia patients residing in the community. Heliyon 2023, 9, e22303. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, M.I.; Reyes, L.F.; Anzueto, A. Complication of Community-Acquired Pneumonia (Including Cardiac Complications). Semin. Respir. Crit. Care Med. 2016, 37, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Capelastegui, A.; España Yandiola, P.P.; Quintana, J.M.; Bilbao, A.; Diez, R.; Pascual, S.; Pulido, E.; Egurrola, M. Predictors of short-term rehospitalization following discharge of patients hospitalized with community-acquired pneumonia. Chest 2009, 136, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Haldane, V.; De Foo, C.; Abdalla, S.M.; Jung, A.S.; Tan, M.; Wu, S.; Chua, A.; Verma, M.; Shrestha, P.; Singh, S.; et al. Health systems resilience in managing the COVID-19 pandemic: Lessons from 28 countries. Nat. Med. 2021, 27, 964–980. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Mo, J.; Lim, Z.Y.; Lu, S.Y.; Low, S.G.; Xu, B.; Loo, Y.X.; Koh, C.W.; Kong, L.Y.; Towle, R.M.; et al. Impact of COVID-19 Measures on Discharge Planning and Continuity of Integrated Care in the Community for Older Patients in Singapore. Int. J. Integr. Care 2022, 22, 13. [Google Scholar] [CrossRef] [PubMed]
- Calvillo-King, L.; Arnold, D.; Eubank, K.J.; Lo, M.; Yunyongying, P.; Stieglitz, H.; Halm, E.A. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: Systematic review. J. Gen. Intern. Med. 2013, 28, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Liapikou, A.; Cilloniz, C.; Torres, A. Drugs that increase the risk of community-acquired pneumonia: A narrative review. Expert Opin. Drug Saf. 2018, 17, 991–1003. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total Cohort | No Death or Readmission Within 30-Days | Death or Readmission |
