Efficacy of Sialendoscopy with Steroid Irrigation for Non-Lithiasic Chronic Sialadenitis: A Systematic Review and Proportional Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Literature Search
2.3. Data Extraction
2.4. Outcome Assessment
2.5. Statistical Analysis
2.6. Assessment of Quality
2.7. Subgroup Analysis
2.8. Sensitivity Analysis
2.9. Analysis of Publication Bias
3. Results
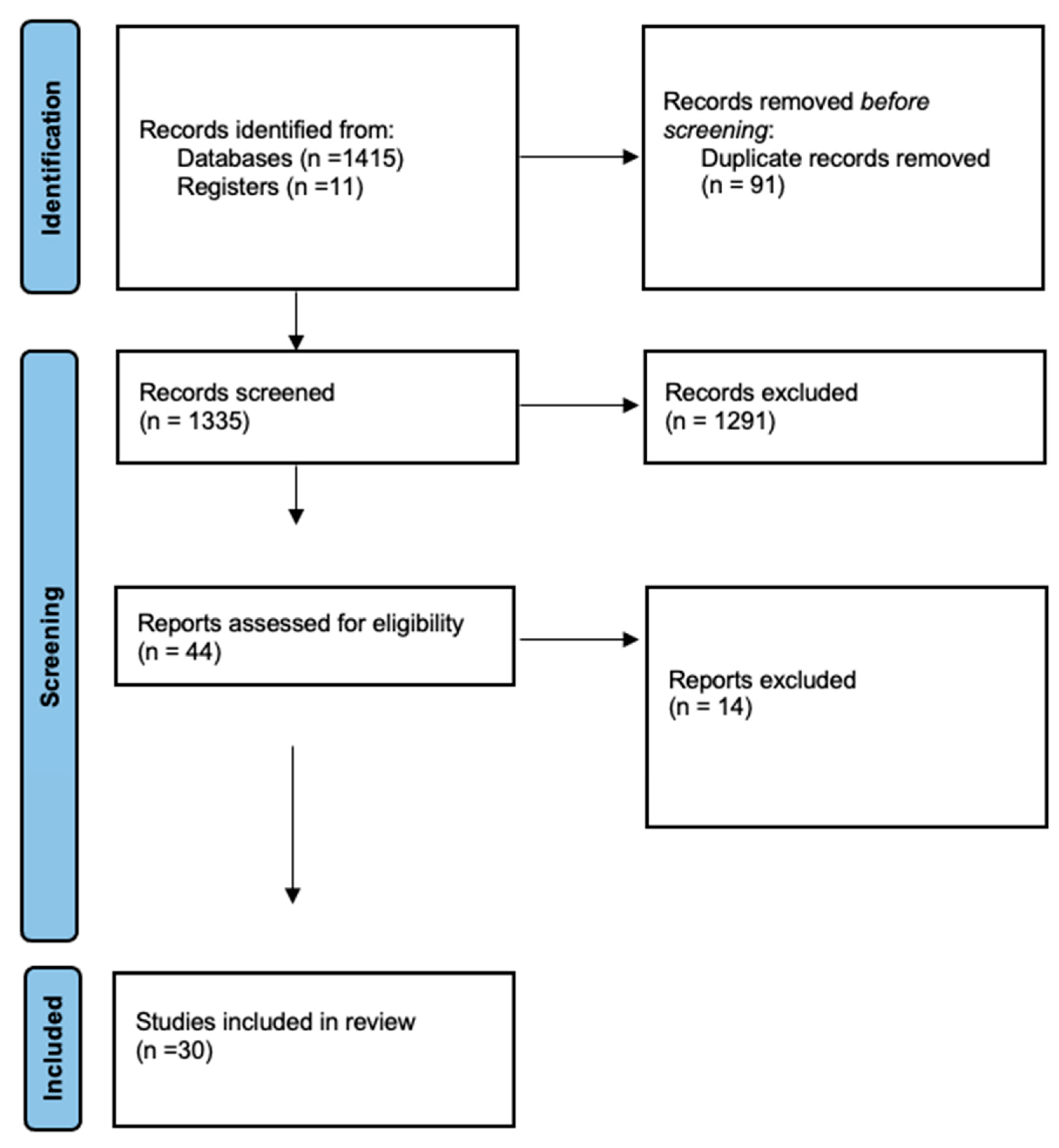
3.1. Study Selection
3.2. Study Characteristics
3.3. Quality Assessment
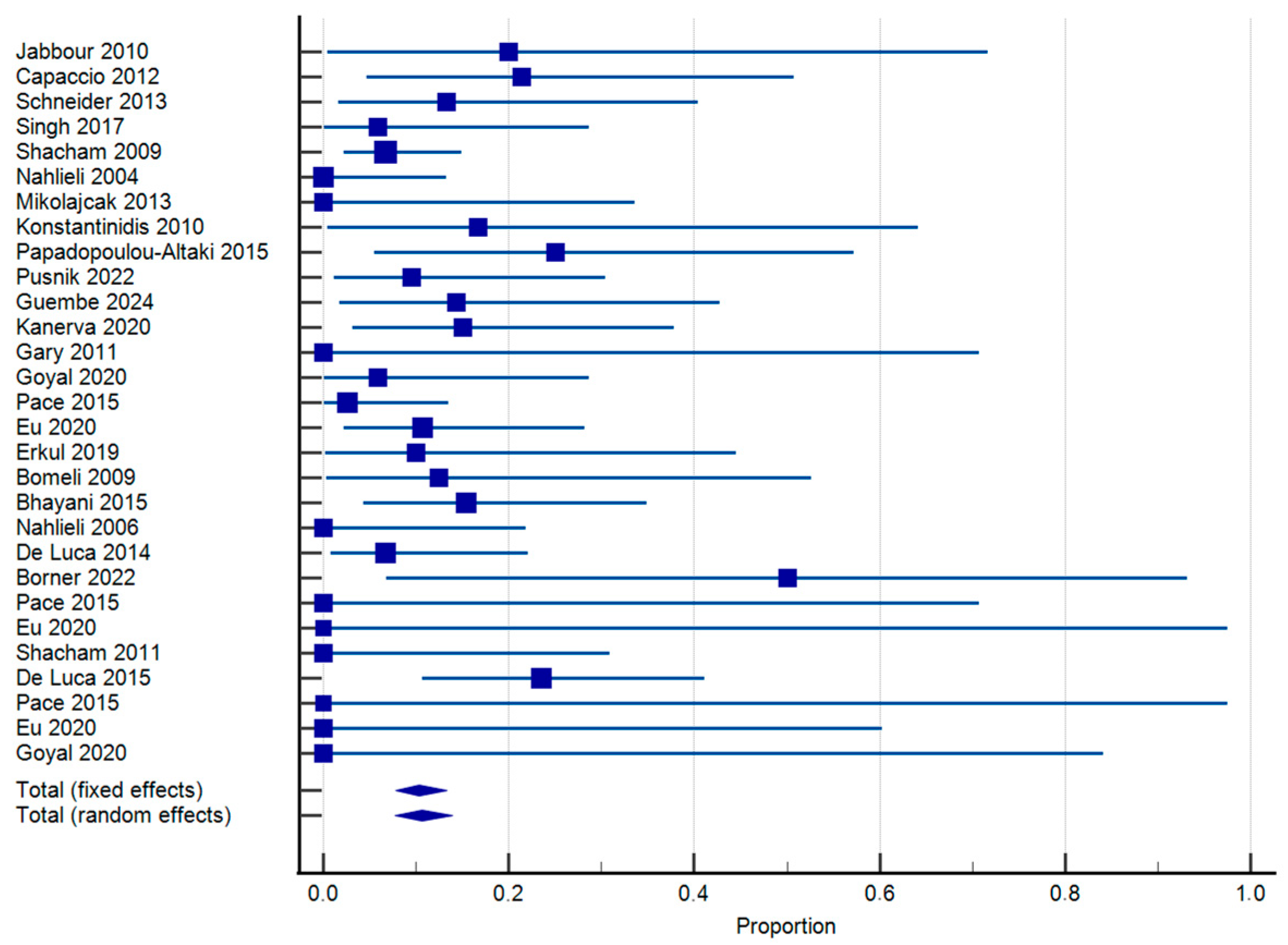
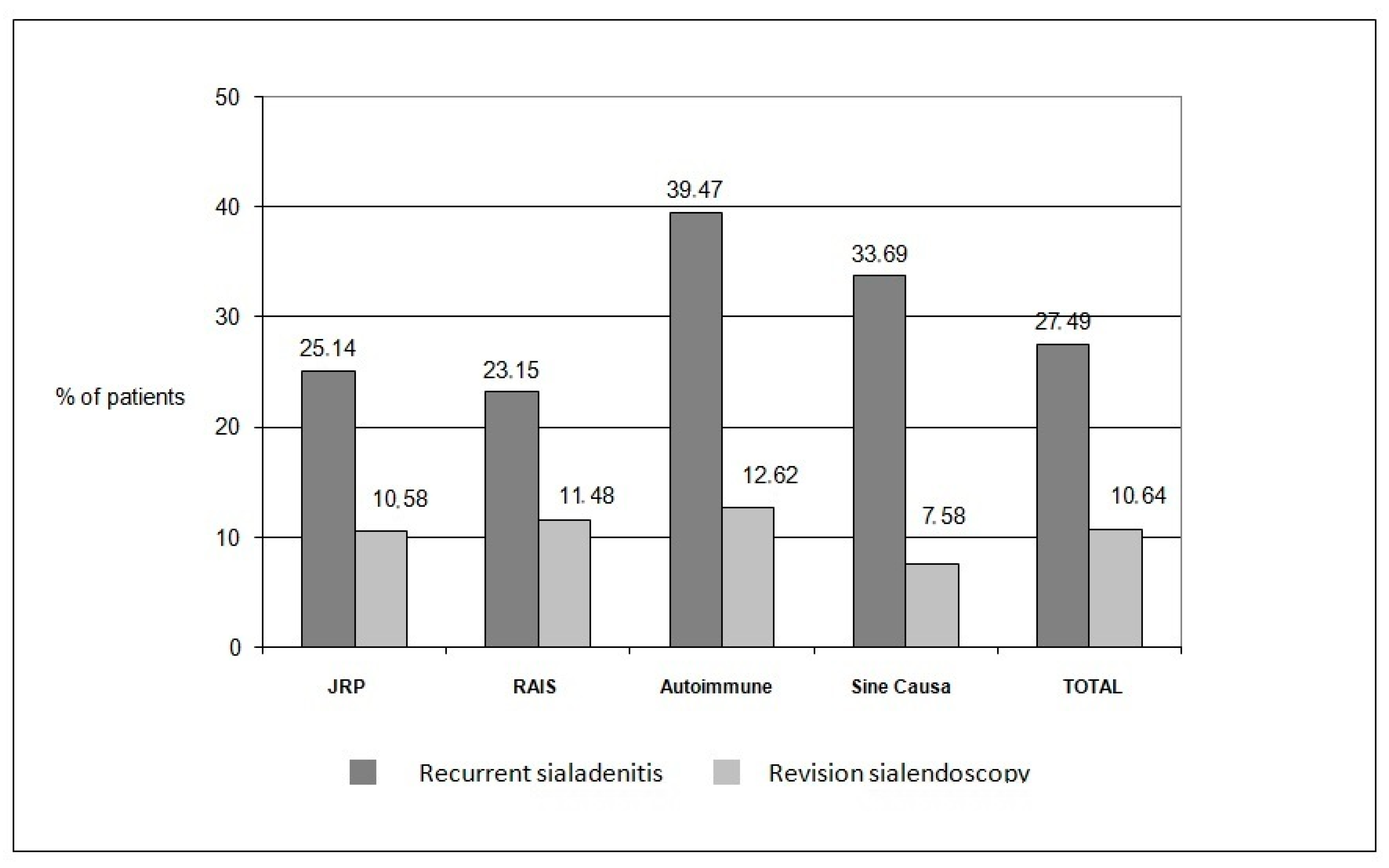
3.4. Synthesis of Results
- Recurrence of episodes of sialadenitis
- Revision sialendoscopy
- Major complications
- Sensitivity analysis
3.5. Assessment of Publication Bias
4. Discussion
- Strengths and Limitations
- Implications for future research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Registration and Protocol
References
- Harrison, J.D. Causes, Natural History, and Incidence of Salivary Stones and Obstructions. Otolaryngol. Clin. N. Am. 2009, 42, 927–947. [Google Scholar] [CrossRef] [PubMed]
- Bhatty, M.A.; Piggot, T.A.; Soames, J.V.; McLean, N.R. Chronic non-specific parotid sialadenitis. Br. J. Plast. Surg. 1998, 51, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Nahlieli, O.; Shacham, R.; Yoffe, B.; Eliav, E. Diagnosis and treatment of strictures and kinks in salivary gland ducts. J. Oral Maxillofac. Surg. 2001, 59, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Marchal, F.; Dulguerov, P.; Becker, M.; Barki, G.; Disant, F.; Lehmann, W. Specificity of parotid sialendoscopy. Laryngoscope 2001, 111, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Zenk, J.; Bozzato, A.; Bumm, K.; Iro, H. Sialoscopy in cases of unclear swelling of the major salivary glands. Otolaryngol.