Left Ventricular Ejection Fraction Predicts Outcomes in Different Subgroups of Patients Undergoing Coronary Angiography
Abstract
1. Introduction
2. Methods
2.1. Study Population, Design, and Data Collection
2.2. Inclusion and Exclusion Criteria
2.3. LVEF Stratification
2.4. Study Endpoints
2.5. Statistical Methods
3. Results
3.1. Study Population
3.2. Prognostic Impact of LVEF
3.3. Multivariable Cox Regression Analyses
3.4. Subgroup Analyses
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Rossignol, P.; Demissei, B.; Sharma, A.; Girerd, N.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.; Hillege, H.L.; et al. Coronary angiography in worsening heart failure: Determinants, findings and prognostic implications. Heart 2018, 104, 606–613. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, 1757–1780. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Anavekar, N.; Skali, H.; McMurray, J.J.V.; Swedberg, K.; Yusuf, S.; Granger, C.B.; Michelson, E.L.; Wang, D.; Pocock, S.; et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005, 112, 3738–3744. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.S.; Xu, H.; Matsouaka, R.A.; Bhatt, D.L.; Heidenreich, P.A.; Hernandez, A.F.; Devore, A.D.; Yancy, C.W.; Fonarow, G.C. Heart Failure with Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J. Am. Coll. Cardiol. 2017, 70, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Solomon, S.D. The middle child in heart failure: Heart failure with mid-range ejection fraction (40–50%). Eur. J. Heart Fail. 2014, 16, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2020, 17, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.J.; Heitner, J.F.; Sweitzer, N.K.; Anand, I.S.; Kim, H.Y.; Harty, B.; Boineau, R.; Clausell, N.; Desai, A.S.; Diaz, R.; et al. Baseline characteristics of patients in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial. Circ. Heart Fail. 2013, 6, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, S.L.; Kober, L.; Jhund, P.S.; Solomon, S.D.; Kjekshus, J.; McKelvie, R.S.; Zile, M.R.; Granger, C.B.; Wikstrand, J.; Komajda, M.; et al. International geographic variation in event rates in trials of heart failure with preserved and reduced ejection fraction. Circulation 2015, 131, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.E.; Enserro, D.; Brouwers, F.P.; Kizer, J.R.; Shah, S.J.; Psaty, B.M.; Bartz, T.M.; Santhanakrishnan, R.; Lee, D.S.; Chan, C.; et al. Predicting heart failure with preserved and reduced ejection fraction: The International Collaboration on Heart Failure Subtypes. Circ. Heart Fail. 2016, 9, e003116. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.I.; Atherton, J.J.; Krishnan, A.; Hammett, C.; Stewart, P.; Mallouhi, M.; Vollbon, W.; Thomas, L.; Prasad, S.B. Diastolic Dysfunction and Survival in Patients with Preserved or Mildly Reduced Left Ventricular Ejection Fraction Following Myocardial Infarction. J. Am. Soc. Echocardiogr. 2025, 38, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, L.; Schupp, T.; Steinke, P.; Dudda, J.; Abumayyaleh, M.; Weidner, K.; Bertsch, T.; Rusnak, J.; Akin, I.; Behnes, M. Age-Related Outcomes in Patients Undergoing Coronary Angiography: In Which Subgroups Does Age Matter? Results from a Large-Scale Retrospective Registry. J. Clin. Med. 2025, 14, 928. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, L.; Schupp, T.; Steinke, P.; Weidner, K.; Bertsch, T.; Rusnak, J.; Jannesari, M.; Siegel, F.; Duerschmied, D.; Behnes, M.; et al. Sex-Based Differences and Outcomes in Unselected Patients Undergoing Coronary Angiography. J. Clin. Med. 2025, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- De Vita, A.; Covino, M.; Pontecorvo, S.; Buonamassa, G.; Marino, A.G.; Marano, R.; Natale, L.; Liuzzo, G.; Burzotta, F.; Franceschi, F. Coronary CT Angiography in the Emergency Department: State of the Art and Future Perspectives. J. Cardiovasc. Dev. Dis. 2025, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Bershtein, L.L.; Sumin, A.N.; Kutina, A.V.; Lunina, M.D.; Evdokimov, D.S.; Nayden, T.V.; Evdokimova, E.D.; Zbyshevskaya, E.V.; Evtushenko, A.E.; Piltakyan, V.K.; et al. The Value of Clinical Variables and the Potential of Longitudinal Ultrasound Carotid Plaque Assessment in Major Adverse Cardiovascular Event Prediction After Uncomplicated Acute Coronary Syndrome. Life 2025, 15, 431. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Cho, D.H.; Kim, M.N.; Park, S.M. Rationale and Study Design of Differences in Cardiopulmonary Exercise Capacity According to Coronary Microvascular Dysfunction and Body Composition in Patients with Suspected Heart Failure with Preserved Ejection Fraction. Int. J. Heart Fail. 2021, 3, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Currie, G.; Kiat, H. Beyond the Lumen: Molecular Imaging to Unmask Vulnerable Coronary Plaques. J. Cardiovasc. Dev. Dis. 2025, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Meng, J.; Zhou, Y.; Lv, L.; Zhang, X. Prognostic value of the left ventricular ejection fraction reserve acquired by gated myocardial perfusion SPECT in patients with CAD and reduced stress LVEF. Front Cardiovasc. Med. 2024, 11, 1480501. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.; Martinez, F.; et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Spadafora, L.; Pastena, P.; Cacciatore, S.; Betti, M.; Biondi-Zoccai, G.; D’Ascenzo, F.; De Ferrari, G.M.; De Filippo, O.; Versaci, F.; Sciarretta, S.; et al. One-year prognostic differences and management strategies between ST-elevation and non-ST-elevation myocardial infarction: Insights from the PRAISE Registry. Am. J. Cardiovasc. Drugs, 2025; Online ahead of print. [Google Scholar]

| LVEF ≥ 55% (n = 3343) | LVEF of 45–54% (n = 1541) | LVEF of 35–44% (n = 966) | LVEF < 35% (n = 1038) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Age, median (IQR) | 68 | (57–78) (n = 3343) | 71 | (59–79) (n = 1541) | 72 | (63–80) (n = 966) | 70 | (61–79) (n = 1038) | 0.001 |
| Male sex, n (%) | 1964 | (58.7) | 1063 | (69.0) | 674 | (69.8) | 785 | (75.6) | 0.001 |
| Body mass index, kg/m2, median (IQR) | 27.6 | (24.6–31.1) (n = 3343) | 27.8 | (24.8–31.3) (n = 1541) | 26.8 | (23.9–30.8) (n = 966) | 26.7 | (24.1–30.4) (n = 1038) | 0.001 |
| Cardiovascular risk factors, n (%) | |||||||||
| Arterial hypertension | 2882 | (86.2) | 1428 | (92.7) | 879 | (91.0) | 861 | (82.9) | 0.001 |
| Diabetes mellitus | 793 | (23.7) | 422 | (27.4) | 287 | (29.7) | 278 | (26.8) | 0.001 |
| Hyperlipidemia | 1286 | (38.5) | 613 | (39.8) | 316 | (32.7) | 318 | (30.6) | 0.001 |
| Prior medical history, n (%) | |||||||||
| Congestive heart failure | 167 | (5.0) | 131 | (8.5) | 126 | (13.0) | 208 | (20.0) | 0.001 |
| Pacemaker | 8 | (0.2) | 17 | (1.1) | 23 | (2.4) | 52 | (5.0) | 0.001 |
| COPD | 125 | (3.7) | 57 | (3.7) | 43 | (4.5) | 43 | (4.1) | 0.718 |
| Chronic kidney disease | 142 | (4.2) | 87 | (5.6) | 66 | (6.8) | 88 | (8.5) | 0.001 |
| Liver cirrhosis | 39 | (1.2) | 10 | (0.6) | 13 | (1.3) | 10 | (1.0) | 0.293 |
| Malignancy | 162 | (4.8) | 85 | (5.5) | 81 | (8.4) | 54 | (5.2) | 0.001 |
| Stroke | 31 | (0.9) | 15 | (1.0) | 6 | (0.6) | 4 | (0.4) | 0.281 |
| Comorbidities at index hospitalization, n (%) | |||||||||
| Acute coronary syndrome | |||||||||
| Unstable angina | 1210 | (36.2) | 370 | (24.0) | 140 | (14.5) | 141 | (13.6) | 0.001 |
| STEMI | 210 | (6.3) | 271 | (17.6) | 206 | (21.3) | 144 | (13.9) | 0.001 |
| NSTEMI | 564 | (16.9) | 339 | (22.0) | 217 | (22.5) | 160 | (15.4) | 0.001 |
