Early Structural Degradation of Dermal Elastic Fibers in Women with Mild Obesity Without Parallel Transcriptional Changes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Patients and Skin Sample Collection
2.2. Tissue Processing
2.3. Quantitative Polymerase Chain Reaction
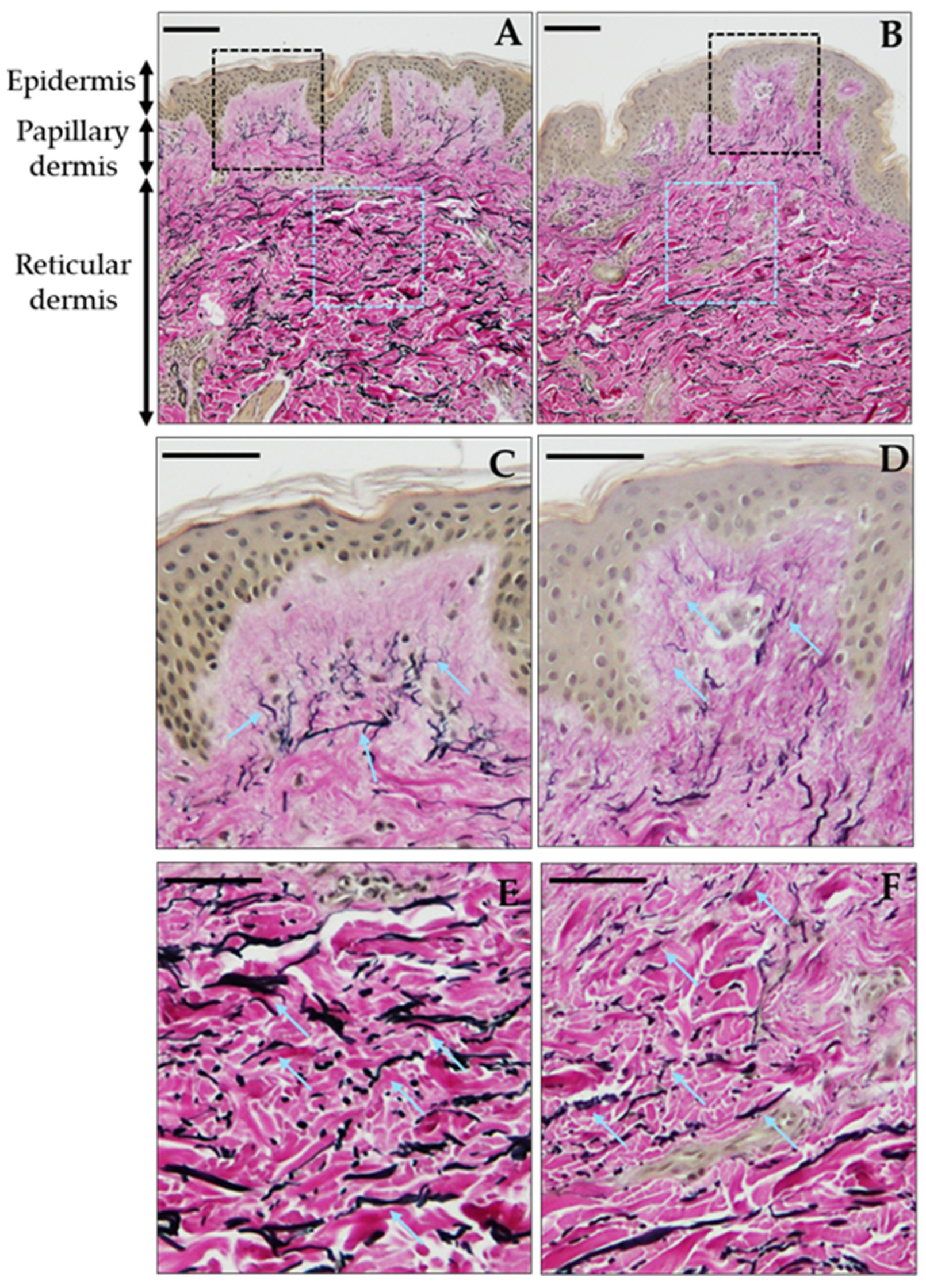
2.4. Histological Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Elastin-Related Gene Expression and Its Association with BMI
3.2.1. Expression of Degradative Genes
3.2.2. Expression of Regenerative Genes
3.3. Early Structural Deterioration of Dermal Elastic Fibers with Mildly Increased BMI
4. Discussion
4.1. Dermal Elastin Alterations in Mild Obesity
4.2. Structural Degradation Despite Limited Transcriptional Changes
4.3. Compensatory Upregulation of ELN Concurrent with Elastic Fiber Loss
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCR | Polymerase chain reaction |
| EVG | Elastica van Gieson |
| BMI | Body mass index |
| ELN | Elastin gene |
| NE | Neutrophil elastase |
| NEP | Neprilysin |
| FBN1 | Fibrillin-1 |
| MMP | Matrix metalloproteinase |
| ACTB | Beta-actin |
References
- Cotta-Pereira, G.; Guerra Rodrigo, F.; Bittencourt-Sampaio, S. Oxytalan, elaunin, and elastic fibers in the human skin. J. Investig. Dermatol. 1976, 66, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Tohgasaki, T.; Kondo, S.; Nishizawa, S.; Ishiwatari, S.; Sakurai, T.; Ishikawa, S.; Takeda, A. Evaluation of elastin fibres in young and aged eyelids and abdominal skin using computational 3D structural analysis. Ski. Health Dis. 2021, 1, e58. [Google Scholar] [CrossRef] [PubMed]
- Gardeazabal, L.; Izeta, A. Elastin and collagen fibres in cutaneous wound healing. Exp. Dermatol. 2024, 33, e15052. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Thibeault, S. Insights into the role of elastin in vocal fold health and disease. J. Voice 2012, 26, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Uitto, J.; Li, Q.; Urban, Z. The complexity of elastic fibre biogenesis in the skin—A perspective to the clinical heterogeneity of cutis laxa. Exp. Dermatol. 2013, 22, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Mithieux, S.M.; Weiss, A.S. Elastin biomaterials in dermal repair. Trends Biotechnol. 2020, 38, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L.; Bernstein, E.F.; Weiss, A.S.; Bates, D.; Humphrey, S.; Silberberg, M.; Daniels, R. Clinical relevance of elastin in the structure and function of skin. Aesthet. Surg. J. Open Forum 2021, 3, ojab019. [Google Scholar] [CrossRef] [PubMed]