-Head Neck Surg. 2005, 133, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Vasaitis, L. IgG4-related disease: A relatively new concept for clinicians. Eur. J. Intern. Med. 2016, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Martellucci, S.; Fusconi, M.; Pagliuca, G.; Greco, A.; De Virgilio, A.; De Vincentiis, M. Trattamento scialendoscopico delle scialoadeniti autoimmuni: Revisione della letteratura. Acta Otorhinolaryngol. Ital. 2017, 37, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Shacham, R.; Puterman, M.B.; Ohana, N.; Nahlieli, O. Endoscopic treatment of salivary glands affected by autoimmune diseases. J. Oral Maxillofac. Surg. 2011, 69, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Mandel, S.J.; Mandel, L. Persistent sialadenitis after radioactive iodine therapy: Report of two cases. J. Oral Maxillofac. Surg. 1999, 57, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Leerdam, C.M.; Martin, H.C.O.; Isaacs, D. Recurrent parotitis of childhood. J. Paediatr. Child Health 2005, 41, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Nahlieli, O.; Shacham, R.; Shlesinger, M.; Eliav, E. Juvenile recurrent parotitis: A new method of diagnosis and treatment. Pediatrics 2004, 114, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.; Toll, E.C.; Hall, F.; Mahadevan, M. Juvenile recurrent parotitis: Review and proposed management algorithm. Int. J. Pediatr. Otorhinolaryngol. 2021, 142, 110617. [Google Scholar] [CrossRef] [PubMed]
