Investigation of Growth Differentiation Factor 15 as a Prognostic Biomarker for Major Adverse Limb Events in Peripheral Artery Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Design
2.3. Patient Recruitment
2.4. Baseline Variables
2.5. Plasma GDF15 Concentration Measurement
2.6. Follow-Up and Outcomes
2.7. Model Development and Evaluation
2.8. Statistical Analysis
3. Results
3.1. Patients
3.2. Outcomes
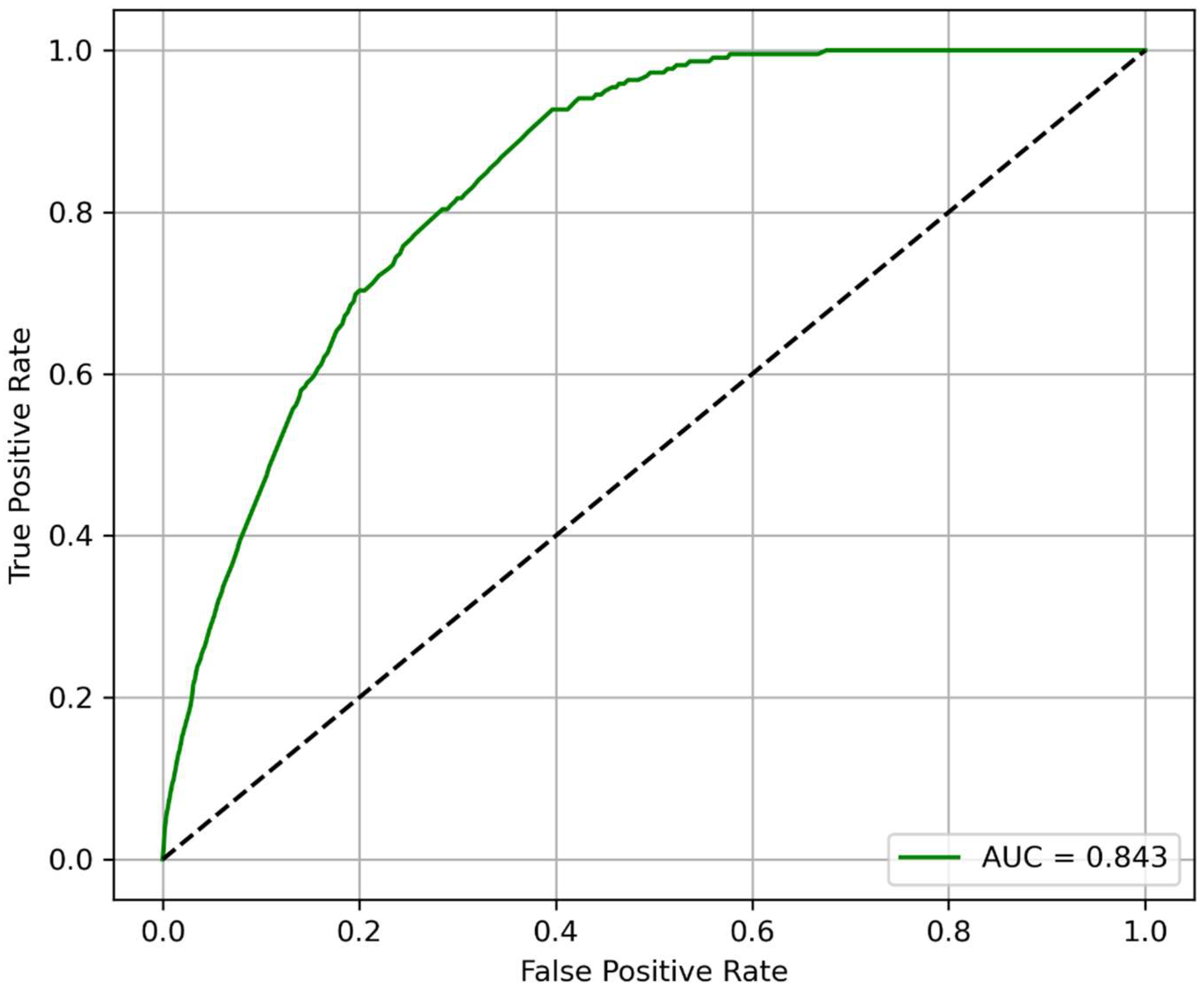
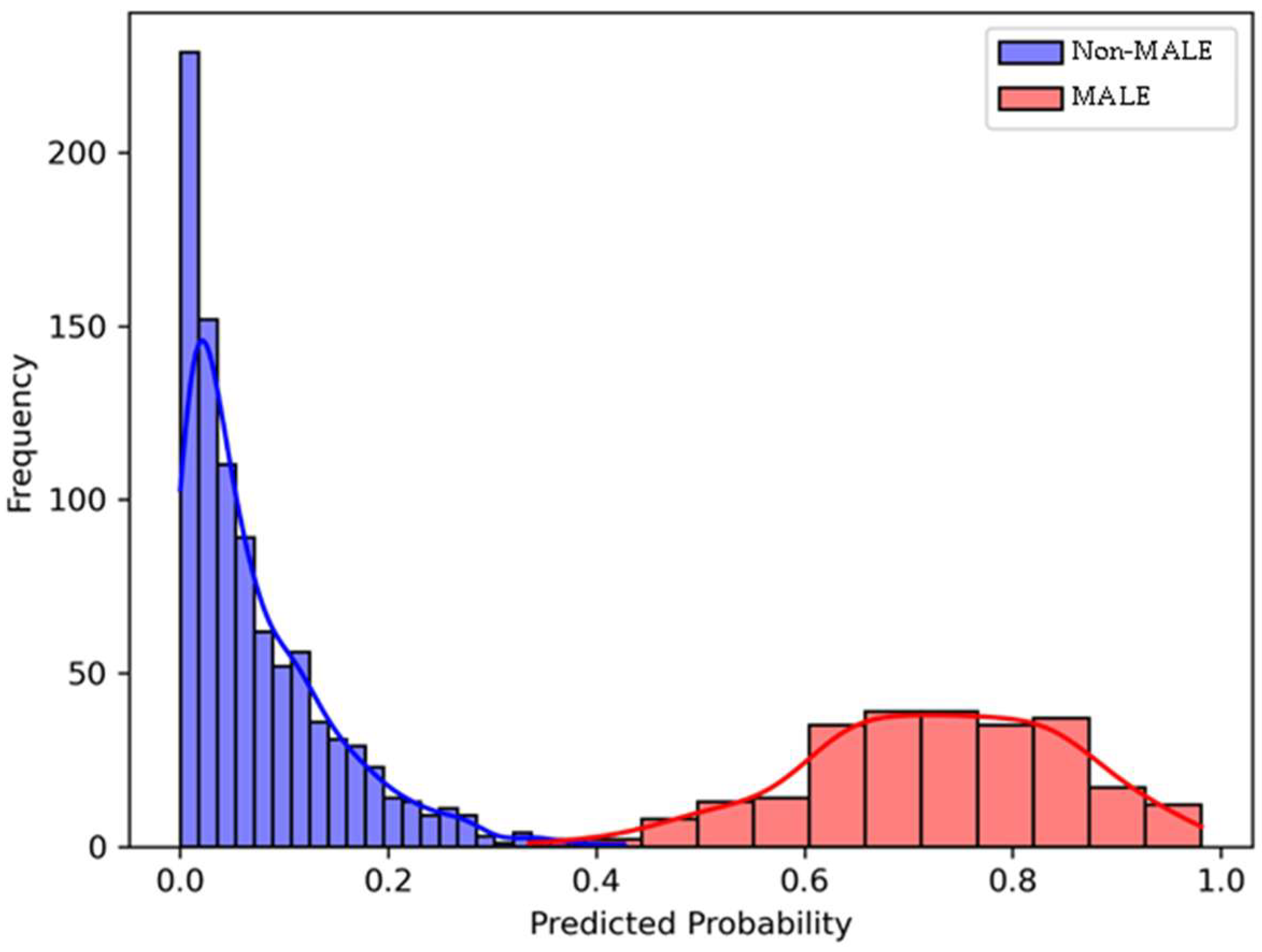
3.3. Model Performance for Predicting 2-Year MALE
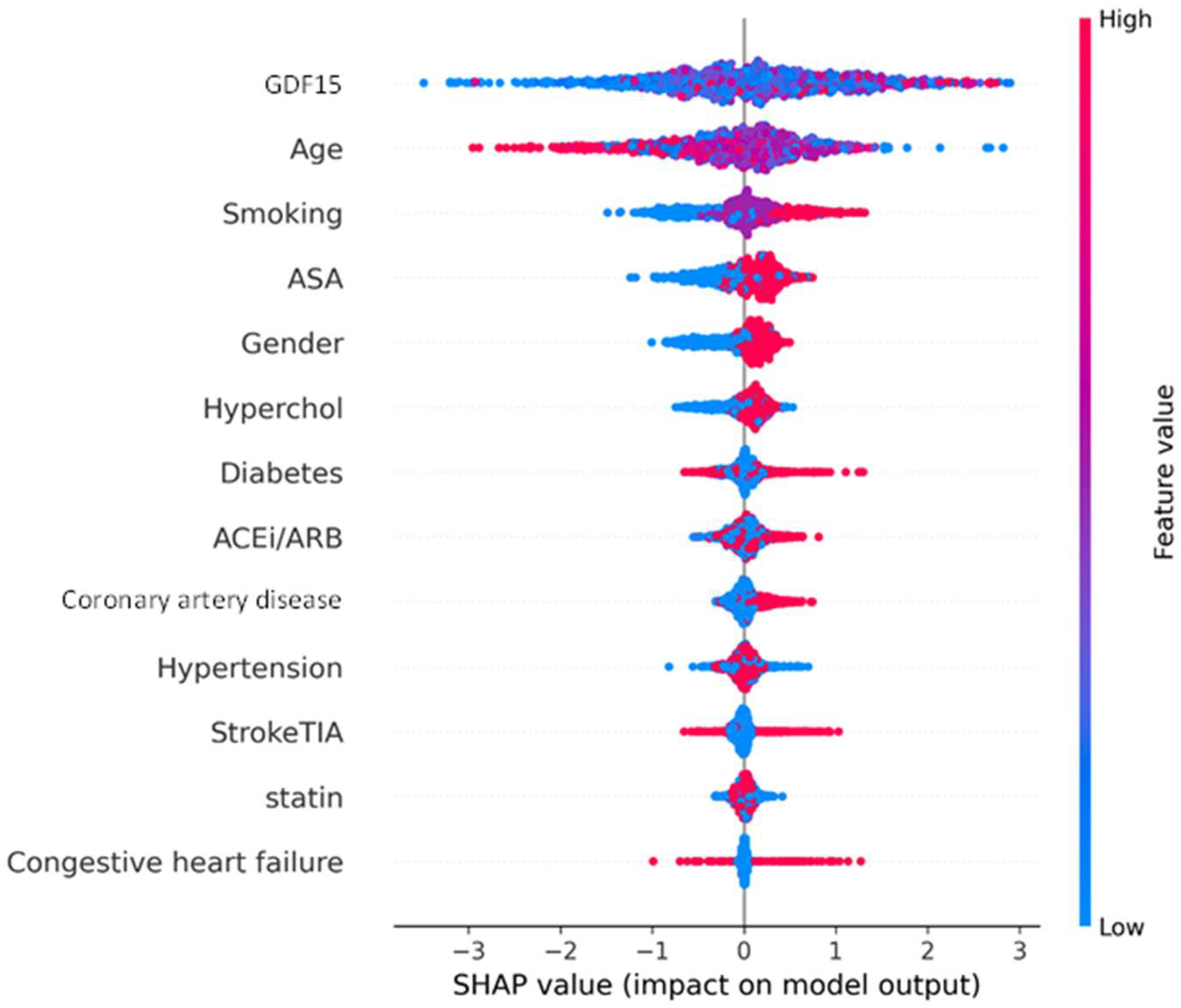
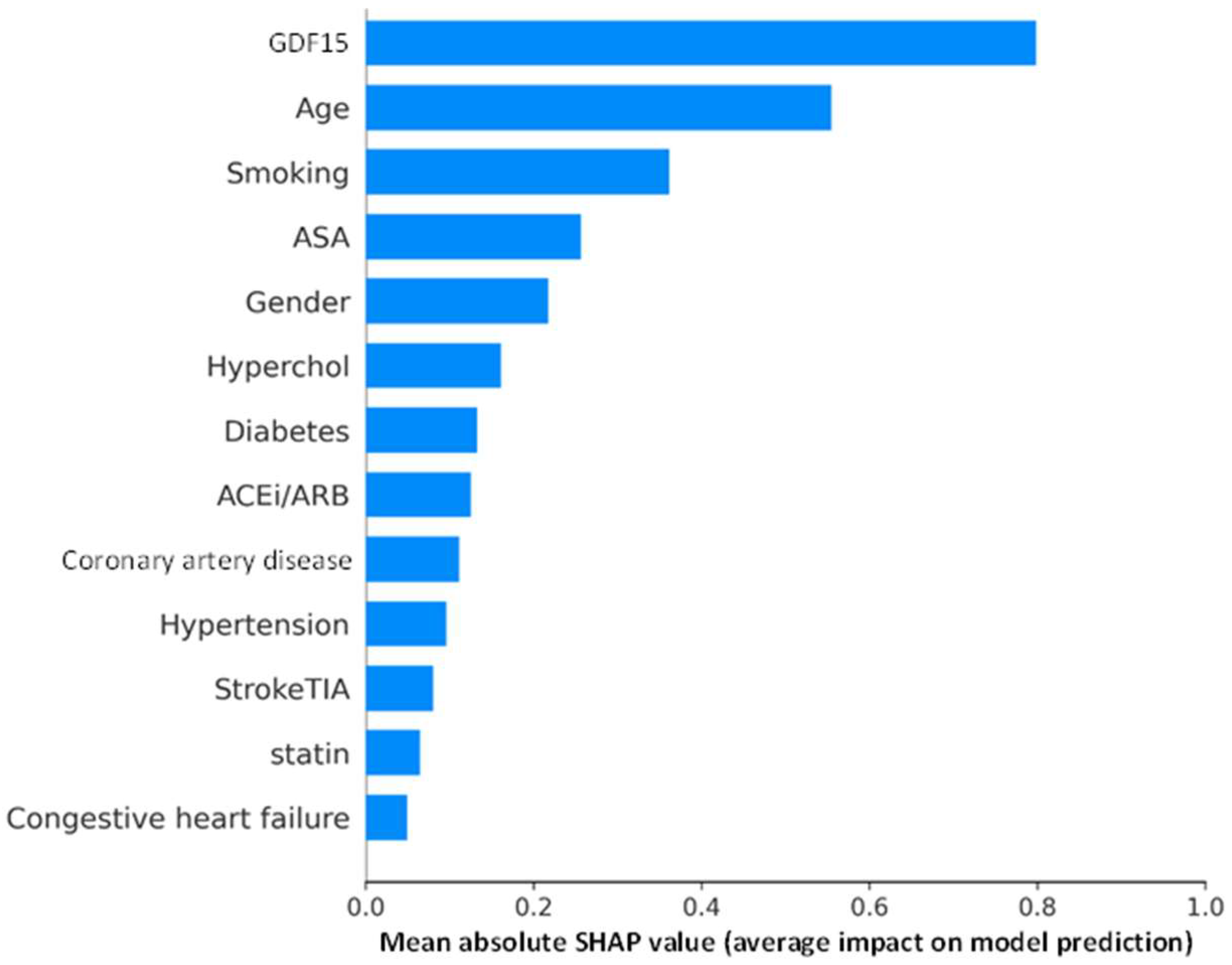
3.4. SHAP Analysis of XGBoost Model for Explainability
4. Discussion
4.1. Summary of Findings
4.2. Comparison with Previous Studies
4.3. Explanation of Findings
4.4. Implications
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zemaitis, M.R.; Boll, J.M.; Dreyer, M.A. Peripheral Arterial Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Olin, J.W.; Sealove, B.A. Peripheral Artery Disease: Current Insight into the Disease and Its Diagnosis and Management. Mayo Clin. Proc. 2010, 85, 678–692. [Google Scholar] [CrossRef]
- Mehta, A.; Dhindsa, D.S.; Hooda, A.; Nayak, A.; Massad, C.S.; Rao, B.; Makue, L.F.; Rajani, R.R.; Alabi, O.; Quyyumi, A.A.; et al. Premature Atherosclerotic Peripheral Artery Disease: An Underrecognized and Undertreated Disorder with a Rising Global Prevalence. Trends Cardiovasc. Med. 2021, 31, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Zamzam, A.; Syed, M.H.; Rotstein, O.D.; Eikelboom, J.; Klein, D.J.; Singh, K.K.; Abdin, R.; Qadura, M. Validating Fatty Acid Binding Protein 3 as a Diagnostic and Prognostic Biomarker for Peripheral Arterial Disease: A Three-Year Prospective Follow-up Study. eClinicalMedicine 2023, 55, 101766. [Google Scholar] [CrossRef] [PubMed]
- Asrih, M.; Wei, S.; Nguyen, T.T.; Yi, H.; Ryu, D.; Gariani, K. Overview of Growth Differentiation Factor 15 in Metabolic Syndrome. J. Cell Mol. Med. 2023, 27, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Kempf, T.; Wallentin, L. Growth Differentiation Factor 15 as a Biomarker in Cardiovascular Disease. Clin. Chem. 2017, 63, 140–151. [Google Scholar] [CrossRef]
- di Candia, A.M.; de Avila, D.X.; Moreira, G.R.; Villacorta, H.; Maisel, A.S. Growth Differentiation Factor-15, a Novel Systemic Biomarker of Oxidative Stress, Inflammation, and Cellular Aging: Potential Role in Cardiovascular Diseases. Am. Heart J. Plus Cardiol. Res. Pract. 2021, 9, 100046. [Google Scholar] [CrossRef]
- Wesseling, M.; de Poel, J.H.C.; de Jager, S.C.A. Growth Differentiation Factor 15 in Adverse Cardiac Remodelling: From Biomarker to Causal Player. ESC Heart Fail. 2020, 7, 1488–1501. [Google Scholar] [CrossRef]
- Wang, J.; Wei, L.; Yang, X.; Zhong, J. Roles of Growth Differentiation Factor 15 in Atherosclerosis and Coronary Artery Disease. J. Am. Heart Assoc. 2019, 8, e012826. [Google Scholar] [CrossRef]