- Monteiro Rodrigues, L.M.; Palma, L.; Santos, O.; Almeida, M.A.; Bujan, J.; Tavares, L. Excessive weight favours skin physiology—Up to a point: Another expression of the obesity paradox. Ski. Pharmacol. Physiol. 2017, 30, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Hirt, P.A.; Castillo, D.E.; Yosipovitch, G.; Keri, J.E. Skin changes in the obese patient. J. Am. Acad. Dermatol. 2019, 81, 1037–1057. [Google Scholar] [CrossRef] [PubMed]
- Yew, Y.W.; Mina, T.; Ng, H.K.; Lam, B.C.C.; Riboli, E.; Lee, E.S.; Lee, J.; Ngeow, J.; Elliott, P.; Thng, S.T.G.; et al. Investigating causal relationships between obesity and skin barrier function in a multi-ethnic Asian general population cohort. Int. J. Obes. 2023, 47, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Coon, D.; Gusenoff, J.A.; Kannan, N.; El Khoudary, S.R.; Naghshineh, N.; Rubin, J.P. Body mass and surgical complications in the postbariatric reconstructive patient: Analysis of 511 cases. Ann. Surg. 2009, 249, 397–401. [Google Scholar] [CrossRef] [PubMed]
- VanGilder, C.; MacFarlane, G.; Meyer, S.; Lachenbruch, C. Body mass index, weight, and pressure ulcer prevalence: An analysis of the 2006–2007 International Pressure Ulcer Prevalence Surveys. J. Nurs. Care Qual. 2009, 24, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Zhang, H.; Ma, Y.; Wei, Y.; Tao, H.; Yang, Q.; Yang, Z.; Han, L. Dose–response relationships between body-mass index and pressure injuries occurrence in hospitalized patients: A multi-center prospective study. J. Tissue Viability 2024, 33, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Orpheu, S.C.; Coltro, P.S.; Scopel, G.P.; Gomez, D.S.; Rodrigues, C.J.; Modolin, M.L.A.; Faintuch, J.; Gemperli, R.; Ferreira, M.C. Collagen and elastic content of abdominal skin after surgical weight loss. Obes. Surg. 2010, 20, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Fearmonti, R.M.; Blanton, M.; Bond, J.E.; Pestana, I.A.; Selim, M.A.; Erdmann, D. Changes in dermal histomorphology following surgical weight loss versus diet-induced weight loss in the morbidly obese patient. Ann. Plast. Surg. 2012, 68, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Sami, K.; Elshahat, A.; Moussa, M.; Abbas, A.; Mahmoud, A. Image analyzer study of the skin in patients with morbid obesity and massive weight loss. ePlasty 2015, 15, e4. [Google Scholar] [PubMed]
