Abstract
Background/Objectives: Colorectal cancer (CRC) is one of the major epidemiological oncological confronts with established risk factors, including male sex. Still, CRC is reported among the leading malignancies in the female population. The necessity for possible, easily accessible prognostic factors is required to improve patient outcomes. This study aimed to assess sex-related differences in nine-month four-stage CRC results of palliative systemic therapy. Methods: A total of 67 patients (39 males) with a median age of 70 (64–76) years were referred for first-line palliative chemotherapy due to end-stage colorectal cancer diagnosis. The CRC advancement was evaluated by computed tomography (CT) before and 9 months after chemotherapy. The demographical and clinical characteristics were evaluated for nine-month therapy outcomes, including mortality risk and CT scan results. Results: The nine-month mortality risk in female and male groups was indifferent, reaching 21% (6 patients) and 21% (8 patients), respectively (p = 0.935). Among survivors, therapy response was observed in 6 (21%) female and 20 (51%) male patients (p = 0.056). In multivariable analysis, the male sex (OR: 3.91, 95% CI: 1.09–14.05, p = 0.037) and RDW (OR: 0.61, 95% CI: 0.42–0.88, p = 0.008) were found to be significant for disease response to systemic therapy based on CT scan results. The ROC curve for predictive role yields a sensitivity of 71.1%, specificity of 57.8%, and an area under the curve (AUC) of 0.726. Conclusions: Our analysis points out the possible favorable role of the male sex on nine-month systemic therapy response in palliative CRC. The RDW-CV can be regarded as a possible indicator of chemotherapy response in colorectal cancer. The mortality risk within 9 months of systemic therapy is comparable between males and females.
1. Introduction
Colorectal cancer (CRC) is one of the major epidemiological oncological confronts worldwide [1]. Despite a growing body of research focused on early diagnosis and risk factors identification, the late onset is still one of the medical challenges related to inferior prognosis [2,3]. CRC represents one of the most common malignancies with established risk factors such as older age, male predominance, hereditary predisposition, physical inactivity, and excessive adipose tissue accumulation [4]. Up to one out of ten new CRC cases are linked with non-polyposis colorectal cancer, familial adenomatous polyposis, or other hereditary syndromes [5].
CRC is reported as one of the leading malignancies in the female population [6]. In recent years, the CRC mortality rate has been reported to decrease in males by 5% and female patients by almost 10% [7]. It is considered that estrogens may diminish colorectal cancer risk by modulating anti-tumor immune responses [8].
Metastatic CRC is a dissimilar disease requiring complex and dynamic management, including applied therapies, including triplet chemotherapy when the more aggressive approach is indicated [9]. Numerous possible prognostic factors were applied to improve patient outcomes, including simple inflammatory markers from peripheral blood count analysis [10,11,12].
The oncologic diseases are believed to induce chronic inflammatory activation [13]. Innate immunity may promote malignant progression [14]. The interleukin-6, tumor necrosis factor-alpha, and C-reactive Protein (CRP) were found to be related to tumor progression [15].
In recent studies [16], inflammation-related biomarkers obtained from peripheral blood count analysis have been proposed as useful predictors of worse outcomes. Next to identities such as neutrophil-to-lymphocyte ratio (NLR) and monocyte-to-lymphocyte ratio (MLR), an adverse relation between red cell distribution width (RDW) and CRC has been suggested [17].
The red cell distribution width (RDW) describes the heterogeneity of circulating red blood cells. Initially, this parameter was applied to diagnose anemia. Its utility in various pathological conditions’ mortality prediction has been noticed, including cardiovascular, pulmonary, gastrointestinal tract, and kidney diseases [18,19].
The clinical utility of the hemoglobin to RDW ratio in differential diagnosis of CRC from benign colorectal lesions has been postulated [20]. In the recent Korzinek et al. [21] study, the diagnostic value of higher levels of RDW was confirmed, differentiating CRC from adenomatous lesions.