- Capaccio, P.; Torretta, S.; Di Pasquale, D.; Rossi, V.; Pignataro, L. The role of interventional sialendoscopy and intraductal steroid therapy in patients with recurrent sine causa sialadenitis: A prospective cross-sectional study. Clin. Otolaryngol. 2017, 42, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, E.R.; Lykke, E.; Wagner, N.; Nielsen, T.; Waersted, S.; Arndal, H. The introduction of sialendoscopy has significantly contributed to a decreased number of excised salivary glands in Denmark. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.G.; Hwang, K.G.; Papadaki, M.E.; Troulis, M.J. Sialadenitis without sialolithiasis treated by sialendoscopy. J. Oral Maxillofac. Surg. 2015, 73, 1748–1752. [Google Scholar] [CrossRef] [PubMed]
- Katz, P. Endoscopy of the salivary glands. Ann. Radiol. 1991, 34, 110–113. [Google Scholar] [PubMed]
- Nahlieli, O.; Neder, A.; Baruchin, A.M. Salivary gland endoscopy: A new technique for diagnosis and treatment of sialolithiasis. J. Oral Maxillofac. Surg. 1994, 52, 1240–1242. [Google Scholar] [CrossRef] [PubMed]
- Nahlieli, O.; Baruchin, A.M. Long-term experience with endoscopic diagnosis and treatment of salivary gland inflammatory diseases. Laryngoscope 2000, 110, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Nahlieli, O.; Baruchin, A.M. Sialoendoscopy: Three years’ experience as a diagnostic and treatment modality. J. Oral Maxillofac. Surg. 1997, 55, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Vashishta, R.; Gillespie, M.B. Salivary endoscopy for idiopathic chronic sialadenitis. Laryngoscope 2013, 123, 3016–3020. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zheng, L.; Yang, C.; Shen, N. Causes of chronic obstructive parotitis and management by sialoendoscopy. Oral Surgery Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2008, 105, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Ryan, W.R.; Plonowska, K.A.; Gurman, Z.R.; Aubin-Pouliot, A.; Chang, J.L. One-Year symptom outcomes after