- Rocha, R.I.; Junior, W.C.; Modolin, M.L.A.; Takahashi, G.G.; Caldini, E.T.E.G.; Gemperli, R. Skin changes due to massive weight loss: Histological changes and the causes of the limited results of contouring surgeries. Obes. Surg. 2021, 31, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Cálix, M.; Menéndez, R.; Baley, M.; Cadena, A.; Carrillo, C.; García-Jiménez, J. Histological changes in skin and subcutaneous cellular tissue in patients with massive weight loss after bariatric surgery. Aesthetic Plast. Surg. 2024, 48, 5060–5066. [Google Scholar] [CrossRef] [PubMed]
- Joudatt, L.L.C.; Zotarelli-Filho, I.J.; de Quadros, L.G.; Lopes, A.C.P.; de Lima André, J.; Joudatt, J.; Junior, R.L.K. Histological skin assessment of patients submitted to bariatric surgery: A prospective longitudinal cohort study. Obes. Surg. 2023, 33, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Ibuki, A.; Akase, T.; Nagase, T.; Minematsu, T.; Nakagami, G.; Horii, M.; Sagara, H.; Komeda, T.; Kobayashi, M.; Shimada, T.; et al. Skin fragility in obese diabetic mice: Possible involvement of elevated oxidative stress and upregulation of matrix metalloproteinases. Exp. Dermatol. 2012, 21, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Makihara, H.; Hidaka, M.; Sakai, Y.; Horie, Y.; Mitsui, H.; Ohashi, K.; Goshima, Y.; Akase, T. Reduction and fragmentation of elastic fibers in the skin of obese mice is associated with altered mRNA expression levels of fibrillin-1 and neprilysin. Connect. Tissue Res. 2017, 58, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Dahlbäck, K.; Ljungquist, A.; Löfberg, H.; Dahlbäck, B.; Engvall, E.; Sakai, L.Y. Fibrillin immunoreactive fibers constitute a unique network in the human dermis: Immunohistochemical comparison of the distributions of fibrillin, vitronectin, amyloid P component, and orcein stainable structures in normal skin and elastosis. J. Investig. Dermatol. 1990, 94, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.K.; Simpson, A.; Steer, R.; Cain, S.A.; Kielty, C.M. Elastic fibres in health and disease. Expert. Rev. Mol. Med. 2013, 15, e8. [Google Scholar] [CrossRef] [PubMed]
- Standeven, K.F.; Hess, K.; Carter, A.M.; Rice, G.I.; Cordell, P.A.; Balmforth, A.J.; Lu, B.; Scott, D.J.; Turner, A.J.; Hooper, N.M.; et al. Neprilysin, obesity and the metabolic syndrome. Int. J. Obes. 2011, 35, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Ezure, T.; Amano, S. Increment of subcutaneous adipose tissue is associated with decrease of elastic fibres in the dermal layer. Exp. Dermatol. 2015, 24, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Labour and Welfare. The National Health and Nutrition Survey in Japan, 2023. Available online: https://www.mhlw.go.jp/content/001435384.pdf (accessed on 5 May 2025).