In Peng et al.’s study [22], the RDW was related to increased postoperative complications risk, but not overall survival among CRC patients undergoing radical surgery. In contrast, Zhao et al. [23] in their analysis performed on 6.224 CRC patients postulated the prognostic value of preoperative higher RDW on overall and disease-free survival in CRC patients treated by radical resection in China. Wen et al. [24], in their meta-analysis, pointed out the differences regarding the RDW methodology on their prognostic value. As the RDW-coefficient of variation (RDW-CV) is a relative measure presented in percentages, the RDW standard deviation (RDW-SD) is an absolute measure given in femtolitres. The prognostic value of RDW-SD as an independent overall survival and disease-free survival (DFS) was identified; the RDW-CV was recognized as a DFS marker.
This study aimed to assess sex-related differences in the nine-month four-stage CRC results of palliative systemic therapy. We aim to point out possible predictors of systemic therapy in palliative CRC.
2. Materials and Methods
Our retrospective analysis included 67 (39 (%) males) with a median age of 70 (64–76) years referred for first-line palliative chemotherapy due to end-stage colorectal cancer diagnosis between 2022 and 2024 in the Clinical Oncology and Immuno-Oncology Department with Day Outpatient Sub-Department and Reception Unit of Greater Poland Cancer Centre. Only patients with a diagnosed 4th-grade CRC, defined by disease spread beyond the gastrointestinal system, including other organs as lungs, liver, or peritoneum, who were treated with the same protocol of systemic therapy within a nine-month period, were enrolled in the study.
The CRC advancement was evaluated by computed tomography (CT) before and 9 months after chemotherapy. The demographical and clinical characteristics were evaluated for nine-month therapy outcomes, including mortality risk and CT scan results.
The exclusion criteria included second- or third-line systemic therapy or requirement for systemic therapy changes within nine months due to intolerance/side effects or other reasons. Any missing data was regarded as exclusion criteria.
The analyzed group was divided according to sex differences, as presented in Table 1.

Table 1.
A comparison of sex-related groups.
2.1. Bioethics Committee
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Poznan University of Medical Sciences, Poznan, Poland (protocol code 405/24 from 19 June 2024, for studies involving humans.
2.2. Statistical Analysis
Since the data did not follow a normal distribution, continuous variables were reported as medians and interquartile ranges (Q1–Q3). Categorical data were presented as numbers and percentages. The Mann–Whitney test compared interval parameters between proximal and non-proximal groups. Categorical data were compared using a chi-square test of independence. Both univariate and multivariable models were used to predict the efficiency of chemotherapy protocols based on CT imaging. The multivariable model was assessed using the best subset method. The results were presented as odds ratio (OR) and 95% confidence intervals (95% CI). The receiver operator curve (ROC) was performed to check the accuracy of confirming multivariable analysis results in the prediction model. Statistical analysis was performed using JASP version 0.14.1 (University of Amsterdam, The Netherlands), with a significance level set at p < 0.05 (https://jasp-stats.org).
3. Results
The nine-month mortality risk in female and male groups was indifferent, reaching 21% (6 patients) and 21% (8 patients), respectively (p = 0.935), as presented in Figure 1.
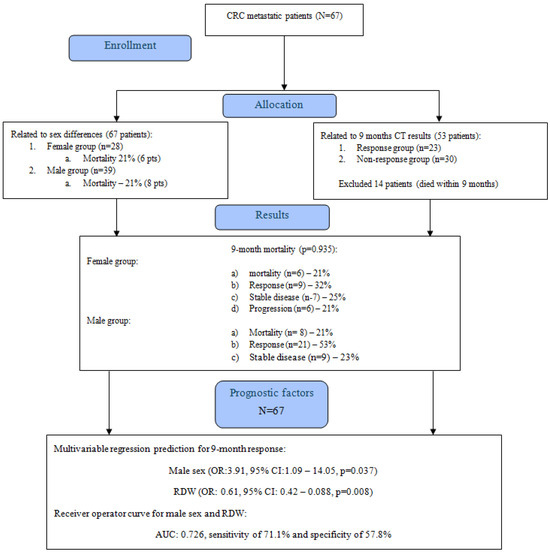
Figure 1.
CONSORT flow diagram.
Fifty-three (79%) patients underwent surgical procedures before chemotherapy, including tumor resection (thirty-five (52%) patients) and hemicolectomy (eighteen (27%) patients). The female and male groups did not differ regarding the surgical intervention, as presented in Table 2.

Table 2.
Applied therapy in the analyzed group.