- Grenon, S.M.; Vittinghoff, E.; Owens, C.D.; Conte, M.S.; Whooley, M.; Cohen, B.E. Peripheral Artery Disease and Risk of Cardiovascular Events in Patients with Coronary Artery Disease: Insights from the Heart and Soul Study. Vasc. Med. 2013, 18, 176–184. [Google Scholar] [CrossRef]
- De Haan, J.J.; Haitjema, S.; den Ruijter, H.M.; Pasterkamp, G.; de Borst, G.J.; Teraa, M.; Verhaar, M.C.; Gremmels, H.; de Jager, S.C.A. Growth Differentiation Factor 15 Is Associated with Major Amputation and Mortality in Patients with Peripheral Artery Disease. J. Am. Heart Assoc. 2017, 6, e006225. [Google Scholar] [CrossRef]
- Yuan, S.; Titova, O.E.; Zhang, K.; Chen, J.; Li, X.; Klarin, D.; Åkesson, A.; Damrauer, S.M.; VA Million Veteran Program; Larsson, S.C. Circulating Proteins and Peripheral Artery Disease Risk: Observational and Mendelian Randomization Analyses. Eur. Heart J. Open 2023, 3, oead056. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, B.; Huang, S.; Chen, Y.; Yan, J. Causal Association between Circulating Inflammatory Proteins and Peripheral Artery Disease: A Bidirectional Two-Sample Mendelian Randomization Study. Front. Immunol. 2024, 15, 1432041. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, J.; Qian, F.; Yao, P.; Xu, K.; Wu, P.; Li, R.; Qiu, Z.; Li, R.; Zhu, K.; et al. Large-Scale Plasma Proteomics Improves Prediction of Peripheral Artery Disease in Individuals with Type 2 Diabetes: A Prospective Cohort Study. Diabetes Care 2025, 48, 381–389. [Google Scholar] [CrossRef]