| Atrial fibrillation | 764 | (22.9) | 419 | (27.2) | 308 | (31.9) | 359 | (34.6) | 0.001 |
| Atrial flutter | 63 | (1.9) | 30 | (1.9) | 24 | (2.5) | 30 | (2.9) | 0.198 |
| Acute decompensated heart failure | 207 | (6.2) | 154 | (10.0) | 210 | (21.7) | 720 | (69.5) | 0.001 |
| Cardiogenic shock | 18 | (0.5) | 24 | (1.6) | 32 | (3.3) | 152 | (14.6) | 0.001 |
| Atrioventricular block | 85 | (2.5) | 37 | (2.4) | 28 | (2.9) | 22 | (2.1) | 0.721 |
| Cardiopulmonary resuscitation | 83 | (2.5) | 64 | (4.2) | 76 | (7.9) | 173 | (16.7) | 0.001 |
| Out of hospital | 62 | (1.9) | 43 | (2.8) | 55 | (5.7) | 112 | (10.8) | 0.001 |
| In hospital | 21 | (0.6) | 21 | (1.4) | 21 | (2.2) | 61 | (5.9) | 0.001 |
| Valvular heart disease | 498 | (14.9) | 258 | (16.7) | 213 | (22.0) | 301 | (29.0) | 0.001 |
| Stroke | 98 | (2.9) | 64 | (4.2) | 38 | (3.9) | 57 | (5.5) | 0.001 |
| LVEF ≥ 55% (n = 3343) | LVEF of 45–54% (n = 1541) | LVEF of 35–44% (n = 966) | LVEF < 35% (n = 1038) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Coronary angiography, n (%) | |||||||||
| No evidence of coronary artery disease | 1331 | (39.8) | 359 | (23.3) | 196 | (20.3) | 251 | (24.2) | 0.001 |
| One-vessel disease | 703 | (21.0) | 312 | (20.2) | 199 | (20.6) | 167 | (16.1) | |
| Two-vessel disease | 617 | (18.5) | 353 | (22.9) | 201 | (20.8) | 219 | (21.1) | |
| Three-vessel disease | 692 | (20.7) | 517 | (33.5) | 370 | (39.3) | 401 | (38.6) | |
| CABG | 32 | (1.0) | 46 | (3.0) | 41 | (4.2) | 78 | (7.5) | 0.001 |
| Chronic total occlusion | 147 | (4.4) | 138 | (9.0) | 125 | (12.9) | 133 | (12.8) | 0.001 |
| Diseased vessels, n (%) | |||||||||
| Right coronary artery | 1250 | (37.4) | 825 | (53.5) | 527 | (54.6) | 570 | (54.9) | 0.001 |
| Left main trunk | 246 | (7.4) | 168 | (10.9) | 153 | (15.8) | 171 | (16.5) | 0.001 |
| Left anterior descending | 1498 | (44.8) | 907 | (58.9) | 640 | (66.3) | 653 | (62.9) | 0.001 |
| Left circumflex | 1092 | (32.7) | 745 | (48.3) | 475 | (49.2) | 524 | (50.5) | 0.001 |
| Ramus intermedius | 266 | (8.0) | 194 | (12.6) | 129 | (13.4) | 159 | (15.3) | 0.001 |
| PCI, n (%) | 1223 | (36.6) | 801 | (52.0) | 464 | (48.0) | 442 | (42.6) | 0.001 |
| Right coronary artery | 484 | (14.5) | 360 | (23.4) | 162 | (16.8) | 143 | (13.8) | 0.001 |
| Left main trunk | 86 | (2.6) | 49 | (3.2) | 52 | (5.4) | 58 | (5.6) | 0.001 |
| Left anterior descending | 624 | (18.7) | 376 | (24.4) | 268 | (27.7) | 240 | (23.1) | 0.001 |
| Left circumflex | 394 | (11.8) | 290 | (18.8) | 152 | (15.7) | 139 | (13.4) | 0.001 |