3.1. Systemic Therapy
The systemic therapy was based on the patient’s clinical assessment and genetic results related to the presence of kras/nras/braf mutations. Standard therapy included the following: Folfox4 or Folfiri with or without EGFR inhibitors, Folfiri with Bevacizumab, Fluorouracil as monotherapy combined with folinic acid, capecitabine, or Irinotecan as monotherapy. EGFR-CTH therapy was based on CTH and anti-EGFR (cetuksymab or panitumumab) or VEGF-anti-angiogenic (bevacizumab) drugs according to National Comprehensive Cancer Network guidelines [25].
The standard dose was as follows:
- FOLFOX4—oxaliplatin 85 mg/m2 on day 1.
- leucovorin 100 mg/m2/day on days 1 and 2.
- 5-FU bolus 400 mg/m2/day followed by continuous infusion, repeated every fourth night with dose of 600 mg/m2/day on days 1 and 2, respectively.
- Folfiri—Irinotecan 180 mg/m2 on day 1, leucovorin 100 mg/m 2/day on days 1 and 2, and 5-FU bolus 400 mg/m2/day followed by continuous infusion 600 mg/m2/day on days 1 and 2, repeated every 2 weeks.
- Capecitabine 850–1250 mg/m2 orally twice daily for 14 days repeat every 3 weeks; monotherapy of irinotecan 180 mg/m2 was repeated every 2 weeks.
- EGFR-CTH therapy was based on CTH (Folfox4 or Folfiri) and anti-EGFR (cetuksymab or panitumumab).
- The anti-EGFR standard dose was: panitumumab 6 mg/kg given parenteraly on day 1 and repeated every 2 weeks.
- Cetuximab 500 mg/m2 perenterally on day 1, and every fourth nights.
- VEGF-CTH therapy was based on CTH (Folfox4 or Folfir) and anti-angiogenic drug bevacizumab—the standard dose of bevacizumab was 5–10 mg/kg on day 1, and repeated every 2 weeks.
- Fluorouracil as monotherapy combined with folinic acid.
The maximal doses of the applied protocols as initial and after nine months of therapy were not statistically different within the time and between the analyzed groups, as presented in Table 2.
3.2. Survival Group
Among survivors, therapy response was observed in 6 (21%) female and 20 (51%) male patients (p = 0.056). A significantly higher partial response based on CT scan results was observed in the male group (p = 0.008). The 27% risk for disease progression in the female group compared to 3% in the male counterparts was noticed, as presented in Table 3.

Table 3.
Nine months of chemotherapy results among female and male patients in CRC cancer.
3.3. Nine-Month Response Analysis
The group was subdivided according to CT scan imaging to identify possible clinical factors that may influence the 9-month palliative chemotherapy response. There were 53 patients with a median age of 69 (64–73) years who underwent further investigation, as presented in Table 4.

Table 4.
Response and non-response groups comparison.
3.4. Uni- and Multivariable Analysis for Systemic Therapy Response
Uni- and multivariable regression analysis for possible predicting factors on nine chemotherapy responses in the analyzed group was performed. Demographical factors such as age, sex, and BMI were followed by co-morbidities (arterial hypertension and diabetes mellitus) and a family-positive history of oncology disease. Surgical intervention prior to the systemic therapy and EGFR-CTH therapy were analyzed, followed by laboratory findings as neutrophil to lymphocyte ratio (NLR), red cell distribution width (RDW), and serum creatinine.
In univariable analysis, the RWD-CV was found as a single predictive factor (OR: 0.64, 95% CI: 0.46–0.90, p = 0.011). In multivariable analysis, the male sex (OR: 3.91, 95% CI: 1.09–14.05, p = 0.037) and RDW (OR: 0.61, 95% CI: 0.42–0.88, p = 0.008) were found significant for disease response to systemic therapy (Table 5).

Table 5.
Uni- and multivariable analysis for response to chemotherapy in CRC patients.
3.5. Receiver Operator Curve (ROC) for 9-Month Chemotherapy Response Predictors
ROC analysis for nine months of response to chemotherapy in palliative CRC patients presented in multivariable regression analysis revealed male sex and RDW, yielding a sensitivity of 71.1%, specificity of 57.8%, and an area under the curve (AUC) of 0.726 as presented in Figure 2. The cut-off value for RD-CV in our predictive model was 14%.