- Parvar, S.L.; Fitridge, R.; Dawson, J.; Nicholls, S.J. Medical and Lifestyle Management of Peripheral Arterial Disease. J. Vasc. Surg. 2018, 68, 1595–1606. [Google Scholar] [CrossRef]
- Cornejo Del Río, V.; Mostaza, J.; Lahoz, C.; Sánchez-Arroyo, V.; Sabín, C.; López, S.; Patrón, P.; Fernández-García, P.; Fernández-Puntero, B.; Vicent, D.; et al. Prevalence of Peripheral Artery Disease (PAD) and Factors Associated: An Epidemiological Analysis from the Population-Based Screening PRE-Diabetes and Type 2 DIAbetes (SPREDIA-2) Study. PLoS ONE 2017, 12, e0186220. [Google Scholar] [CrossRef]
- Duval, S.; Massaro, J.M.; Jaff, M.R.; Boden, W.E.; Alberts, M.J.; Califf, R.M.; Eagle, K.A.; D’Agostino, R.B.; Pedley, A.; Fonarow, G.C.; et al. An Evidence-Based Score to Detect Prevalent Peripheral Artery Disease (PAD). Vasc. Med. 2012, 17, 342–351. [Google Scholar] [CrossRef]
- Gouda, P.; Ramasundarahettige, C.; Anand, S.; Muhlhoffer, E.; Berkowitz, S.; Fox, K.A.; Eikelboom, J.; Welsh, R. Clinical Factors Associated with Peripheral Artery Disease in Patients with Documented Coronary Artery Disease: A Post Hoc Analysis of the COMPASS Trial. Atherosclerosis 2021, 331, 38–44. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Collins, G.S.; Moons, K.G.M.; Dhiman, P.; Riley, R.D.; Beam, A.L.; Calster, B.V.; Ghassemi, M.; Liu, X.; Reitsma, J.B.; van Smeden, M.; et al. TRIPOD+AI Statement: Updated Guidance for Reporting Clinical Prediction Models That Use Regression or Machine Learning Methods. BMJ 2024, 385, e078378. [Google Scholar] [CrossRef]