| Ramus intermedius | 48 | (1.4) | 28 | (1.8) | 19 | (2.0) | 23 | (2.2) | 0.314 |
| CABG | 8 | (0.2) | 12 | (0.8) | 7 | (0.7) | 22 | (2.1) | 0.001 |
| Sent to CABG, n (%) | 121 | (3.6) | 73 | (4.7) | 69 | (7.1) | 53 | (5.1) | 0.001 |
| Procedural data | |||||||||
| Number of stents, n (%) | 2 | (1–3) (n = 1210) | 2 | (1–3) (n = 794) | 2 | (1–4) (n = 454) | 2 | (1–4) (n = 437) | 0.001 |
| Stent length, mm, median (IQR) | 40 | (23–68) (n = 1073) | 44 | (24–79) (n = 710) | 50 | (28–83) (n = 398) | 48 | (24–86) (n = 362) | 0.001 |
| Baseline laboratory values, median (IQR) | |||||||||
| Sodium, mmol/L | 139 | (138–141) (n = 3315) | 139 | (138–141) (n = 1532) | 139 | (138–141) (n = 960) | 139 | (137–141) (n = 1030) | 0.379 |
| Potassium, mmol/L | 3.90 | (3.70–4.13) (n = 3286) | 3.95 | (3.74–4.18) (n = 1525) | 3.97 | (3.75–4.21) (n = 956) | 4.03 | (3.78–4.30) (n = 1022) | 0.001 |
| Creatinine, mg/dL | 0.960 | (0.815–1.160) (n = 3318) | 1.020 | (0.865–1.251) (n = 1534) | 1.11 | (0.90–1.46) (n = 960) | 1.253 | (0.990–1.800) (n = 1030) | 0.001 |
| eGFR, mL/min/1.73 m2 | 72.64 | (57.18–87.11) (n = 3318) | 69.71 | (52.10–84.52) (n = 1534) | 63.83 | (45.27–80.74) (n = 960) | 57.35 | (39.74–76.20) (n = 1030) | 0.001 |
| Urea, mg/dL | 34.50 | (27.80–44.75) (n = 3279) | 36.78 | (29.46–49.34) (n = 1520) | 43.15 | (31.78–62.15) (n = 957) | 49.75 | (36.96–74.41) (n = 1026) | 0.001 |
| Hemoglobin, g/dL | 13.45 | (12.10–14.60) (n = 3322) | 13.30 | (11.70–14.55) (n = 1532) | 12.78 | (11.10–14.20) (n = 964) | 12.96 | (11.10–14.40) (n = 1033) | 0.001 |
| WBC count, ×109/L | 8.41 | (6.87–10.45) (n = 3322) | 8.93 | (7.20–11.15) (n = 1532) | 9.57 | (7.36–12.12) (n = 964) | 9.76 | (7.75–12.86) (n = 1033) | 0.001 |
| Platelet count, ×109/L | 238 | (196–286) (n = 3321) | 234 | (191–279) (n = 1532) | 234 | (192–287) (n = 964) | 228 | (182–285) (n = 1033) | 0.001 |
| HbA1c, % | 5.8 | (5.4–6.4) (n = 1644) | 5.8 | (5.4–6.6) (n = 881) | 5.9 | (5.5–6.9) (n = 524) | 6.0 | (5.5–6.9) (n = 566) | 0.001 |
| LDL-cholesterol, mg/dL | 110 | (83–140) (n = 2271) | 109 | (81–140) (n = 1118) | 98 | (74–126) (n = 678) | 94 | (71–123) (n = 712) | 0.001 |
| C-reactive protein, mg/L | 15.35 | (6.70–53.55) (n = 2012) | 24.15 | (9.50–70.00) (n = 1117) | 34.72 | (11.23–96.25) (n = 792) | 45.25 | (14.30–109.10) (n = 863) | 0.001 |
| Albumin, g/L | 35.40 | (32.48–38.00) (n = 3265) | 34.45 | (31.21–37.05) (n = 1516) | 33.00 | (29.35–36.15) (n = 954) | 31.70 | (27.50–35.15) (n = 1024) | 0.001 |