|---|---|---|---|
| 3984 | 3108 (78.1) | 876 (21.9) | |
| Age years, mean (SD) | 73.4 (17.7) | 72.8 (18.1) | 75.5 (16.1) |
| Age group, n (%) | |||
| <40 | 254 (6.3) | 216 (6.9) | 38 (4.3) |
| 40–59 | 521 (13.1) | 428 (13.7) | 93 (10.6) |
| 60–79 | 1451 (36.4) | 1137 (36.6) | 314 (35.8) |
| >80 | 1758 (44.1) | 1327 (42.7) | 431 (49.2) |
| Sex male, n (%) | 2186 (54.8) | 1677 (53.9) | 509 (58.1) |
| Residence home, n (%) | 3768 (94.5) | 2954 (95.1) | 814 (92.9) |
| Charlson index, mean (SD) | 2.6 (2.8) | 2.2 (2.6) | 3.8 (3.3) |
| CURB-65 score, mean (SD) | 1.6 (1.1) | 1.6 (1.1) | 1.8 (0.9) |
| Severe CAP †, n (%) | 871 (21.9) | 654 (21.0) | 217 (24.8) |
| HFRS, mean (SD) | 4.9 (4.5) | 4.8 (4.4) | 5.8 (4.7) |
| Frail, n (%) | 1542 (38.7) | 1140 (36.7) | 402 (45.9) |
| Microbiological aetiology n (%) | |||
| No pathogen detected | 3382 (84.8) | 2695 (84.9) | 687 (84.5) |
| Bacterial | 285 (7.2) | 226 (7.2) | 59 (7.3) |
| Viral | 234 (5.9) | 191 (6.0) | 43 (5.2) |
| Polymicrobial | 46 (1.2) | 29 (0.9) | 17 (2.1) |
| Others | 37 (0.9) | 30 (1.0) | 7 (0.9) |
| Antibiotics prescribed n (%) | |||
| Penicillin | 1024 (25.7) | 814 (25.6) | 210 (25.8) |
| Cephalosporin | 562 (14.1) | 446 (14.1) | 116 (14.3) |
| Macrolide | 1465 (36.7) | 1156 (36.5) | 309 (38.1) |
| Quinolone | 94 (2.4) | 82 (2.6) | 12 (1.5) |