- Gul, F.; Janzer, S.F. Peripheral Vascular Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison, H.C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef] [PubMed]
- Luminex Assays. Multiplex Immunoassays. Available online: https://www.bio-techne.com/ (accessed on 6 May 2023).
- MAGPIX® System|xMAP Instrument|Luminex Corporation. Available online: https://www.luminexcorp.com/magpix-system/ (accessed on 18 December 2021).
- Luminex Assays—CA. Available online: https://www.thermofisher.com/ca/en/home/life-science/antibodies/immunoassays/procartaplex-assays-luminex.html (accessed on 18 December 2021).
- xPONENT® Software for xMAP® Instruments; Luminex Corporation: Austin, TX, USA, 2025.
- Anand, S.S.; Caron, F.; Eikelboom, J.W.; Bosch, J.; Dyal, L.; Aboyans, V.; Abola, M.T.; Branch, K.R.H.; Keltai, K.; Bhatt, D.L.; et al. Major Adverse Limb Events and Mortality in Patients with Peripheral Artery Disease: The COMPASS Trial. J. Am. Coll. Cardiol. 2018, 71, 2306–2315. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Song, Y.-Y.; Lu, Y. Decision Tree Methods: Applications for Classification and Prediction. Shanghai Arch. Psychiatry 2015, 27, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Elfanagely, O.; Toyoda, Y.; Othman, S.; Mellia, J.A.; Basta, M.; Liu, T.; Kording, K.; Ungar, L.; Fischer, J.P. Machine Learning and Surgical Outcomes Prediction: A Systematic Review. J. Surg. Res. 2021, 264, 346–361. [Google Scholar] [CrossRef]