| INR | 1.04 | (0.99–1.10) (n = 3264) | 1.05 | (1.01–1.12) (n = 1509) | 1.08 | (1.02–1.18) (n = 940) | 1.13 | (1.05–1.31) (n = 1012) | 0.001 |
| NT-pro BNP, pg/mL | 670 | (193–2214) (n = 1003) | 1623 | (499–3635) (n = 586) | 3410 | (1602–8554) (n = 446) | 5208 | (2375–11,869) (n = 603) | 0.001 |
| All-cause mortality, in-hospital | 31 | (0.9) | 38 | (2.5) | 46 | (4.8) | 172 | (16.6) | 0.001 |
| Patients discharged alive | 3312 | (99.1) | 1503 | (97.5) | 920 | (95.2) | 866 | (83.4) | |
| Medication at discharge, n (%) | |||||||||
| ACE inhibitor | 1542 | (46.6) | 844 | (56.3) | 542 | (59.2) | 494 | (57.5) | 0.001 |
| ARB | 889 | (26.9) | 384 | (25.6) | 195 | (21.3) | 123 | (14.3) | 0.001 |
| Beta-blocker | 2049 | (61.9) | 1162 | (77.5) | 774 | (84.6) | 755 | (87.9) | 0.001 |
| Aldosterone antagonist | 149 | (4.5) | 113 | (7.5) | 266 | (29.1) | 502 | (58.4) | 0.001 |
| ARNI | 1 | (0.0) | 4 | (0.3) | 11 | (1.2) | 59 | (6.9) | 0.001 |
| SGLT2 inhibitor | 91 | (2.8) | 71 | (4.7) | 76 | (8.3) | 88 | (10.2) | 0.001 |
| Statin | 2385 | (72.1) | 1189 | (79.3) | 711 | (77.7) | 628 | (73.1) | 0.001 |
| ASA | 2096 | (63.4) | 1041 | (69.4) | 613 | (67.0) | 500 | (58.2) | 0.001 |
| P2Y12 inhibitor | 1376 | (41.6) | 866 | (57.8) | 510 | (55.7) | 374 | (43.5) | 0.001 |
| OAK | 784 | (23.7) | 436 | (29.1) | 304 | (33.2) | 345 | (40.2) | 0.001 |
| Follow-up data, median (IQR) | |||||||||
| Hospitalization time | 6 | (4–11) (n = 3343) | 7 | (4–11) (n = 1541) | 9 | (5–15) (n = 966) | 11 | (6–19) (n = 1038) | 0.001 |
| ICU time | 0 | (0-0) (n = 3343) | 0 | (0-0) (n = 1541) | 0 | (0.0) (n = 966) | 0 | (0-0) (n = 1038) | 0.001 |
| Primary endpoint, n (%) | |||||||||
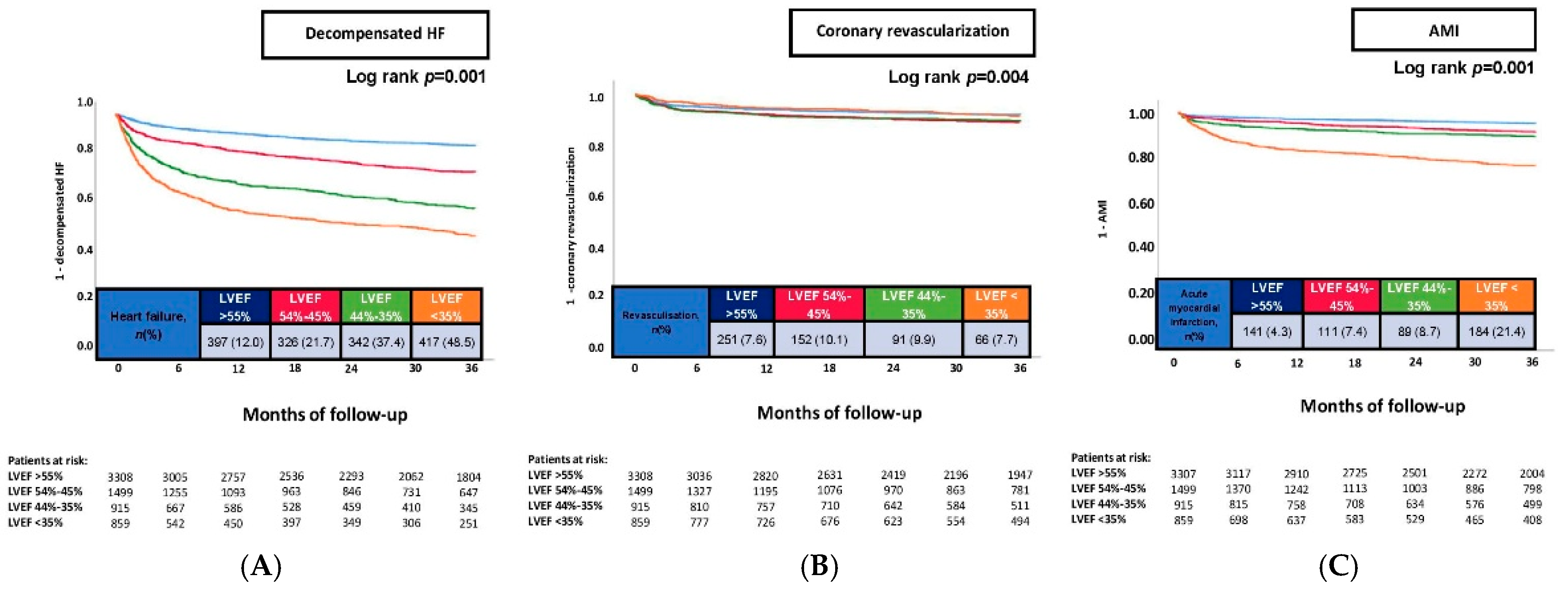
| Heart failure, at 36 months | 397 | (12.0) | 326 | (21.7) | 342 | (37.4) | 417 | (48.5) | 0.001 |
| Secondary endpoints, n (%) | |||||||||
| Acute myocardial infarction, at 36 months | 141 | (4.3) | 111 | (7.4) | 89 | (8.7) | 184 | (21.4) | 0.001 |
| Coronary revascularization, at 36 months | 251 | (7.6) | 152 | (10.1) | 91 | (9.9) | 66 | (7.7) | 0.008 |
| HF-Related Rehospitalization | Coronary Revascularization | AMI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p-Value | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Age (per year increase) | 1.012 | 1.008–1.017 | 0.001 | 1.014 | 0.903–1.139 | 0.808 | 0.990 | 0.983–0.997 | 0.005 |
| Male sex | 1.020 | 0.887–1.173 | 0.778 | 1.025 | 0.906–1.161 | 0.695 | 0.923 | 0.764–1.116 | 0.408 |
| Diabetes mellitus | 1.151 | 1.004–1.319 | 0.044 | 1.332 | 1.186–1.496 | 0.001 | 1.234 | 1.031–1.477 | 0.022 |
