Ultrasound Pattern of Indeterminate Thyroid Nodules with Prevalence of Oncocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Institutional Setting and Management of ITNs
2.2. Case Selection
2.3. Measures and Reference Standard
2.4. Statistical Analysis
3. Results
3.1. Case Study
3.2. US Assessment
3.3. Cytological Assessment
3.4. Differences Between O-ITN and Non-O-ITN
3.5. Translation from ICCRTC to Other Cytological Classification Systems
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ITN | Indeterminate thyroid nodule |
| O-ITN | Oncocyte-rich indeterminate thyroid nodule |
| US | Ultrasound |
| ICCRTC | Italian Consensus for the Classification and Reporting of Thyroid Cytology |
| BSRTC | Bethesda System for Reporting Thyroid Cytopathology |
| PTC | Papillary thyroid carcinoma |
| MTC | Medullary thyroid carcinoma |
| OTC | Oncocytic thyroid carcinoma |
References
- Wong, K.S.; Angell, T.E.; Barletta, J.A.; Krane, J.F. Hürthle cell lesions of the thyroid: Progress made and challenges remaining. Cancer Cytopathol. 2021, 129, 347–362. [Google Scholar] [CrossRef] [PubMed]
- Máximo, V.; Soares, P.; Lima, J.; Cameselle-Teijeiro, J.; Sobrinho-Simões, M. Mitochondrial DNA Somatic Mutations (Point Mutations and Large Deletions) and Mitochondrial DNA Variants in Human Thyroid Pathology. Am. J. Pathol. 2002, 160, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Nardi, F.; Basolo, F.; Crescenzi, A.; Fadda, G.; Frasoldati, A.; Orlandi, F.; Palombini, L.; Papini, E.; Zini, M.; Pontecorvi, A.; et al. Italian consensus for the classification and reporting of thyroid cytology. J. Endocrinol. Investig. 2014, 37, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.Z.; Baloch, Z.W.; Cochand-Priollet, B.; Schmitt, F.C.; Vielh, P.; VanderLaan, P.A. The 2023 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2023, 33, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Tappouni, R.R.; Itri, J.N.; McQueen, T.S.; Lalwani, N.; Ou, J.J. ACR TI-RADS: Pitfalls, Solutions, and Future Directions. RadioGraphics 2019, 39, 2040–2052. [Google Scholar] [CrossRef] [PubMed]
- Poller, D.N.; Megadmi, H.; Ward, M.J.A.; Trimboli, P. Hürthle Cells on Fine-Needle Aspiration Cytology Are Important for Risk Assessment of Focally PET/CT FDG Avid Thyroid Nodules. Cancers 2020, 12, 3544. [Google Scholar] [CrossRef] [PubMed]
- Schatz-Siemers, N.; Brandler, T.C.; Oweity, T.; Sun, W.; Hernandez, A.; Levine, P. Hürthle cell lesions on thyroid fine needle aspiration cytology: Molecular and histologic correlation. Diagn. Cytopathol. 2019, 47, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Piticchio, T.; Sellasie, S.W.; D’aRrigo, F.; Galeano, F.; Barca, I.; Prinzi, A.; Le Moli, R.; Scappaticcio, L.; Amendola, S.; Guidobaldi, L.; et al. Clinical management of indeterminate thyroid nodules needs to be revisited. New evidence for a personalized approach to the problem. J. Endocrinol. Investig. 2024, 48, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Hegedüs, L.; Czarniecka, A.; Paschke, R.; Russ, G.; Schmitt, F.; Soares, P.; Solymosi, T.; Papini, E. 2023 European Thyroid Association Clinical Practice Guidelines for thyroid nodule management. Eur. Thyroid J. 2023, 12, e230067. [Google Scholar] [CrossRef] [PubMed]