sialolithiasis treatment with sialendoscopy-assisted salivary duct surgery. Laryngoscope 2019, 129, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Iro, H.; Klintworth, N.; Psychogios, G.; Zenk, J. Results of minimally invasive gland-preserving treatment in different types of parotid duct stenosis. Arch. Otolaryngol.-Head Neck Surg. 2012, 138, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Erkul, E.; Gillespie, M.B. Sialendoscopy for non-stone disorders: The current evidence. Laryngoscope Investig. Otolaryngol. 2016, 1, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Izumi, M.; Eguchi, K.; Nakamura, H.; Takagi, Y.; Kawabe, Y.; Nakamura, T. Corticosteroid irrigation of parotid gland for treatment of xerostomia in patients with Sjogren’s syndrome. Ann. Rheum. Dis. 1998, 57, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Jokela, J.; Haapaniemi, A.; Mäkitie, A.; Saarinen, R. Sialendoscopy in treatment of adult chronic recurrent parotitis without sialolithiasis. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Marchal, F.; Becker, M.; Dulguerov, P.; Lehmann, W. Interventional sialendoscopy: A targeted problem and its solution. Laryngoscope 2000, 110, 318. [Google Scholar] [CrossRef] [PubMed]
- Baurmash, H.D. Chronic recurrent parotitis: A closer look at its origin, diagnosis, and management. J. Oral Maxillofac. Surg. 2004, 62, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Nahlieli, O. Complications of sialendoscopy: Personal experience, literature analysis, and suggestions. J. Oral Maxillofac. Surg. 2015, 73, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- MedCalc Statistical Software, version 23.0.6; MedCalc Software Ltd.: Ostend, Belgium. Available online: https://www.medcalc.org (accessed on 2 July 2025).
