Inhaled Corticosteroids and Risk of Staphylococcus aureus Isolation in Bronchiectasis: A Register-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
- The Danish National Patient Register is a nationwide register that holds data on all hospital admissions since 1977 and hospital outpatient visits since 1995. The register was used to identify patients with BE and to identify comorbidities [13].
- The Danish National Database of Reimbursed Prescriptions includes information on all reimbursed prescriptions redeemed at hospital or community pharmacies since 1995. The register was used to stratify the population based on ICS and identify other treatments and comorbidities [14].
- Microbiological data from the Clinical Microbiology Departments in Region Zealand and the Capital Region (consisting of approximately 2.7 million inhabitants). These data were used to identify lower respiratory tract cultures positive for S. aureus.
- The Danish Civil Registration System includes individual information, including unique personal identification number, sex, date of birth, and vital status [15].
2.2. Study Population
2.3. Exposure to ICS
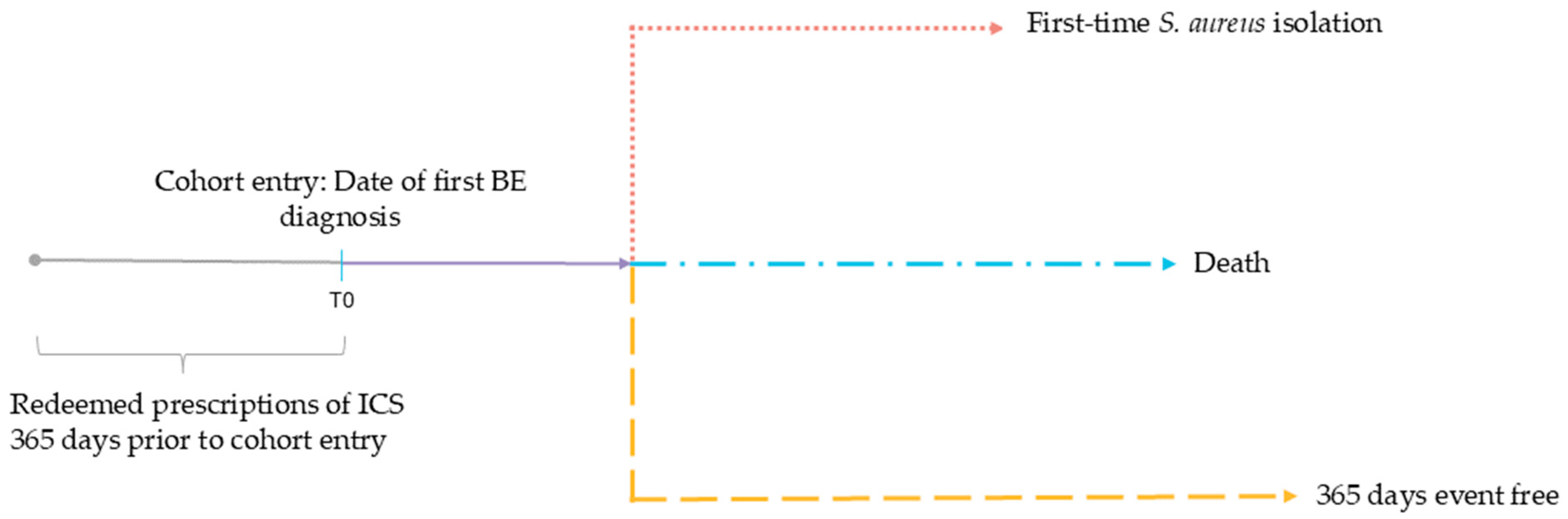
2.4. Outcome and Follow-Up
2.5. Statistical Analysis
2.6. Ethics
2.7. Generative Artificial Intelligence
3. Results
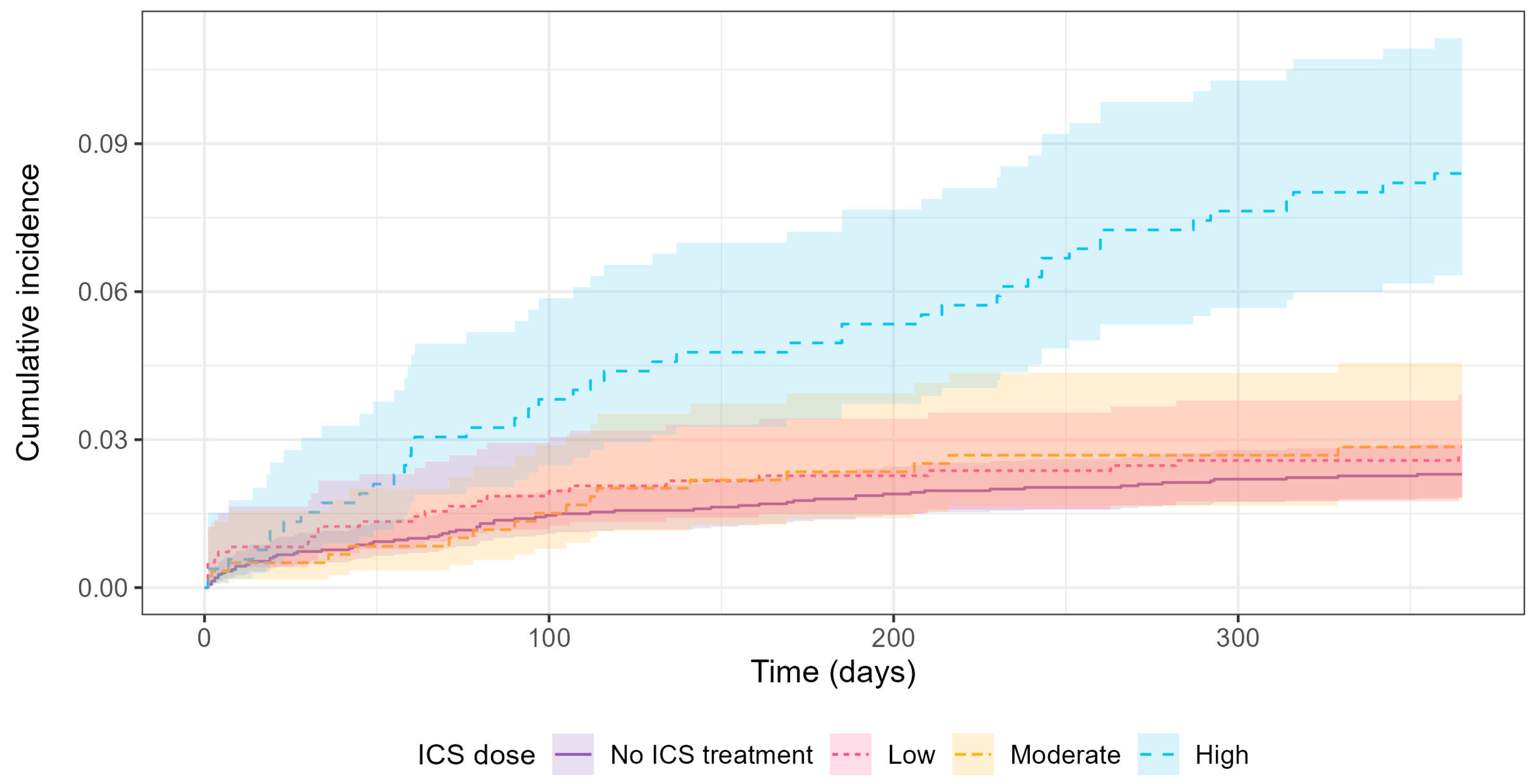
3.1. Outcome and Regression Results
3.2. Sensitivity Analyses
4. Discussion
Strengths, Limitations, and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASMD | absolute standardized mean difference |
| ATC | Anatomical Therapeutic Chemical Classification System |
| BE | non-cystic fibrosis bronchiectasis |
| BMI | body mass index |
| CCI | Charlson Comorbidity Index |
| CI | confidence interval |
| COPD | chronic obstructive pulmonary disease |
| First-time S. aureus isolation | first-time S. aureus isolation from a lower respiratory tract sample |
| H. influenzae | Haemophilus influenzae |