- Matrone, A.; Gambale, C.; Biagini, M.; Prete, A.; Vitti, P.; Elisei, R. Ultrasound features and risk stratification systems to identify medullary thyroid carcinoma. Eur. J. Endocrinol. 2021, 185, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Słowińska-Klencka, D.; Wysocka-Konieczna, K.; Klencki, M.; Popowicz, B. Usability of EU-TIRADS in the Diagnostics of Hürthle Cell Thyroid Nodules with Equivocal Cytology. J. Clin. Med. 2020, 9, 3410. [Google Scholar] [CrossRef] [PubMed]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Kure, S.; Ohashi, R. Thyroid Hürthle Cell Carcinoma: Clinical, Pathological, and Molecular Features. Cancers 2020, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Sparano, C.; Rotondi, M.; Verdiani, V.; Brunori, P.; Castiglione, F.; Bartoli, C.; Perigli, G.; Badii, B.; Vezzosi, V.; Simontacchi, G.; et al. Classic and Follicular Variant of Papillary Thyroid Microcarcinoma: 2 Different Phenotypes Beyond Tumor Size. J. Endocr. Soc. 2022, 6, bvac157. [Google Scholar] [CrossRef] [PubMed]
- Tallini, G.; Tuttle, R.M.; Ghossein, R.A. The History of the Follicular Variant of Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2017, 102, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Kyriazidis, N.; Faquin, W.C.; Soylu, S.; Kamani, D.; Saade, R.; Torchia, N.; Lubitz, C.C.; Davies, L.; Stathatos, N.; et al. The Presence of Hürthle Cells Does Not Increase the Risk of Malignancy in Most Bethesda Categories in Thyroid Fine-Needle Aspirates. Thyroid 2020, 30, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.S.; Jo, V.Y.; Lowe, A.C.; Faquin, W.C.; Renshaw, A.A.; Shah, A.A.; Roh, M.H.; Stelow, E.B.; Krane, J.F. Malignancy risk for solitary and multiple nodules in Hürthle cell–predominant thyroid fine-needle aspirations: A multi-institutional study. Cancer Cytopathol. 2020, 128, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Ferrarazzo, G.; Cappelli, C.; Piccardo, A.; Castellana, M.; Barizzi, J. Thyroid Nodules with Indeterminate FNAC According to the Italian Classification System: Prevalence, Rate of Operation, and Impact on Risk of Malignancy. An Updated Systematic Review and Meta-analysis. Endocr. Pathol. 2022, 33, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, L.A.; Ganly, I.; Fugazzola, L.; Buczek, E.; Faquin, W.C.; Haugen, B.R.; McIver, B.; McMullen, C.P.; Newbold, K.; Rocke, D.J.; et al. Molecular Alterations and Comprehensive Clinical Management of Oncocytic Thyroid Carcinoma. JAMA Otolaryngol. –Head Neck Surg. 2024, 150, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Abi-Raad, R.; Prasad, M.L.; Adeniran, A.J.; Cai, G. Copy number variations identified in thyroid FNA specimens are associated with Hürthle cell cytomorphology. Cancer Cytopathol. 2022, 130, 415–422. [Google Scholar] [CrossRef] [PubMed]