- Wallace, B.C.; Dahabreh, I.J.; Trikalinos, T.A.; Lau, J.; Trow, P.; Schmid, C.H. End-Users: R as a Computational Back-End. J. Stat. Softw. 2012, 49, 1–15. [Google Scholar] [CrossRef]
- Migliavaca, C.B.; Stein, C.; Colpani, V.; Munn, Z.; Falavigna, M. Quality assessment of prevalence studies: A systematic review. J. Clin. Epidemiol. 2020, 127, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Deeks, J.J.; Altman, D.G. Chapter 9: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Green, S., Eds.; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- Moga, C.; Guo, B.; Harstall, C. Development of a Quality Appraisal Tool for Case Series Studies Using a Modified Delphi Technique. IHE Publ [Internet]. March 2012; pp. 1–71. Available online: https://www.ihe.ca/files/development_of_a_quality_appraisal_tool_for_case_series_studies_poster.pdf (accessed on 1 November 2024).
- Lele, S.J.; Hamiter, M.; Fourrier, T.L.; Nathan, C.A. Sialendoscopy With Intraductal Steroid Irrigation in Patients With Sialadenitis Without Sialoliths. Ear Nose Throat J. 2019, 98, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Bhayani, M.K.; Acharya, V.; Kongkiatkamon, S.; Farah, S.; Roberts, D.B.; Sterba, J.; Chambers, M.S.; Lai, S.Y. Sialendoscopy for Patients with Radioiodine-Induced Sialadenitis and Xerostomia. Thyroid 2015, 25, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Douglas, J.E.; Wen, Z.; Thomas, W.W. Management of Chronic Sialadenitis due to Sjogren’s Syndrome and Radioactive Iodine Therapy Using Sialendoscopy. ORL 2023, 85, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Bomeli, S.R.; Schaitkin, B.; Carrau, R.L.; Walvekar, R.R. Interventional sialendoscopy for treatment of radioiodine-induced sialadenitis. Laryngoscope 2009, 119, 864–867. [Google Scholar] [CrossRef] [PubMed]
- Gary, C.; Kluka, E.A.; Schaitkin, B.; Walvekar, R.R. Interventional sialendoscopy for treatment of juvenile recurrent parotitis. J. Indian Assoc. Pediatr. Surg. 2011, 16, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, N.; Tibesar, R.; Lander, T.; Sidman, J. Sialendoscopy in children. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Capaccio, P.; Sigismund, P.E.; Luca, N.; Marchisio, P.; Pignataro, L. Modern management of juvenile recurrent parotitis. J. Laryngol. Otol. 2012, 126, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Berlucchi, M.; Rampinelli, V.; Ferrari, M.; Grazioli, P.; Redaelli De Zinis, L.O. Sialoendoscopy for treatment of juvenile recurrent parotitis: The Brescia experience. Int. J. Pediatr. Otorhinolaryngol. 2018, 105, 163–166. [Google Scholar] [CrossRef] [PubMed]
- De Luca, R.; Trodella, M.; Vicidomini, A.; Colella, G.; Tartaro, G. Endoscopic management of salivary gland obstructive diseases in patients with Sjögren’s syndrome. J. Cranio-Maxillofac. Surg. 2015, 43, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- De Luca, R.; Vicidomini, A.; Trodella, M.; Tartaro, G.; Colella, G. Sialoendoscopy: A viable treatment for I131 induced sialoadenitis. Br. J. Oral Maxillofac. Surg. 2014, 52, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczak, S.; Meyer, M.F.; Beutner, D.; Luers, J.C. Treatment of chronic recurrent juvenile parotitis using sialendoscopy. Acta Otolaryngol. 2014, 134, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.; Koch, M.; Künzel, J.; Gillespie, M.B.; Grundtner, P.; Iro, H.; Zenk, J. Juvenile recurrent parotitis: A retrospective comparison of sialendoscopy versus conservative therapy. Laryngoscope 2014, 124, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou-Alataki, E.; Chatziavramidis, A.; Vampertzi, O.; Alataki, S.; Konstantinidis, I. Evaluation and management of juvenile recurrent parotitis in children from Northern Greece. Hippokratia 2015, 19, 356–359. [Google Scholar] [PubMed]