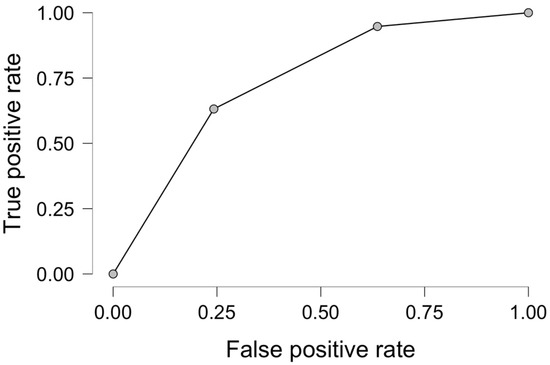
Figure 2.
Receiver operator curve analysis for male sex and RDW-CV as predictive factors for 9-month response to systemic therapy in palliative CRC.
4. Discussion
The results from our analysis point out possible predictive factors for systemic therapy response in palliative CRC. The male sex and RDW-CV were found to be significant. The study involved a relatively limited number of patients treated for nine months with chemotherapeutic protocols that were non-significantly modified throughout the analyzed period.
The recent publications [26] indicate a 5-year survival improvement in localized and regional CRC, suggesting the need for either therapy innovations or new prognostic factor definitions. A recent study by Hamers et al. suggested the impact of palliative CRC screening on their survival [26]. Xie et al. [27], in their analysis, revealed a novel BAP1-MAFF signaling axis that may be fundamental for CRC growth and could be regarded as either a prognostic marker or a potential therapeutic target. The possible prognostic role of liver metastatic involvement in disseminated CRC was proposed by Cohen et al. in the ARCAD pooled analysis [28].
The male sex is characterized by a higher incidence and inferior outcomes in CRC cancers [29]. Lifestyle, drinking habits, and gut microbiota can explain sexual dimorphism in CRC epidemiology [30].
Among other conditions, obesity is considered one of the known qualities allied to the male CRC population [31]. Sex differences may imply disease incidence, clinicopathological qualities, therapeutic tolerability, and outcomes [32]. Palliative chemotherapy was found in our analysis to be related to comparable mortality rates in both sexes. In previous reports, the higher risk for adverse effects of systemic therapy in females was postulated [33]. The obtained results were in coordination with previous reports [34]. The novelty of our analysis is based on male-sex-related favorable responses to applied therapy in four-stage CRC.
RDW is a simple and easily accessed parameter from peripheral whole blood count analysis that can be applied in clinical practice for CRC patients referred for systemic therapy. The clinical utility of RDW in breast tumor differentiative diagnosis was postulated by Lu et al. [35]. Nocini et al. [36], in their meta-analysis, presented the significance of RDW for survival prediction in laryngeal cancer patients. The relations between various pathological conditions and anisocytosis (reflected by RDW value) are widely postulated [37]. The RDW values may be modified by either malnutrition or acute infection [38]. In our analysis, none of the patients reported infection episodes either at the time of study enrollment or characterized by malnutrition. Cancerogenesis impairs erythropoiesis, resulting in RDW disturbances, by oxidative stress, peritumoral inflammatory activation, and poor nutritional status. Our analysis indicates an inverse relationship between chemotherapy response. The RDW-CV values can be explained by lesser degrees of hematological disturbances and abnormal red blood cell production. The heterogeneous red cell population results in impaired oxygen transportation, leading to peripheral tissue malfunction.
Chronic inflammatory system activation is considered one of the significant CRC drivers. Chronic inflammatory bowel diseases are considered potential risk factors of colitis-associated colorectal cancer (CA-CRC). It is believed that therapies targeting key inflammatory pathways and immune responses could lead to a reduction in CRC risk as part of personalized treatment in inflammatory bowel diseases [39]. In the report [40], the therapeutic response in CRC patients was suggested to be succeeded by cytotoxic lymphocytes and antigen-presenting macrophages modification by interferon. In Grellier et al. review [41], the involvement of gut microbia and the benefits of its potential modification on carcinogenic risk in inflammatory bowel disease was presented. Previous reports also postulated the link between inflammatory markers such as interleukin-8 and disease progression [42].