| Doxycycline | 285 (7.1) | 234 (7.4) | 51 (6.3) |
| Others | 554 (13.1) | 439 (13.8) | 115 (14.2) |
| Hospital admissions past 12 months, mean (SD) | 0.7 (1.4) | 0.5 (1.3) | 1.1 (1.8) |
| ED visits past 6 months, mean (SD) | 0.5 (0.5) | 0.5 (0.5) | 0.7 (0.5) |
| LOS, median (IQR) | 3.9 (2.3, 6.6) | 3.8 (2.2, 6.3) | 4.2 (2.5, 7.3) |
| 30-day mortality, n (%) | 246 (6.1) | 0 | 246 (28.1) |
| †† 30-day readmissions n (%) | 630 (15.8) | 0 | 630 (71.9) |
| Number of Unstable Vitals on Discharge | p Value | |||||
|---|---|---|---|---|---|---|
| Any | 0 | 1 | 2 | ≥3 | ||
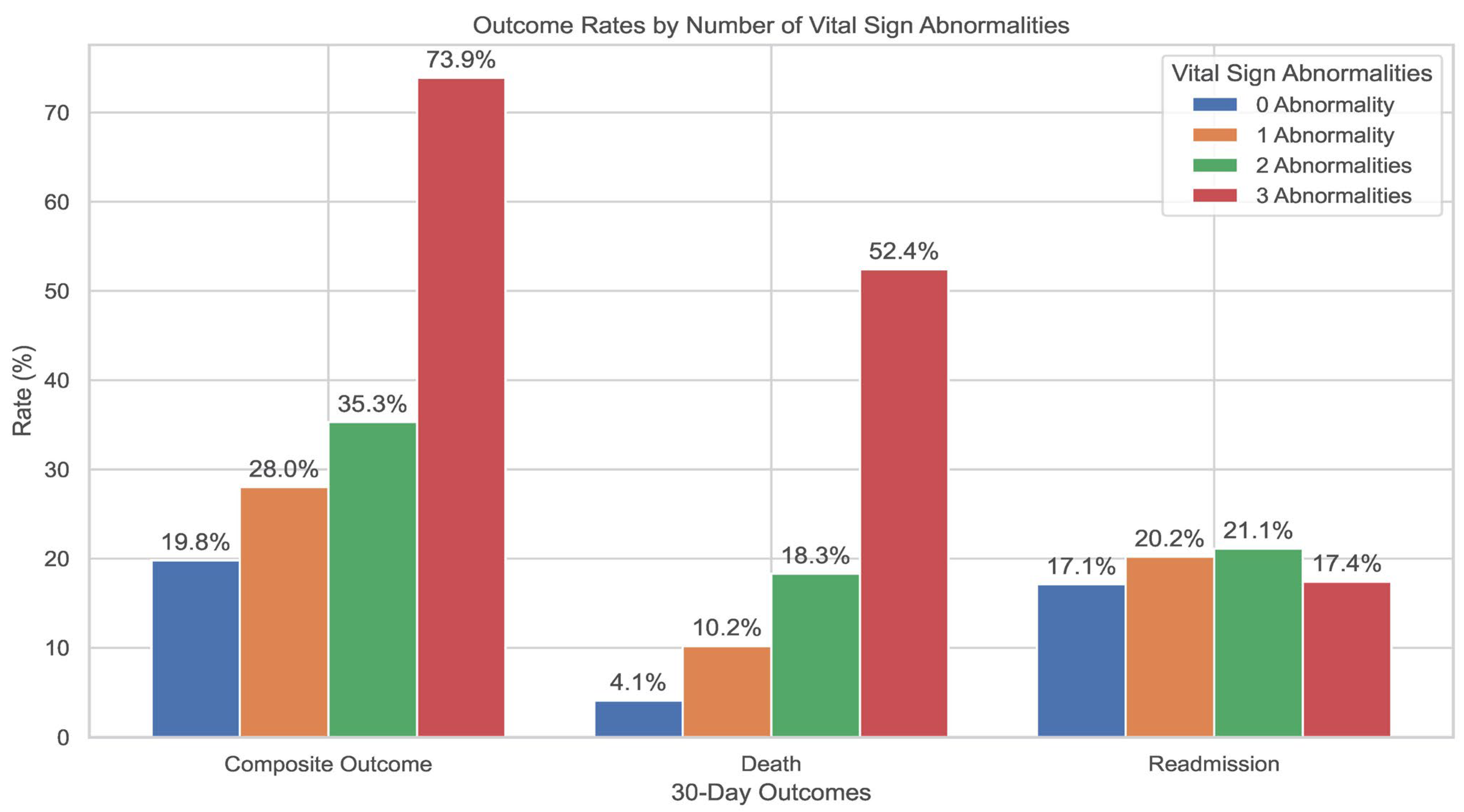
| Composite outcome | <0.001 | |||||
| Unadjusted Odds ratio (95% CI) | 1.77 (1.49–2.11) | Baseline | 1.57 (1.30–1.90) | 2.21 (1.53–3.19) | 11.47 (4.50–29.21) | |
| † Adjusted Odds ratio (95% CI) | 1.73 (1.42–2.09) | Baseline | 1.55 (1.25–1.90) | 2.12 (1.39–3.22) | 14.17 (4.45–45.09) | |
| Mortality | <0.001 | |||||
| Unadjusted Odds ratio (95% CI) | 3.32 (2.55–4.33) | Baseline | 2.52 (1.86–3.41) | 5.49 (3.45–8.73) | 28.25 (12.13–65.78) | |
| † Adjusted Odds ratio (95% CI) | 3.70 (2.73–5.00) | Baseline | 2.78 (1.98–3.90) | 6.79 (3.90–11.81) | 52.45 (16.43–167.42) | |
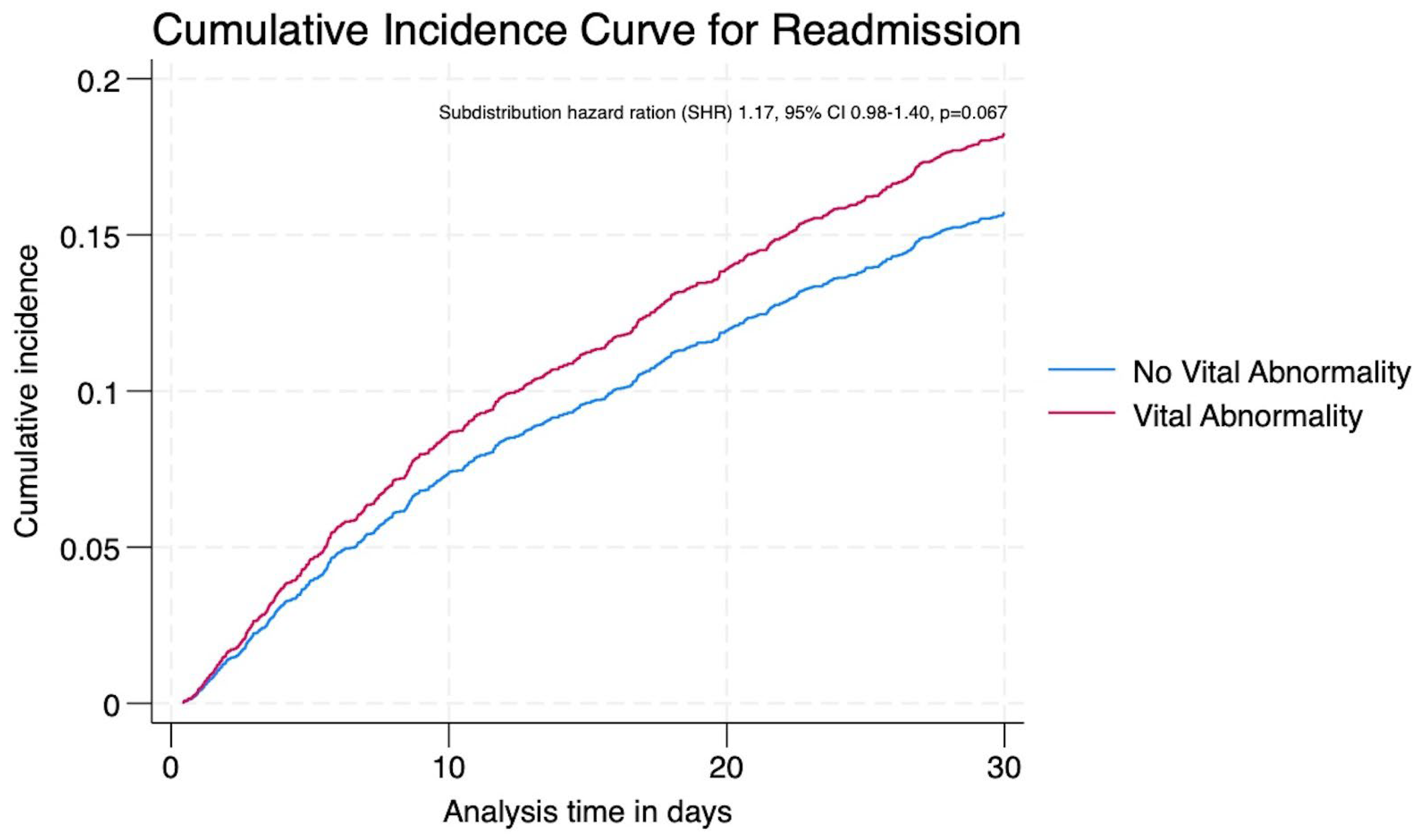
| Readmissions | >0.05 | |||||
| Unadjusted Odds ratio (95% CI) | 1.29 (1.05–1.59) | Baseline | 1.27 (1.01–1.59) | 1.26 (0.78–2.06) | 3.46 (0.97–12.33) | |
| † Adjusted Odds ratio (95% CI) | 1.16 (0.93–1.44) | Baseline | 1.17 (0.93–1.48) | 1.19 (0.73–1.92) | 0.70 (0.17–2.81) | |
| Vital Sign Abnormality | aOR * | 95% CI | p Value |
|---|---|---|---|