- Bektaş, M.; Tuynman, J.B.; Costa Pereira, J.; Burchell, G.L.; van der Peet, D.L. Machine Learning Algorithms for Predicting Surgical Outcomes After Colorectal Surgery: A Systematic Review. World J. Surg. 2022, 46, 3100–3110. [Google Scholar] [CrossRef]
- Senders, J.T.; Staples, P.C.; Karhade, A.V.; Zaki, M.M.; Gormley, W.B.; Broekman, M.L.D.; Smith, T.R.; Arnaout, O. Machine Learning and Neurosurgical Outcome Prediction: A Systematic Review. World Neurosurg. 2018, 109, 476–486.e1. [Google Scholar] [CrossRef]
- Ruopp, M.D.; Perkins, N.J.; Whitcomb, B.W.; Schisterman, E.F. Youden Index and Optimal Cut-Point Estimated from Observations Affected by a Lower Limit of Detection. Biom. J. 2008, 50, 419–430. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, R.; Bajorath, J. Interpretation of Machine Learning Models Using Shapley Values: Application to Compound Potency and Multi-Target Activity Predictions. J. Comput. Aided Mol. Des. 2020, 34, 1013–1026. [Google Scholar] [CrossRef]
- Riley, R.D.; Ensor, J.; Snell, K.I.E.; Harrell, F.E.; Martin, G.P.; Reitsma, J.B.; Moons, K.G.M.; Collins, G.; van Smeden, M. Calculating the Sample Size Required for Developing a Clinical Prediction Model. BMJ 2020, 368, m441. [Google Scholar] [CrossRef]
- Python Documentation by Version. Available online: https://www.python.org/doc/versions/ (accessed on 21 May 2025).