The accessibility to RDW is one of the strong points of our clinical practice analysis. The diagnostic utility of RDW in clinical practice regarding the tumor location was suggested in the Fancellu et al. study [43]. In the early stage of CRC, the propensity matching analysis performed by Cheng et al. [44] presented a negative association between RDW and patients’ survival. Next to higher levels of eosinophils and RDW, other parameters derived from whole blood count analysis, such as lower levels of hemoglobin, mean platelet volume (MPV), and mean corpuscular hemoglobin concentration (MCHC), were suggested as possible negative predictors in CRC patients [45].
The circulating microRNA as a possible predictor for systemic response in palliative CRC treatment was noticed in the Zhang et al. study [46]. Mhaidat et al. [47] pointed out the predictive value of protein 78 (GRP78) in colorectal cancer response to chemotherapy. The modulatory role of genetic variations on applied therapy in four-stage CRC was found in Zhang et al.’s analysis [48]. All those markers are sophisticated and highly accurate, but may be limited in everyday practice. Our analysis highlights the possible clinical utility of a simple and easily accessible marker, obtained from peripheral blood analysis, that can be applied in everyday oncological practice. The male patients with RDW-CV values below 14% can be regarded as a subgroup of CRC patients with a higher risk of unfavorable response to applied systemic therapy.
Study Limitation
The retrospective features of the single-center analysis point out significant limitations requiring confirmation in large-volume prospective studies. The analysis was performed on the elderly population and included patients on various systemic therapies.
5. Conclusions
Our analysis points out the possible favorable role of the male sex on nine-month systemic therapy in palliative CRC. The RDW can be regarded as a possible indicator of chemotherapy response in colorectal cancer. The mortality risk within 9 months of systemic therapy is comparable between male and female sex. The further analysis is required on large-scale population to confirm the presented results.
Author Contributions
Conceptualization, M.J. and T.U.; methodology, M.J.; software, M.J.; validation, M.J.; formal analysis, T.U., E.G. and M.R.; investigation, M.J.; resources, M.J.; data curation, M.J., E.G. and M.R.; writing—original draft preparation, M.J.; writing—review and editing, E.G., M.R. and T.U.; visualization, M.J.; supervision, T.U.; project administration, T.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee Poznan University of Medical Sciences, Poznan, Poland (protocol code 405/24 from 19 June 2024).
Informed Consent Statement
Patient consent was waived due to the retrospective character of the analysis.
Data Availability Statement
Data supporting the presented results will be available after a reasonable request for three years after publication by contacting the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AUC | Area Under the Curve |
| BMI | Body Mass Index |
| CRC | Colorectal cancer |
| CRP | C-reactive Protein |
| CI | Confidence interval |
| COPD | Chronic Obstructive Pulmonary Disease |
| CT | Computed tomography |
| EGFR-CTH | Epidermal growth factor receptor-cystathionine gamma-lyase |
| Hb | Hemoglobin |
| Hct | Hematocrit |
| MLR | Monocyte-to-lymphocyte ratio |
| NLR | Neutrophil-to-lymphocyte ratio |
| Plt | Platelets |
| Q | Quartile |
| RDW | Red cell distribution width |
| ROC | Receiver Operator Curve |
| VEGF | Vascular Endothelial Growth Factor |
| WBC | White blood cell count |
References
- Baidoun, F.; Elshiwy, K.; Elkeraie, Y.; Merjaneh, Z.; Khoudari, G.; Sarmini, M.T.; Gad, M.; Al-Husseini, M.; Saad, A. Colorectal cancer epidemiology: Recent trends and impact on outcomes. Curr. Drug Targets 2021, 22, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Fedewa, S.; Siegel, R. Early colorectal cancer detection-Current and evolving challenges in evidence, guidelines, policy, and practices. Adv. Cancer Res. 2021, 151, 69–107. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; Spinelli, A.; Ciardiello, D.; Realis Luc, M.; de Pascale, S.; Bertani, E.; Fazio, N.; Fumagalli Romario, U. Prognosis of early-onset versus late-onset sporadic colorectal cancer: Systematic review and meta-analysis. Eur. J. Cancer 2025, 215, 115172. [Google Scholar] [CrossRef] [PubMed]