| HR | hazard ratio |
| ICD-10 | International Classification of Diseases and Related Health Problems 10th Revision |
| ICS | inhaled corticosteroids |
| IPTW | inverse probability of treatment weighted model |
| IQR | inter-quartile range |
| LABA | long-acting beta agonist |
| M. catarrhalis | Moraxella catarrhalis |
| OCS | oral corticosteroids |
| P. aeruginosa | Pseudomonas aeruginosa |
| Py | person-year |
| SD | standard deviation |
| S. aureus | Staphylococcus aureus |
| S. pneumoniae | Streptococcus pneumoniae |
References
- Aliberti, S.; Goeminne, P.C.; E O′DOnnell, A.; Aksamit, T.R.; Al-Jahdali, H.; Barker, A.F.; Blasi, F.; Boersma, W.G.; Crichton, M.L.; De Soyza, A.; et al. Criteria and definitions for the radiological and clinical diagnosis of bronchiectasis in adults for use in clinical trials: International consensus recommendations. Lancet Respir. Med. 2022, 10, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.D.; Chang, A.B.; Chotirmall, S.H.; Dhar, R.; McShane, P.J. Bronchiectasis. Nat. Rev. Dis. Primers 2018, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- A Flume, P.; Chalmers, J.D.; Olivier, K.N. Advances in bronchiectasis: Endotyping, genetics, microbiome, and disease heterogeneity. Lancet 2018, 392, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Quint, J.K.; Millett, E.R.; Joshi, M.; Navaratnam, V.; Thomas, S.L.; Hurst, J.R.; Smeeth, L.; Brown, J.S. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: A population-based cohort study. Eur. Respir. J. 2016, 47, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Polverino, E.; Goeminne, P.C.; McDonnell, M.J.; Aliberti, S.; Marshall, S.E.; Loebinger, M.R.; Murris, M.; Cantón, R.; Torres, A.; Dimakou, K.; et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 2017, 50, 1700629. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.; Petsky, H.L.; Bell, S.; Kolbe, J.; Chang, A.B. Inhaled corticosteroids for bronchiectasis. Cochrane Database Syst. Rev. 2018, 2018, CD000996. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; McShane, P.J.; Aliberti, S.; Chalmers, J.D. Bronchiectasis management in adults: State of the art and future directions. Eur. Respir. J. 2024, 63, 2400518. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Lai, C.-C.; Yang, W.-C.; Lin, C.-C.; Chen, L.; Wang, H.-C.; Yu, C.-J. The association between inhaled corticosteroid and pneumonia in COPD patients: The improvement of patients’ life quality with COPD in Taiwan (IMPACT) study. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2775–2783. [Google Scholar] [CrossRef] [PubMed]
- Ottesen, T.G.; Rovsing, A.H.; Ulrik, C.S. Local and systemic adverse effects of inhaled corticosteroids - Does ciclesonide differ from other inhaled corticosteroids? Respir. Med. 2025, 238, 107962. [Google Scholar] [CrossRef] [PubMed]
- Heerfordt, C.K.; Eklöf, J.; Sivapalan, P.; Ingebrigtsen, T.S.; Biering-Sørensen, T.; Harboe, Z.B.; Petersen, J.K.; Andersen, C.Ø.; Boel, J.B.; Bock, A.K.; et al. Inhaled Corticosteroids in Patients with Chronic Obstructive Pulmonary Disease and Risk of Acquiring Streptococcus pneumoniae Infection. A Multiregional Epidemiological Study. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Eklöf, J.; Ingebrigtsen, T.S.; Sørensen, R.; Saeed, M.I.; Alispahic, I.A.; Sivapalan, P.; Boel, J.B.; Bangsborg, J.; Ostergaard, C.; Dessau, R.B.; et al. Use of inhaled corticosteroids and risk of acquiring Pseudomonas aeruginosa in patients with chronic obstructive pulmonary disease. Thorax 2022, 77, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, R.H.; Heerfordt, C.K.; Boel, J.B.; Dessau, R.B.; Ostergaard, C.; Sivapalan, P.; Eklöf, J.; Jensen, J.-U.S. Inhaled corticosteroids and risk of lower respiratory tract infection with Moraxella catarrhalis in patients with chronic obstructive pulmonary disease. BMJ Open Respir. Res. 2023, 10, e001726. [Google Scholar] [CrossRef] [PubMed]