- Horie, Y.; Makihara, H.; Horikawa, K.; Takeshige, F.; Ibuki, A.; Satake, T.; Yasumura, K.; Maegawa, J.; Mitsui, H.; Ohashi, K.; et al. Reduced skin lipid content in obese Japanese women mediated by decreased expression of rate-limiting lipogenic enzymes. PLoS ONE 2018, 13, e0193830. [Google Scholar] [CrossRef] [PubMed]
- Makihara, H.; Maezawa, M.; Kaiga, K.; Satake, T.; Muto, M.; Tsunoda, Y.; Shimada, T.; Akase, T. mRNA expression levels of cytochrome P450 CYP1A2, CYP3A4, and CYP3A5 in the epidermis: A focus on individual differences among Japanese individuals. Xenobiotica 2024, 54, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Just, M.; Ribera, M.; Monsó, E.; Lorenzo, J.C.; Ferrándiz, C. Effect of smoking on skin elastic fibres: Morphometric and immunohistochemical analysis. Br. J. Dermatol. 2007, 156, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Mora Huertas, A.C.; Schmelzer, C.E.H.; Luise, C.; Sippl, W.; Pietzsch, M.; Hoehenwarter, W.; Heinz, A. Degradation of tropoelastin and skin elastin by neprilysin. Biochimie 2018, 146, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Berton, A.; Godeau, G.; Emonard, H.; Baba, K.; Bellon, P.; Hornebeck, W.; Bellon, G. Analysis of the ex vivo specificity of human gelatinases A and B towards skin collagen and elastic fibers by computerized morphometry. Matrix Biol. 2000, 19, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Son, E.D.; Lee, J.Y.; Lee, S.; Kim, M.S.; Lee, B.G.; Chang, I.S.; Chung, J.H. Topical application of 17beta-estradiol increases extracellular matrix protein synthesis by stimulating tgf-Beta signaling in aged human skin in vivo. J. Investig. Dermatol. 2005, 124, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, K.; Nakagawa, H.; Moriwaki, S.; Kakuo, S.; Ohuchi, A.; Takema, Y.; Imokawa, G. Ovariectomy is sufficient to accelerate spontaneous skin ageing and to stimulate ultraviolet irradiation-induced photoageing of murine skin. Br. J. Dermatol. 2004, 151, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Hany, M.; Zidan, A.; Ghozlan, N.A.; Ghozlan, M.N.; Abouelnasr, A.A.; Sheta, E.; Hamed, Y.; Kholosy, H.; Soffar, M.; Midany, W.M.E.; et al. Comparison of Histological Skin Changes After Massive Weight Loss in Post-bariatric and Non-bariatric Patients. Obes. Surg. 2024, 34, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Amaral, C.; da Costa, J.R.; Costa, M.O.; Verbicario, J.P.; Dias, L.; Gontijo-de-Amorim, N.F.; Charles-de-Sá, L.; Takiya, C.M. M1 Polarized Macrophages Persist in Skin of Post-Bariatric Patients after 2 Years. Aesthetic Plast. Surg. 2022, 46, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Triwatcharikorn, J.; Itthipanichpong, Y.; Washrawirul, C.; Chuenboonngarm, N.; Chongpison, Y.; Udomsawaengsup, S.; Boonchaya-Anant, P.; Rerknimitr, P. Skin manifestations and biophysical changes following weight reduction induced by bariatric surgery: A 2-year prospective study. J. Dermatol. 2023, 50, 1635–1639. [Google Scholar] [CrossRef] [PubMed]
- Sephel, G.C.; Davidson, J.M. Elastin production in human skin fibroblast cultures and its decline with age. J. Investig. Dermatol. 1986, 86, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Krymchenko, R.; Coşar Kutluoğlu, G.; van Hout, N.; Manikowski, D.; Doberenz, C.; van Kuppevelt, T.H.; Daamen, W.F. Elastogenesis in focus: Navigating elastic fibers synthesis for advanced dermal biomaterial formulation. Adv. Healthc. Mater. 2024, 13, e2400484. [Google Scholar] [CrossRef] [PubMed]