- Sninsky, J.A.; Shore, B.M.; Lupu, G.V.; Crockett, S.D. Risk factors for colorectal polyps and cancer. Gastrointest. Endosc. Clin. N. Am. 2022, 32, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Prz. Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Santucci, C.; Mignozzi, S.; Malvezzi, M.; Boffetta, P.; Collatuzzo, G.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2024 with focus on colorectal cancer. Ann. Oncol. 2024, 35, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Santiago, Y.; Garay-Canales, C.A.; Nava-Castro, K.E.; Morales-Montor, J. Sexual dimorphism in colorectal cancer: Molecular mechanisms and treatment strategies. Biol. Sex Differ. 2024, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.M.; Bahl, A.; Sharma, G.; Pramanik, R.; Wadhwa, J.; Bajpai, P.; Jandyal, S.; Dubey, A.P.; Sarin, A.; Dadhich, S.C.; et al. Management of metastatic colorectal cancer (mCRC): Real-world recommendations. South Asian J. Cancer 2024, 13, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Iaciu, C.I.; Emilescu, R.A.; Cotan, H.T.; Nitipir, C. Systemic neutrophil-to-lymphocyte ratio as a prognostic biomarker for colon cancer. Chirurgia 2023, 118, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.J.; Burge, M.; Feeney, K.; Gibbs, P.; Jones, K.; Marx, G.; Molloy, M.P.; Price, T.; Reece, W.H.H.; Segelov, E.; et al. The prognostic role of inflammatory markers in patients with metastatic colorectal cancer treated with bevacizumab: A translational study [ASCENT]. PLoS ONE 2020, 15, e0229900. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Nozawa, H.; Sasaki, K.; Hongo, K.; Hiyoshi, M.; Tada, N.; Murono, K.; Nirei, T.; Kawai, K.; Sunami, E.; et al. Elevated neutrophil to lymphocyte ratio predicts poor prognosis in advanced colorectal cancer patients receiving oxaliplatin-based chemotherapy. Oncology 2012, 82, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Karin, M.; Sun, B. Targeting cancer-promoting inflammation-have anti-inflammatory therapies come of age? Nat. Rev. Clin. Oncol. 2021, 18, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Li, T.; Niu, M.; Mei, Q.; Zhao, B.; Chu, Q.; Dai, Z.; Wu, K. Exploiting innate immunity for cancer immunotherapy. Mol. Cancer 2023, 22, 187. [Google Scholar] [CrossRef] [PubMed]
- Pavese, I.; Satta, F.; Todi, F.; Di Palma, M.; Piergrossi, P.; Migliore, A.; Piselli, P.; Borghesi, R.; Mancino, G.; Brunetti, E.; et al. High serum levels of TNF-α and IL-6 predict the clinical outcome of treatment with human recombinant erythropoietin in anaemic cancer patients. Ann. Oncol. 2010, 21, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Kawada, K.; Obama, K. Inflammation-related biomarkers for the prediction of prognosis in colorectal cancer patients. Int. J. Mol. Sci. 2021, 22, 8002. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xin, S.; Xu, B. Value research of NLR, PLR, and RDW in prognostic assessment of patients with colorectal cancer. J. Healthc. Eng. 2022, 2022, 7971415. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Huang, X.; Xue, M.; Zhong, Z.; Wang, R.; Zhang, W.; Wang, L.; Qiao, Y.; Ling, F.; Zhang, Q.; et al. Prognostic significance of increased preoperative red cell distribution width (RDW) and changes in RDW for colorectal cancer. Cancer Med. 2023, 12, 13361–13373. [Google Scholar] [CrossRef] [PubMed]
- Sobieraj, M.; Urbanowicz, T.; Olasińska-Wiśniewska, A.; Gładki, M.; Michalak, M.; Filipiak, K.J.; Węclewska, A.; Bartkowska-Śniatkowska, A.; Tykarski, A.; Bobkowski, W.; et al. Anisocytosis as a possible predictor of low cardiac output syndrome in children undergoing mitral valve surgery. Adv. Med. Sci. 2024, 69, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Y.Y.; Qin, Y.Y.; Lin, F.Q. The combined detection of hematological indicators is used for the differential diagnosis of colorectal cancer and benign-colorectal lesions. Cancer Biomark. 2024, 39, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Koržinek, M.; Ćelap, I.; Fabijanec, M.; Žanić, T.; Ljubičić, N.; Baršić, N.; Verbanac, D.; Barišić, K.; Rajković, M.G. Complete blood count parameters and inflammation-related biomarkers in patients with colorectal carcinoma. Acta Pharm. 2025, 74, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Li, Z.W.; Liu, F.; Liu, X.R.; Wang, C.Y. Predictive value of red blood cell distribution width and hematocrit for short-term outcomes and prognosis in colorectal cancer patients undergoing radical surgery. World J. Gastroenterol. 2024, 30, 1714–1726. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Shen, X.; Hua, Q.; Yang, L.; Zhou, R.; Zhou, C.; Xu, P. Red cell distribution width-a potential prognostic indicator for colorectal cancer patients after radical resection in China. J. Gastrointest. Oncol. 2023, 14, 1746–1758. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.L.; Zhou, X.; Xiao, D.C. Is red blood cell distribution width a prognostic factor for colorectal cancer? A meta-analysis. Front. Surg. 2022, 9, 945126. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 24 November 1997).