- Lynge, E.; Sandegaard, J.L.; Rebolj, M. The Danish national patient register. Scand. J. Public. Health 2011, 39, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Kildemoes, H.W.; Sørensen, H.T.; Hallas, J. The Danish national prescription registry. Scand. J. Public. Health 2011, 39, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Pedersen, L.; Sørensen, H.T. The Danish Civil Registration System as a tool in epidemiology. Eur. J. Epidemiol. 2014, 29, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, S.K.; Christiansen, C.F.; Christensen, S.; Lash, T.L.; Sørensen, H.T. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med. Res. Methodol. 2011, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies. Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- The National Institute for Health and Care Excellence (NICE). Asthma: Diagnosis, Monitoring and Chronic Asthma Management. In NICE Guidance; The National Institute for Health and Care Excellence (NICE): London, UK, 2017; pp. 1–38. Available online: https://www.nice.org.uk/guidance/ng245/resources/asthma-diagnosis-monitoring-and-chronic-asthma-management-bts-nice-sign-pdf-66143958279109 (accessed on 21 May 2025).
- Chesnaye, N.C.; Stel, V.S.; Tripepi, G.; Dekker, F.W.; Fu, E.L.; Zoccali, C.; Jager, K.J. An introduction to inverse probability of treatment weighting in observational research. Clin. Kidney J. 2022, 15, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Sjoberg, D.D.; Baillie, M.; Fruechtenicht, C.; Haesendonckx, S.; Treis, T. Ggsurvfit: Flexible Time-to-Event Figures. 2025. Available online: https://github.com/pharmaverse/ggsurvfit (accessed on 21 May 2025).
- Cefalu, M.; Ridgeway, G.; McCaffrey, D.; Morral, A.; Griffin, B.A.; Burgette, L. Twang: Toolkit for Weighting and Analysis of Nonequivalent Groups. 2024. Available online: https://cran.r-project.org/web/packages/twang/index.html (accessed on 6 May 2025).