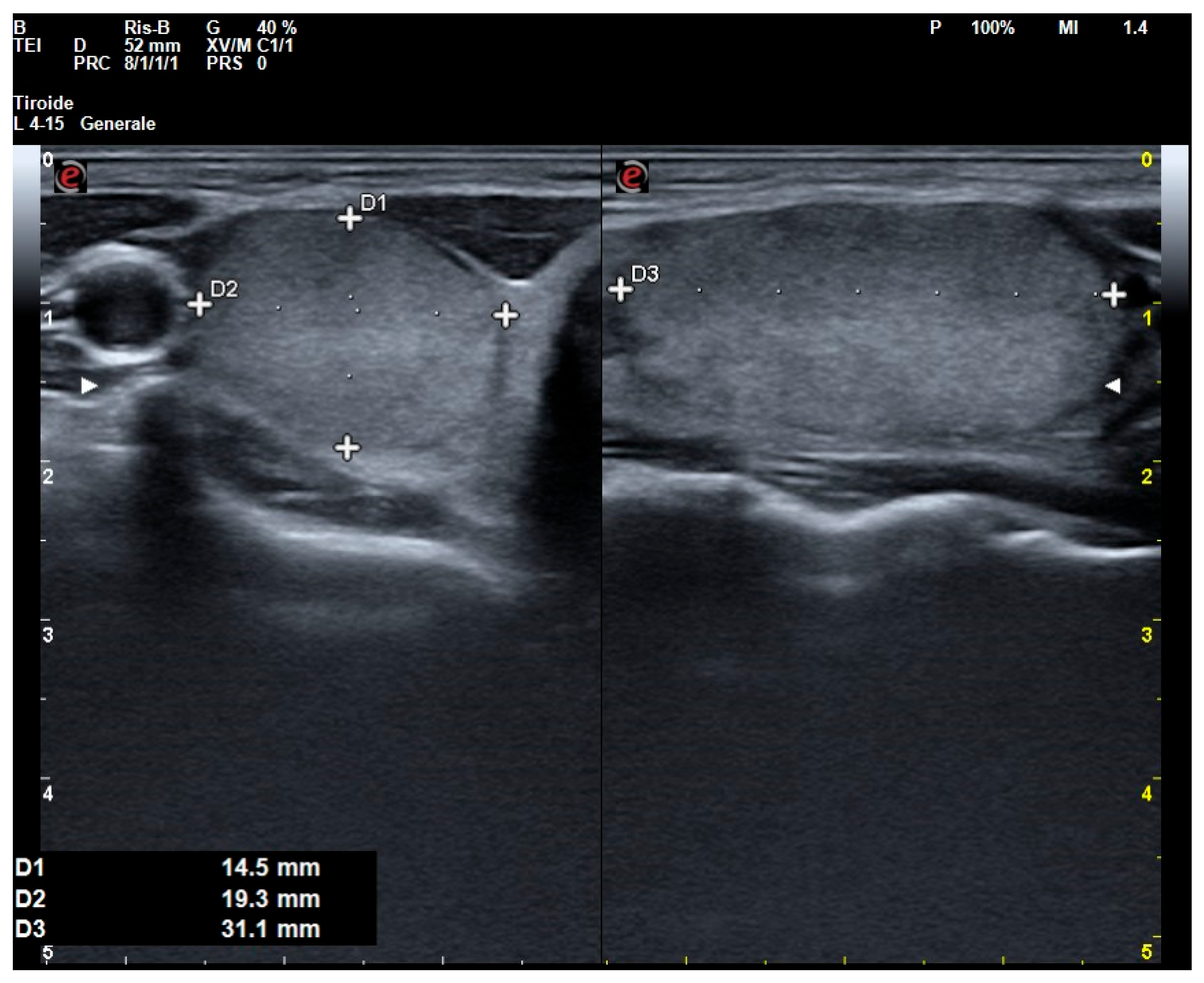
| Demographic Features | |
| Cases, n | 177 |
| Age, years (IQR) | 58.0 (49.0–65.0) |
| Gender (male; female), % | 25.4; 74.6 |
| Nodule’s US data | |
| Maximum ITN diameter, mm (IQR) | 15.0 (10.0–20.0) |
| ACR TI-RADS (1; 2; 3; 4; 5), % | 0.0; 1.1; 26.0; 61.0; 11.9 |
| Composition (cystic or almost completely cystic, spongiform, mixed cystic and solid, or solid or almost completely solid), % | 0.0; 0.0; 4.0; 96.0 |
| Echogenicity (anechoic, hyperechoic or isoechoic, hypoechoic, or very hypoechoic), % | 0.0; 35.0; 58.8; 6.2 |
| Shape (wider than tall or taller than wide), % | 94.9; 5.1 |
| Margin (smooth, ill-defined, lobulated or irregular, or extra-thyroidal extension), % | 93.2; 0.0; 6.8; 0.0 |
| Echogenic foci (none or large comet-tail artifacts, macrocalcifications, peripheral rim calcifications, or punctate echogenic foci), % | 83.1; 11.3; 4.0; 1.6 |
| Lobe (right; left; isthmus), % | 44.9; 47.2; 8.0 |
| Position in the lobe (upper; middle; lower), % | 30.7; 29.7; 39.6 |
| Nodule’s cytological characterization | |
| Cytological classification-ICCRTC (TIR3A; TIR3B), % | 72.3; 27.7 |
| Cytological classification-BSRTC (AUS-n; AUS-o; FN; SFM), % | 42.4; 35.6; 17.5; 4.5 |
| Nuclear alterations (absent; minor atypia; major atypia), % | 10.2; 68.9; 20.9 |
| Scant/absent colloid (yes; no), % | 16.9; 83.1 |
| Oncocyte predominance (yes; no), % | 47.5; 52.5 |
| Microfollicular arrangement (yes; no), % | 32.8; 67.2 |
| High cellularity (yes; no), % | 48.0; 52.0 |
| Non-O-ITN (n = 92) | O-ITN (n = 85) | p-Value | |
|---|---|---|---|
| Age, years (IQR) | 58.0 (48–64) | 59.0 (50–67) | 0.53 |
| Gender (male; female), % | 22.8; 77.2 | 28.2; 71.8 | 0.41 |
| Maximum ITN diameter, mm (IQR) | 14.0 (9.7–19.3) | 16.0 (11–23) | 0.04 |
| ACR TI-RADS classes (1; 2; 3; 4; 5), % | 0.0; 2.2; 13; 67.4; 17.4 | 0.0; 2.4; 37.6; 54.1; 5.9 | <0.01 |
| Composition (cystic or almost completely cystic, spongiform, mixed cystic and solid, or solid or almost completely solid), % | 0.0; 0.0; 3.3; 96.7 | 0.0; 0.0; 4.7; 95.3 | 0.62 |
| Echogenicity (anechoic, hyperechoic or isoechoic, hypoechoic, or very hypoechoic), % | 0.0; 26.1; 66.3; 7.6 | 0.0; 44.7; 50.6; 4.7 | 0.03 |
| Shape (wider than tall or taller than wide), % | 91.3; 8.7 | 98.8; 1.2 | 0.02 |
| Margin (smooth, ill-defined, lobulated or irregular, or extra-thyroidal extension), % | 89.1; 0.0; 10.9; 0.0 | 97.6; 0.0; 2.4; 0.0 | 0.02 |
| Echogenic foci (none or large comet-tail artifacts, macrocalcifications, peripheral (rim) calcifications, or punctate echogenic foci), % | 75.0; 17.4; 5.4; 2.2 | 91.8; 4.7; 2.4; 1.2 | 0.03 |