| Prior coronary artery disease | 1.631 | 1.350–1.971 | 0.001 | 1.175 | 0.883–1.564 | 0.268 | 1.215 | 0.919–1.606 | 0.171 |
| Prior myocardial infarction | 0.989 | 0.672–1.456 | 0.956 | 1.622 | 1.250–2.105 | 0.001 | 1.648 | 1.043–2.605 | 0.032 |
| Prior CABG | 1.278 | 1.022–1.597 | 0.032 | 1.234 | 0.882–1.728 | 0.220 | 1.248 | 0.923–1.688 | 0.150 |
| Chronic kidney disease | 1.319 | 1.066–1.631 | 0.011 | 1.588 | 1.328–1.899 | 0.001 | 0.959 | 0.676–1.361 | 0.816 |
| STEMI | 0.924 | 0.693–1.232 | 0.588 | 0.803 | 0.545–1.182 | 0.266 | 0.798 | 0.621–1.027 | 0.079 |
| NSTEMI | 0.945 | 0.796–1.123 | 0.520 | 0.959 | 0.758–1.213 | 0.727 | 0.751 | 0.595–0.948 | 0.016 |
| Atrial fibrillation | 1.223 | 1.066–1.403 | 0.004 | 1.185 | 1.055–1.331 | 0.004 | 0.974 | 0.796–1.193 | 0.801 |
| Decompensated heart failure | 1.607 | 1.374–1.880 | 0.001 | 1.490 | 1.287–1.725 | 0.001 | 1.451 | 1.161–1.812 | 0.001 |
| LVEF of 54–45% | 1.826 | 1.573-2.121 | 0.001 | 1.172 | 0.952-1.443 | 0.136 | 1.667 | 1.292-2.150 | 0.001 |
| LVEF of 44–35% | 2.948 | 2.523-3.446 | 0.001 | 1.084 | 0.836-1.405 | 0.543 | 1.988 | 1.497-2.640 | 0.001 |
| LVEF < 35% | 3.731 | 3.168-4.394 | 0.001 | 0.867 | 0.632-1.190 | 0.378 | 4.184 | 3.200-5.471 | 0.001 |
| LVEF ≥ 55% | (Reference group) | (Reference group) | (Reference group) | ||||||
| Heart-Failure-Related Rehospitalization | ||||
|---|---|---|---|---|
| Variable | HR | 95% CI | p-Value | |
| Age ≥ 70 years | LVEF < 35% | 2.338 | 1.793–3.050 | 0.001 |
| LVEF of 44–35% | 2.007 | 1.558–2.586 | 0.001 | |
| LVEF of 54–45% | 1.512 | 1.182–1.935 | 0.001 | |
| LVEF ≥ 55% | (Reference group) | |||
| Age < 70 years | LVEF < 35% | 3.874 | 2.783–5.393 | 0.001 |
| LVEF of 44–35% | 2.925 | 2.043–4.189 | 0.001 | |
| LVEF of 54–45% | 1.802 | 1.256–2.585 | 0.002 | |
| LVEF ≥ 55% | (Reference group) | |||
| Male sex | LVEF < 35% | 3.363 | 2.459–4.599 | 0.001 |
| LVEF of 44–35% | 2.599 | 1.900–3.555 | 0.001 | |
| LVEF of 54–45% | 1.786 | 1.317–2.422 | 0.001 | |
| LVEF ≥ 55% | (Reference group) | |||
| Female sex | LVEF < 35% | 3.061 | 2.048–4.579 | 0.001 |
| LVEF of 44–35% | 2.217 | 1.515–3.246 | 0.001 | |
| LVEF of 54–45% | 1.455 | 1.021–2.074 | 0.038 | |
| LVEF ≥ 55% | (Reference group) | |||
| Unstable angina | LVEF < 35% | 4.214 | 2.800–6.340 | 0.001 |
| LVEF of 44–35% | 3.531 | 2.247–5.550 | 0.001 | |
| LVEF of 54–45% | 1.979 | 1.234–3.174 | 0.005 | |
| LVEF ≥ 55% | (Reference group) | |||
| STEMI | LVEF < 35% | 4.028 | 2.351–6.892 | 0.001 |