- Martinez-Garcia, M.A.; Miravitlles, M. Bronchiectasis in COPD patients: More than a comorbidity? Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 1401–1411. [Google Scholar] [CrossRef] [PubMed]
- Gensler, L.S. Glucocorticoids: Complications to Anticipate and Prevent. Neurohospitalist 2013, 3, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Freund, O.; Hadad, Y.; Bergeron, A.; Fried, S.; Pomerantz, G.; Shaffer, A.; Paz, D.; Barel, N.; Perluk, T.M.; Amit, O.; et al. Bronchiectasis after allogeneic hematopoietic cell transplantation–an underdiagnosed complication. Respir. Med. 2025, 245, 108208. [Google Scholar] [CrossRef] [PubMed]
- Goussault, H.; Salvator, H.; Catherinot, E.; Chabi, M.-L.; Tcherakian, C.; Chabrol, A.; Didier, M.; Rivaud, E.; Fischer, A.; Suarez, F.; et al. Primary immunodeficiency-related bronchiectasis in adults: Comparison with bronchiectasis of other etiologies in a French reference center. Respir. Res. 2019, 20, 275. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Corticosteroid effects on cell signalling. Eur. Respir. J. 2006, 27, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Singanayagam, A.; Glanville, N.; Cuthbertson, L.; Bartlett, N.W.; Finney, L.J.; Turek, E.; Bakhsoliani, E.; Calderazzo, M.A.; Trujillo-Torralbo, M.-B.; Footitt, J.; et al. Inhaled corticosteroid suppression of cathelicidin drives dysbiosis and bacterial infection in chronic obstructive pulmonary disease. Sci. Transl. Med. 2019, 11, eaav3879. [Google Scholar] [CrossRef] [PubMed]
- Contoli, M.; Pauletti, A.; Rossi, M.R.; Spanevello, A.; Casolari, P.; Marcellini, A.; Forini, G.; Gnesini, G.; Marku, B.; Barnes, N.; et al. Long-term effects of inhaled corticosteroids on sputum bacterial and viral loads in COPD. Eur. Respir. J. 2017, 50, 1700451. [Google Scholar] [CrossRef] [PubMed]

| No ICS, n = 3003 | Low-Dose ICS, n = 970 | Moderate-Dose ICS, n = 596 | High-Dose ICS, n = 524 | Total, n = 5093 | |
|---|---|---|---|---|---|
| Age, mean (SD) | 62.5 (14.4) | 60.3 (14.5) | 62.6 (13.3) | 62.1 (13.5) | 62.0 (14.2) |
| Female sex, n (%) | 1816 (60.5) | 624 (64.3) | 376 (63.1) | 341 (65.1) | 3157 (62.0) |
| CCI score | |||||
| 0–2, n (%) | 1633 (54.4) | 518 (53.4) | 227 (38.1) | 196 (37.4) | 2574 (50.5) |
| 3–4, n (%) | 1144 (38.1) | 366 (37.7) | 282 (47.3) | 261 (49.8) | 2053 (40.3) |
| 5+, n (%) | 226 (7.5) | 86 (8.9) | 87 (14.6) | 67 (12.8) | 466 (9.1) |
| Heart failure, n (%) | 116 (3.9) | 40 (4.1) | 29 (4.9) | 32 (6.1) | 217 (4.3) |
| Myocardial infarction, n (%) | 53 (1.8) | 20 (2.1) | 12 (2.0) | 21 (4.0) | 106 (2.1) |
| Peripheral vascular disease, n (%) | 95 (3.2) | 26 (2.7) | 13 (2.2) | 23 (4.4) | 157 (3.1) |
| Cerebrovascular disease, n (%) | 120 (4.0) | 30 (3.1) | 24 (4.0) | 16 (3.1) | 190 (3.7) |
| Dementia, n (%) | 10 (0.3) | <4 | <4 | <4 | − |
| COPD/asthma, n (%) | 616 (20.5) | 435 (44.8) | 426 (71.5) | 416 (79.4) | 1893 (37.2) |
| Connective tissue disease, n (%) | 210 (7.0) | 53 (5.5) | 45 (7.6) | 39 (7.4) | 347 (6.8) |
| Ulcer disease, n (%) | 43 (1.4) | 10 (1.0) | 11 (1.8) | 11 (2.1) | 75 (1.5) |