- Almine, J.F.; Wise, S.G.; Weiss, A.S. Elastin signaling in wound repair. Birth Defects Res. C Embryo Today 2012, 96, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Lee, S.H.; Youn, C.S.; Choi, H.R.; Rhie, G.E.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C.; Chung, J.H. Ultraviolet radiation increases tropoelastin mRNA expression in the epidermis of human skin in vivo. J. Investig. Dermatol. 2001, 116, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.D.; Endicott, S.K.; Province, M.A.; Pierce, J.A.; Campbell, E.J. Marked longevity of human lung parenchymal elastic fibers deduced from prevalence of D-aspartate and nuclear weapons-related radiocarbon. J. Clin. Investig. 1991, 87, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Ritz-Timme, S.; Laumeier, I.; Collins, M.J. Aspartic acid racemization: Evidence for marked longevity of elastin in human skin. Br. J. Dermatol. 2003, 149, 951–959. [Google Scholar] [CrossRef] [PubMed]


| Target Gene | Forward Primer | Reverse Primer |
|---|---|---|
| ACTB | 5′-ATTGCCGACAGGATGCAGA-3′ | 5′-GAGTACTTGCGCTCAGGAGGA-3′ |
| ELN | 5′-GAGTGAAGCCTGGGAAAGTG-3′ | 5′-ACTCCTGCTCCAGTGGGAAC-3′ |
| FBN1 | 5′-CTTGAAGGGAGAAGGGCTGG-3′ | 5′-AGGGACCACTCGGACGCATA-3′ |
| LOX | 5′-ACTGCACACACACAGGAGTTG-3′ | 5′-GCCTTCTAATACGGTGGAAATT-3′ |
| LOXL1 | 5′-GACTTCGGCAACCTCAAGC-3′ | 5′-TGTTGCAGAACAAGTCGGAC-3′ |
| NE | 5′-GGGCTACCCCGGATGC-3′ | 5′-GCGTTGGTAGTAGAGTCGATC-3′ |
| NEP | 5′-CCTTCTTTAGTGCCAGGCAG-3′ | 5′-TGAGTCCACCAGTCAACGAG-3′ |
| MMP2 | 5′-CCTGAGATCTGAGCCAGGACAT-3′ | 5′-GCCAAATGGAACCGGTGCCTT-3′ |
| MMP9 | 5′-TTCATCTTCACCCGAGACATC-3′ | 5′-CTTGTGCGTGTCAAAGTTCG-3′ |
| Characteristic | N | Average ± SD, n (%) |
|---|---|---|
| Age (year) | 31 | 49.5 ± 4.8 |
| BMI (kg/m2) | 31 | 23.9 ± 3.2 |
| Obese (BMI ≥ 25) | 31 | 9 (29.0) |
| Smoking history | 25 1 | 11 (44.0) 1 |
| Gene | Dermis | Subcutaneous Tissue | ||
|---|---|---|---|---|
| Correlation Coefficient | p | Correlation Coefficient | p | |
| NE | 0.280 2 | 0.127 | 0.176 2 | 0.343 |
| NEP | 0.034 1 | 0.855 | 0.042 2 | 0.822 |
| MMP2 | 0.203 2 | 0.274 | 0.140 2 | 0.451 |
| MMP9 | 0.034 2 | 0.855 | 0.300 2 | 0.101 |
| Gene | Dermis | |
|---|---|---|
| Correlation Coefficient | p | |
| ELN | 0.517 2 | 0.003 |
| FBN1 | 0.303 2 | 0.098 |
| LOX | 0.237 2 | 0.199 |
| LOXL1 | 0.049 1 | 0.792 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makihara, H.; Kaiga, K.; Satake, T.; Muto, M.; Tsunoda, Y.; Mitsui, H.; Ohashi, K.; Akase, T. Early Structural Degradation of Dermal Elastic Fibers in Women with Mild Obesity Without Parallel Transcriptional Changes. J. Clin. Med. 2025, 14, 5220. https://doi.org/10.3390/jcm14155220
Makihara H, Kaiga K, Satake T, Muto M, Tsunoda Y, Mitsui H, Ohashi K, Akase T. Early Structural Degradation of Dermal Elastic Fibers in Women with Mild Obesity Without Parallel Transcriptional Changes. Journal of Clinical Medicine. 2025; 14(15):5220. https://doi.org/10.3390/jcm14155220
Chicago/Turabian StyleMakihara, Hiroko, Kazusa Kaiga, Toshihiko Satake, Mayu Muto, Yui Tsunoda, Hideaki Mitsui, Kenichi Ohashi, and Tomoko Akase. 2025. "Early Structural Degradation of Dermal Elastic Fibers in Women with Mild Obesity Without Parallel Transcriptional Changes" Journal of Clinical Medicine 14, no. 15: 5220. https://doi.org/10.3390/jcm14155220
APA StyleMakihara, H., Kaiga, K., Satake, T., Muto, M., Tsunoda, Y., Mitsui, H., Ohashi, K., & Akase, T. (2025). Early Structural Degradation of Dermal Elastic Fibers in Women with Mild Obesity Without Parallel Transcriptional Changes. Journal of Clinical Medicine, 14(15), 5220. https://doi.org/10.3390/jcm14155220