| LVEF of 44–35% | 2.973 | 1.682–5.255 | 0.001 | |
| LVEF of 54–45% | 1.778 | 1.001–3.159 | 0.050 | |
| LVEF ≥ 55% | (Reference group) | |||
| NSTEMI | LVEF < 35% | 3.420 | 2.349–4.979 | 0.001 |
| LVEF of 44–35% | 2.346 | 1.572–3.501 | 0.001 | |
| LVEF of 54–45% | 1.624 | 1.123–2.348 | 0.010 | |
| LVEF ≥ 55% | (Reference group) | |||
| Decompensated heart failure | LVEF < 35% | 5.612 | 3.950–7.972 | 0.001 |
| LVEF of 44–35% | 3.694 | 2.525–5.402 | 0.001 | |
| LVEF of 54–45% | 1.938 | 1.291–2.910 | 0.001 | |
| LVEF ≥ 55% | (Reference group) | |||
| No/–one-vessel disease | LVEF < 35% | 3.145 | 2.326–4.254 | 0.001 |
| LVEF of 44–35% | 2.255 | 1.630–3.121 | 0.001 | |
| LVEF of 54–45% | 1.405 | 1.035–1.907 | 0.029 | |
| LVEF ≥ 55% | (Reference group) | |||
| Two/three-vessel disease | LVEF < 35% | 4.847 | 3.049–7.708 | 0.001 |
| LVEF of 44–35% | 3.277 | 2.047–5.243 | 0.001 | |
| LVEF of 54–45% | 1.720 | 1.041–2.841 | 0.034 | |
| LVEF ≥ 55% | (Reference group) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steffen, H.J.; Schupp, T.; Abumayyaleh, M.; Kuhn, L.; Steinke, P.; Dudda, J.; Weidner, K.; Rusnak, J.; Jannesari, M.; Siegel, F.; et al. Left Ventricular Ejection Fraction Predicts Outcomes in Different Subgroups of Patients Undergoing Coronary Angiography. J. Clin. Med. 2025, 14, 5219. https://doi.org/10.3390/jcm14155219
Steffen HJ, Schupp T, Abumayyaleh M, Kuhn L, Steinke P, Dudda J, Weidner K, Rusnak J, Jannesari M, Siegel F, et al. Left Ventricular Ejection Fraction Predicts Outcomes in Different Subgroups of Patients Undergoing Coronary Angiography. Journal of Clinical Medicine. 2025; 14(15):5219. https://doi.org/10.3390/jcm14155219
Chicago/Turabian StyleSteffen, Henning Johann, Tobias Schupp, Mohammad Abumayyaleh, Lasse Kuhn, Philipp Steinke, Jonas Dudda, Kathrin Weidner, Jonas Rusnak, Mahboubeh Jannesari, Fabian Siegel, and et al. 2025. "Left Ventricular Ejection Fraction Predicts Outcomes in Different Subgroups of Patients Undergoing Coronary Angiography" Journal of Clinical Medicine 14, no. 15: 5219. https://doi.org/10.3390/jcm14155219
APA StyleSteffen, H. J., Schupp, T., Abumayyaleh, M., Kuhn, L., Steinke, P., Dudda, J., Weidner, K., Rusnak, J., Jannesari, M., Siegel, F., Duerschmied, D., Behnes, M., & Akin, I. (2025). Left Ventricular Ejection Fraction Predicts Outcomes in Different Subgroups of Patients Undergoing Coronary Angiography. Journal of Clinical Medicine, 14(15), 5219. https://doi.org/10.3390/jcm14155219