- de Jager, S.C.A.; Bermúdez, B.; Bot, I.; Koenen, R.R.; Bot, M.; Kavelaars, A.; de Waard, V.; Heijnen, C.J.; Muriana, F.J.G.; Weber, C.; et al. Growth Differentiation Factor 15 Deficiency Protects against Atherosclerosis by Attenuating CCR2-Mediated Macrophage Chemotaxis. J. Exp. Med. 2011, 208, 217–225. [Google Scholar] [CrossRef]
- Dogon, G.; Rigal, E.; Potel, E.; Josse, M.; Rochette, L.; Bejot, Y.; Vergely, C. Growth/Differentiation Factor 15 (GDF15) Expression in the Heart after Myocardial Infarction and Cardioprotective Effect of Pre-Ischemic rGDF15 Administration. Sci. Rep. 2024, 14, 12949. [Google Scholar] [CrossRef]
- Du, W.; Piek, A.; Schouten, E.M.; van de Kolk, C.W.A.; Mueller, C.; Mebazaa, A.; Voors, A.A.; de Boer, R.A.; Silljé, H.H.W. Plasma Levels of Heart Failure Biomarkers Are Primarily a Reflection of Extracardiac Production. Theranostics 2018, 8, 4155–4169. [Google Scholar] [CrossRef] [PubMed]
- Adela, R.; Banerjee, S.K. GDF-15 as a Target and Biomarker for Diabetes and Cardiovascular Diseases: A Translational Prospective. J. Diabetes Res. 2015, 2015, 490842. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Robinson-Cohen, C.; Smith, M.R.; Bellovich, K.A.; Bhat, Z.Y.; Bobadilla, M.; Brosius, F.; de Boer, I.H.; Essioux, L.; Formentini, I.; et al. Growth Differentiation Factor–15 and Risk of CKD Progression. J. Am. Soc. Nephrol. 2017, 28, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.M.; Saudek, V.; O’Rahilly, S. GDF15: A Hormone Conveying Somatic Distress to the Brain. Endocr. Rev. 2020, 41, bnaa007. [Google Scholar] [CrossRef]
- Bakhet, M.; Ul-Haq, Z.; Kamalati, T.; Lucas, A.; Majeed, A.; El-Osta, A. Blood Tests in General Practice: The Use of Routine Data to Characterise Venous Blood Testing in North West London, 2016–2018. Br. J. Gen. Pract. 2020, 70, bjgp20X711605. [Google Scholar] [CrossRef]
- Koh, C.E.; Walker, S.R. Vascular Surgery Consults: A Significant Workload. ANZ J. Surg. 2007, 77, 352–354. [Google Scholar] [CrossRef]
- Bridgwood, B.M.; Sayers, R.D. Peripheral Artery Disease (PAD) in Primary Care-Educational Experiences for PAD Primary Care in England—A Mixed-Method Study. Fam. Pract. 2023, 40, 820–826. [Google Scholar] [CrossRef]
- Nishimiya, K.; Matsumoto, Y.; Shimokawa, H. Recent Advances in Vascular Imaging. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e313–e321. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef]
- Farber, A.; Menard, M.T.; Conte, M.S.; Kaufman, J.A.; Powell, R.J.; Choudhry, N.K.; Hamza, T.H.; Assmann, S.F.; Creager, M.A.; Cziraky, M.J.; et al. Surgery or Endovascular Therapy for Chronic Limb-Threatening Ischemia. N. Engl. J. Med. 2022, 387, 2305–2316. [Google Scholar] [CrossRef]
- Caetano, A.P.; Conde Vasco, I.; Veloso Gomes, F.; Costa, N.V.; Luz, J.H.; Spaepen, E.; Formiga, A.; Coimbra, É.; Neves, J.; Bilhim, T. Successful Revascularization Has a Significant Impact on Limb Salvage Rate and Wound Healing for Patients with Diabetic Foot Ulcers: Single-Centre Retrospective Analysis with a Multidisciplinary Approach. Cardiovasc. Interv. Radiol. 2020, 43, 1449–1459. [Google Scholar] [CrossRef]