| Lobe (right; left; isthmus), % | 45.1; 49.5; 5.5 | 44.7; 44.7; 10.6 | 0.44 |
| Position in the lobe (upper; middle; lower), % | 38.0; 26.0; 36.0 | 41.2; 33.3; 25.5 | 0.49 |
| Cytological classification-ICCRTC (TIR3A; TIR3B), % | 60.9; 39.1 | 84.7; 15.3 | <0.01 |
| Cytological classification-BSRTC (AUS-n; AUS-o; FN; SFM), % | 15.2; 55.4; 23.9; 5.4 | 71.8; 14.1; 10.6; 3.5 | <0.01 |
| Nuclear Alterations (absent; minor atypia; major atypia), % | 8.7; 59.8; 31.5 | 11.8; 78.8; 9.4 | <0.01 |
| Scant/absent colloid (yes; no), % | 27.2; 78.8 | 5.9; 94.1 | <0.01 |
| Microfollicular arrangement (yes; no), % | 57.6; 42.4 | 5.9; 94.1 | <0.01 |
| High cellularity (yes; no), % | 25.0; 75.0 | 72.9; 27.1 | <0.01 |
| ROM, % | 53.3 | 47.1 | 0.67 |
| US | Sensitivity, % | Specificity, % | Accuracy, % |
|---|---|---|---|
| Composition | |||
| Mixed cystic and solid | 4.8 | 96.4 | 53.1 |
| Solid or almost completely solid | 95.2 | 3.2 | 46.9 |
| Echogenicity | |||
| Hyperechoic or isoechoic | 45.2 | 74.2 | 60.5 |
| Hypoechoic | 50 | 33.3 | 41.2 |
| Markedly hypoechoic | 4.8 | 92.5 | 50.8 |
| Shape | |||
| Wider than tall | 98.8 | 8.6 | 51.6 |
| Taller than wide | 1.2 | 91.4 | 48.6 |
| Margin | |||
| Smooth | 98.8 | 10.8 | 52.5 |
| Lobulated or irregular | 1.2 | 89.2 | 47.5 |
| Echogenic foci | |||
| None or large comet-tail artifacts | 91.7 | 24.7 | 56.5 |
| Macrocalcifications | 4.8 | 82.8 | 45.8 |
| Peripheral rim calcifications | 2.4 | 94.6 | 50.8 |
| Punctate echogenic foci | 1.2 | 97.8 | 52 |
| ACR TI-RADS | |||
| 2 | 2.4 | 97.8 | 52.5 |
| 3 | 38.1 | 87.1 | 63.8 |
| 4 | 53.6 | 32.3 | 42.4 |
| 5 | 6.0 | 82.8 | 46.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolde Sellasie, S.; Amendola, S.; Guidobaldi, L.; Pedicini, F.; Nardone, I.; Piticchio, T.; Zaccaria, S.; Uccioli, L.; Trimboli, P. Ultrasound Pattern of Indeterminate Thyroid Nodules with Prevalence of Oncocytes. J. Clin. Med. 2025, 14, 5206. https://doi.org/10.3390/jcm14155206
Wolde Sellasie S, Amendola S, Guidobaldi L, Pedicini F, Nardone I, Piticchio T, Zaccaria S, Uccioli L, Trimboli P. Ultrasound Pattern of Indeterminate Thyroid Nodules with Prevalence of Oncocytes. Journal of Clinical Medicine. 2025; 14(15):5206. https://doi.org/10.3390/jcm14155206
Chicago/Turabian StyleWolde Sellasie, Sium, Stefano Amendola, Leo Guidobaldi, Francesco Pedicini, Isabella Nardone, Tommaso Piticchio, Simona Zaccaria, Luigi Uccioli, and Pierpaolo Trimboli. 2025. "Ultrasound Pattern of Indeterminate Thyroid Nodules with Prevalence of Oncocytes" Journal of Clinical Medicine 14, no. 15: 5206. https://doi.org/10.3390/jcm14155206
APA StyleWolde Sellasie, S., Amendola, S., Guidobaldi, L., Pedicini, F., Nardone, I., Piticchio, T., Zaccaria, S., Uccioli, L., & Trimboli, P. (2025). Ultrasound Pattern of Indeterminate Thyroid Nodules with Prevalence of Oncocytes. Journal of Clinical Medicine, 14(15), 5206. https://doi.org/10.3390/jcm14155206