- Hamers, P.A.H.; Vink, G.R.; Elferink, M.A.G.; Moons, L.M.G.; Punt, C.J.A.; May, A.M.; Koopman, M. Impact of colorectal cancer screening on survival after metachronous metastasis. Eur. J. Cancer 2024, 196, 113429. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Lin, H.; Huang, Y.; Wang, X.; Lin, H.; Xu, M.; Wu, J.; Wu, Y.; Shen, H.; Zhang, Q.; et al. BAP1-mediated MAFF deubiquitylation regulates tumor growth and is associated with adverse outcomes in colorectal cancer. Eur. J. Cancer 2024, 210, 114278. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Raeisi, M.; Chibaudel, B.; Shi, Q.; Yoshino, T.; Zalcberg, J.R.; Adams, R.; Cremolini, C.; Van Cutsem, E.; Heinemann, V.; et al. Prognostic value of liver metastases in colorectal cancer treated by systemic therapy: An ARCAD pooled analysis. Eur. J. Cancer 2024, 207, 114160. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Mullins, C.S.; Schafmayer, C.; Zeißig, S.; Linnebacher, M. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun. 2021, 41, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tu, Y.X.; Chen, L.; Zhang, Y.; Pan, X.L.; Yang, S.Q.; Zhang, S.J.; Li, S.H.; Yu, K.C.; Song, S.; et al. Male-biased gut microbiome and metabolites aggravate colorectal cancer development. Adv. Sci. 2023, 10, e2206238. [Google Scholar] [CrossRef] [PubMed]
- Wele, P.; Wu, X.; Shi, H. Sex-dependent differences in colorectal cancer: With a focus on obesity. Cells 2022, 11, 3688. [Google Scholar] [CrossRef] [PubMed]
- Baraibar, I.; Ros, J.; Saoudi, N.; Salvà, F.; García, A.; Castells, M.R.; Tabernero, J.; Élez, E. Sex and gender perspectives in colorectal cancer. ESMO Open 2023, 8, 101204. [Google Scholar] [CrossRef] [PubMed]
- Hirano, H.; Kataoka, K.; Yamaguchi, T.; Wagner, A.D.; Shimada, Y.; Inomata, M.; Hamaguchi, T.; Takii, Y.; Mizusawa, J.; Sano, Y.; et al. Sex differences in toxicities and survival outcomes among Japanese patients with Stage III colorectal cancer receiving adjuvant fluoropyrimidine monotherapy: A pooled analysis of 4 randomized controlled trials (JCOG2310A). Eur. J. Cancer 2025, 214, 115139. [Google Scholar] [CrossRef] [PubMed]
- Afify, A.Y.; Ashry, M.H.; Hassan, H. Sex differences in survival outcomes of early-onset colorectal cancer. Sci. Rep. 2024, 14, 22041. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Pan, S.; Qi, Y.; Li, X.; Wang, J. The clinical application value of RDW, CA153, and MPV in breast cancer. Clin. Lab. 2021, 67, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Nocini, R.; Sanchis-Gomar, F.; Lippi, G.; Mattiuzzi, C. Red blood cell distribution width (RDW) is a significant predictor of survival in laryngeal cancer patients: Systematic literature review and meta-analysis. J. Med. Biochem. 2023, 42, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Ananthaseshan, S.; Bojakowski, K.; Sacharczuk, M.; Poznanski, P.; Skiba, D.S.; Prahl Wittberg, L.; McKenzie, J.; Szkulmowska, A.; Berg, N.; Andziak, P.; et al. Red blood cell distribution width is associated with increased interactions of blood cells with vascular wall. Sci. Rep. 2022, 12, 13676. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Martín, C.A.; Chong-Aviña, P.A.; Godinez-Breacher, V.; Aportela-Vázquez, V.A.; Bueno-Hernández, G.; Gante-García, M.F.; Pimentel-Luna, K.Y.; Sánchez-Abrego, M. Malnutrition: Muscle wasting, inflammation, RDW, and their relation with adverse outcomes. Cir. Cir. 2024, 92, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Shahgoli, V.K.; Noorolyai, S.; Ahmadpour Youshanlui, M.; Saeidi, H.; Nasiri, H.; Mansoori, B.; Holmskov, U.; Baradaran, B. Inflammatory bowel disease, colitis, and cancer: Unmasking the chronic inflammation link. Int. J. Color. Dis. 2024, 39, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Acha-Sagredo, A.; Andrei, P.; Clayton, K.; Taggart, E.; Antoniotti, C.; Woodman, C.A.; Afrache, H.; Fourny, C.; Armero, M.; Moinudeen, H.K.; et al. A constitutive interferon-high immunophenotype defines response to immunotherapy in colorectal cancer. Cancer Cell 2025, 43, 292–307.e7. [Google Scholar] [CrossRef] [PubMed]
- Grellier, N.; Severino, A.; Archilei, S.; Kim, J.; Gasbarrini, A.; Cammarota, G.; Porcari, S.; Benech, N. Gut microbiota in inflammation and colorectal cancer: A potential Toolbox for Clinicians. Best Pract. Res. Clin. Gastroenterol. 2024, 72, 101942–101951. [Google Scholar] [CrossRef] [PubMed]
- Burz, C.; Bojan, A.; Balacescu, L.; Pop, V.V.; Silaghi, C.; Lupan, I.; Aldea, C.; Sur, D.; Samasca, G.; Cainap, C.; et al. Interleukin 8 as predictive factor for response to chemotherapy in colorectal cancer patients. Acta Clin. Belg. 2021, 76, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Fancellu, A.; Zinellu, A.; Mangoni, A.A.; Popova, A.; Galotti, F.; Feo, C.F.; Attene, F.; Cossu, A.; Palmieri, G.; Paliogiannis, P. Red Blood Cell Distribution Width (RDW) Correlates to the Anatomical Location of Colorectal Cancer. Implications for Clinical Use. J. Gastrointest. Cancer 2022, 53, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.C.; Lin, Y.M.; Liu, C.C.; Wu, K.L.; Lee, K.C. High Red Cell Distribution Width Is Associated with Worse Prognosis in Early Colorectal Cancer after Curative Resection: A Propensity-Matched Analysis. Cancers 2022, 14, 945. [Google Scholar] [CrossRef] [PubMed]
- Alsalman, A.; Al-Mterin, M.A.; Abu-Dayeh, A.; Alloush, F.; Murshed, K.; Elkord, E. Associations of Complete Blood Count Parameters with Disease-Free Survival in Right- and Left-Sided Colorectal Cancer Patients. J. Pers. Med. 2022, 12, 816–829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, K.; Bi, M.; Jiao, X.; Zhang, D.; Dong, Q. Circulating microRNA expressions in colorectal cancer as predictors of response to chemotherapy. Anticancer Drugs 2014, 25, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Mhaidat, N.M.; Alzoubi, K.H.; Almomani, N.; Khabour, O.F. Expression of glucose regulated protein 78 (GRP78) determines colorectal cancer response to chemotherapy. Cancer Biomark. 2015, 15, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, P.; Zhou, X.C.; Bao, G.Q.; Lyu, Z.M.; Liu, X.N.; Wan, S.G.; He, X.L.; Huang, Q.C. Genetic variations in the HIF1A gene modulate response to adjuvant chemotherapy after surgery in patients with colorectal cancer. Asian Pac. J. Cancer Prev. 2014, 15, 4637–4642. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).