- Margolis, J.; Barron, J.J.; Grochulski, W.D. Health Care Resources and Costs for Treating Peripheral Artery Disease in a Managed Care Population: Results from Analysis of Administrative Claims Data. J. Manag. Care Pharm. 2005, 11, 727–734. [Google Scholar] [CrossRef]
- Rockley, M.; Kobewka, D.; Kunkel, E.; Nagpal, S.; McIsaac, D.I.; Thavorn, K.; Forster, A. Characteristics of High-Cost Inpatients with Peripheral Artery Disease. J. Vasc. Surg. 2020, 72, 250–258.e8. [Google Scholar] [CrossRef]
- Wang, D.; Day, E.A.; Townsend, L.K.; Djordjevic, D.; Jørgensen, S.B.; Steinberg, G.R. GDF15: Emerging Biology and Therapeutic Applications for Obesity and Cardiometabolic Disease. Nat. Rev. Endocrinol. 2021, 17, 592–607. [Google Scholar] [CrossRef]
| Patients with PAD (n = 454) | |
|---|---|
| Age, years | 71.11 ± 9.64 |
| Female sex | 139 (30.6) |
| Hypertension | 378 (83.3) |
| Dyslipidemia | 374 (82.4) |
| Diabetes | 192 (42.3) |
| Smoking, past | 241 (65.7) |
| Smoking, current | 126 (34.3) |
| Congestive heart failure | 28 (6.2) |
| Coronary artery disease | 178 (39.2) |
| Previous stroke or transient ischemic attack | 82 (18.1) |
| Statin | 347 (76.4) |
| ACE-I/ARB | 261 (57.5) |
| ASA | 349 (76.9) |
| Patients with PAD (n = 454) | |
|---|---|
| Major adverse limb event | 157 (34.6) |
| Vascular intervention | |
| Endovascular | 92 (20.3) |
| Open | 91 (20.0) |
| Major amputation | 31 (6.8) |
| Acute limb ischemia | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Shaikh, F.; Younes, H.; Abuhalimeh, B.; Zamzam, A.; Abdin, R.; Qadura, M. Investigation of Growth Differentiation Factor 15 as a Prognostic Biomarker for Major Adverse Limb Events in Peripheral Artery Disease. J. Clin. Med. 2025, 14, 5239. https://doi.org/10.3390/jcm14155239
Li B, Shaikh F, Younes H, Abuhalimeh B, Zamzam A, Abdin R, Qadura M. Investigation of Growth Differentiation Factor 15 as a Prognostic Biomarker for Major Adverse Limb Events in Peripheral Artery Disease. Journal of Clinical Medicine. 2025; 14(15):5239. https://doi.org/10.3390/jcm14155239
Chicago/Turabian StyleLi, Ben, Farah Shaikh, Houssam Younes, Batool Abuhalimeh, Abdelrahman Zamzam, Rawand Abdin, and Mohammad Qadura. 2025. "Investigation of Growth Differentiation Factor 15 as a Prognostic Biomarker for Major Adverse Limb Events in Peripheral Artery Disease" Journal of Clinical Medicine 14, no. 15: 5239. https://doi.org/10.3390/jcm14155239
APA StyleLi, B., Shaikh, F., Younes, H., Abuhalimeh, B., Zamzam, A., Abdin, R., & Qadura, M. (2025). Investigation of Growth Differentiation Factor 15 as a Prognostic Biomarker for Major Adverse Limb Events in Peripheral Artery Disease. Journal of Clinical Medicine, 14(15), 5239. https://doi.org/10.3390/jcm14155239