| Hemiplegia/paraplegia, n (%) | 8 (0.3) | <4 | <4 | <4 | − |
| Diabetes without complications, n (%) | 126 (4.2) | 38 (3.9) | 34 (5.7) | 26 (5.0) | 224 (4.4) |
| Diabetes with chronic complications, n (%) | 56 (1.9) | 23 (2.4) | 20 (3.4) | 18 (3.4) | 117 (2.3) |
| Mild liver disease, n (%) | 32 (1.1) | 5 (0.5) | 6 (1.0) | 7 (1.3) | 50 (1.0) |
| Moderate/severe liver disease, n (%) | 9 (0.3) | <4 | <4 | <4 | − |
| Renal disease, n (%) | 56 (1.9) | 18 (1.9) | 12 (2.0) | 11 (2.1) | 97 (1.9) |
| AIDS, n (%) | 8 (0.3) | <4 | <4 | <4 | − |
| OCS treatment | |||||
| No use, n (%) | 2709 (90.2) | 739 (76.2) | 352 (59.1) | 259 (49.4) | 4059 (79.7) |
| Low dose, n (%) | 179 (6.0) | 153 (15.8) | 136 (22.8) | 119 (22.7) | 587 (11.5) |
| High dose, n (%) | 115 (3.8) | 78 (8.0) | 108 (18.1) | 146 (27.9) | 447 (8.8) |
| AB treatment, n (%) | 1985 (66.1) | 731 (75.4) | 466 (78.2) | 457 (87.2) | 3639 (71.5) |
| LABA treatment, n (%) | 478 (15.9) | 489 (50.4) | 412 (69.1) | 415 (79.2) | 1794 (35.2) |
| Hospitalization, n (%) | 775 (25.8) | 238 (24.5) | 182 (30.5) | 171 (32.6) | 1366 (26.8) |
| Outcome | No ICS, n = 3003 | Low-Dose ICS, n = 970 | Moderate-Dose ICS, n = 596 | High-Dose ICS, n = 524 | Total, n = 5093 |
|---|---|---|---|---|---|
| First-time S. aureus isolation, n (%) | 69 (2.3) | 26 (2.7) | 17 (2.9) | 44 (8.4) | 156 (3.1) |
| Death, n (%) | 81 (2.7) | 19 (2.0) | 30 (5.0) | 32 (6.1) | 162 (3.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipsen, A.A.; Frost, K.H.; Eklöf, J.; Tønnesen, L.L.; Vognsen, A.K.; Boel, J.B.; Pinholt, M.; Andersen, C.Ø.; Dessau, R.B.C.; Biering-Sørensen, T.; et al. Inhaled Corticosteroids and Risk of Staphylococcus aureus Isolation in Bronchiectasis: A Register-Based Cohort Study. J. Clin. Med. 2025, 14, 5207. https://doi.org/10.3390/jcm14155207
Filipsen AA, Frost KH, Eklöf J, Tønnesen LL, Vognsen AK, Boel JB, Pinholt M, Andersen CØ, Dessau RBC, Biering-Sørensen T, et al. Inhaled Corticosteroids and Risk of Staphylococcus aureus Isolation in Bronchiectasis: A Register-Based Cohort Study. Journal of Clinical Medicine. 2025; 14(15):5207. https://doi.org/10.3390/jcm14155207
Chicago/Turabian StyleFilipsen, Andrea Arlund, Karen Hougaard Frost, Josefin Eklöf, Louise Lindhardt Tønnesen, Anna Kubel Vognsen, Jonas Bredtoft Boel, Mette Pinholt, Christian Østergaard Andersen, Ram Benny Christian Dessau, Tor Biering-Sørensen, and et al. 2025. "Inhaled Corticosteroids and Risk of Staphylococcus aureus Isolation in Bronchiectasis: A Register-Based Cohort Study" Journal of Clinical Medicine 14, no. 15: 5207. https://doi.org/10.3390/jcm14155207
APA StyleFilipsen, A. A., Frost, K. H., Eklöf, J., Tønnesen, L. L., Vognsen, A. K., Boel, J. B., Pinholt, M., Andersen, C. Ø., Dessau, R. B. C., Biering-Sørensen, T., Johansson, S. L., Jensen, J.-U., & Sivapalan, P. (2025). Inhaled Corticosteroids and Risk of Staphylococcus aureus Isolation in Bronchiectasis: A Register-Based Cohort Study. Journal of Clinical Medicine, 14(15), 5207. https://doi.org/10.3390/jcm14155207