| Temperature ≥ 37.8 °C | 0.91 | 0.59–1.41 | 0.699 |
| Cardiovascular parameters | |||
| Heart rate ≥ 100/min | 1.70 | 1.33–2.17 | <0.001 |
| Systolic blood pressure ≤ 90 mm Hg | 4.51 | 2.51–8.11 | <0.001 |
| Respiratory parameters | |||
| Respiratory rate > 24/min | 3.13 | 2.16–4.54 | <0.001 |
| Oxygen saturation of <90% | 1.54 | 1.08–2.20 | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, Y.; Mangoni, A.A.; Shahi, R.; Horwood, C.; Thompson, C. Clinical Instability at Discharge and Post-Discharge Outcomes in Patients with Community-Acquired Pneumonia: An Observational Study. J. Clin. Med. 2025, 14, 5273. https://doi.org/10.3390/jcm14155273
Sharma Y, Mangoni AA, Shahi R, Horwood C, Thompson C. Clinical Instability at Discharge and Post-Discharge Outcomes in Patients with Community-Acquired Pneumonia: An Observational Study. Journal of Clinical Medicine. 2025; 14(15):5273. https://doi.org/10.3390/jcm14155273
Chicago/Turabian StyleSharma, Yogesh, Arduino A. Mangoni, Rashmi Shahi, Chris Horwood, and Campbell Thompson. 2025. "Clinical Instability at Discharge and Post-Discharge Outcomes in Patients with Community-Acquired Pneumonia: An Observational Study" Journal of Clinical Medicine 14, no. 15: 5273. https://doi.org/10.3390/jcm14155273
APA StyleSharma, Y., Mangoni, A. A., Shahi, R., Horwood, C., & Thompson, C. (2025). Clinical Instability at Discharge and Post-Discharge Outcomes in Patients with Community-Acquired Pneumonia: An Observational Study. Journal of Clinical Medicine, 14(15), 5273. https://doi.org/10.3390/jcm14155273