- Konstantinidis, I.; Chatziavramidis, A.; Tsakiropoulou, E.; Malliari, H.; Constantinidis, J. International Journal of Pediatric Otorhinolaryngology Pediatric sialendoscopy under local anesthesia: Limitations and potentials. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M. Role of Sialendoscopy in Non-neoplastic Parotid Diseases: A Prospective Study of 241 Patients in Indian Population. J. Maxillofac. Oral Surg. 2020, 21, 715. [Google Scholar] [CrossRef] [PubMed]
- Faizal, B.; Abraham, S.M.; Krishnakumar, T. Study of Evaluation of Symptoms of Juvenile Recurrent Parotitis Prior to and After Sialendoscopy. Open Pain J. 2017, 10, 29–36. [Google Scholar] [CrossRef]
- Singh, P.P.; Goyal, M.; Goyal, A. Sialendoscopic Approach in Management of Juvenile Recurrent Parotitis. Indian J. Otolaryngol. Head Neck Surg. 2017, 69, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Borner, U.; Anschuetz, L.; Caversaccio, M.; von Werdt, M.; Panosetti, E.; Keghian, J.; Remacle, M. A Retrospective Analysis of Multiple Affected Salivary Gland Diseases: Diagnostic and Therapeutic Benefits of Interventional Sialendoscopy. Ear Nose Throat J. 2022, 103, NP662–NP670. [Google Scholar] [CrossRef] [PubMed]
- Kanerva, M.; Tapiovaara, L.; Aro, K.; Saarinen, R. Pediatric sialendoscopy: An 11-year study from a single tertiary care center. Int. J. Pediatr. Otorhinolaryngol. 2020, 131, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Guembe, M.A.; Miguel, C.; Estomba, C.; Gutiérrez, C.S.; Arrizabalaga, I.T.; Olano, M.Á.; Quintano, M.V. Utility of sialendoscopy in the management of juvenile recurrent parotitis. Retrosp. Study 2024, 75, 304–309. [Google Scholar]
- Martins-Carvalho, C.; Plouin-Gaudon, I.; Quenin, S.; Lesniak, J.; Froehlich, P.; Marchal, F.; Faure, F. Pediatric sialendoscopy: A 5-Year Experience at a Single Institution. Arch Otolaryngol. Head Neck Surg. 2010, 136, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Pušnik, L.; Jerman, A.; Urbančič, J.; Aničin, A. Sialendoscopy in Management of Juvenile Recurrent Parotitis—A Single Centre Experience. Children 2022, 9, 1632. [Google Scholar] [CrossRef] [PubMed]
- Shacham, R.; Droma, E.B.; London, D.; Bar, T.; Nahlieli, O. Long-Term Experience With Endoscopic Diagnosis and Treatment of Juvenile Recurrent Parotitis. J. Oral Maxillofac. Surg. 2009, 67, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Nahlieli, O.; Nazarian, Y. Sialadenitis following radioiodine therapy—A new diagnostic and treatment modality. Oral Dis. 2006, 12, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Erkul, E. Long-Term Outcomes of Sialendoscopy in the Management of Sialolithiasis and Idiopathic Chronic Sialadenitis with Ductal Scars. Turk. Arch. Otorhinolaryngol. 2019, 57, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Eu, D.; Orl, M. Efficacy of Sialendoscopy in the Management of Noncalculi-Related Sialadenitis. J. Oral Maxillofac. Surg. 2020, 78, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.; Stuermer, K.J.; Luers, J.C. The positive effect of sialendoscopy with irrigation lavage for sialadenitis without sialolithiasis or stenosis. ORL 2018, 80, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Plonowska, K.A.; Ochoa, E.; Ryan, W.R.; Chang, J.L. Sialendoscopy in Chronic Obstructive Sialadenitis Without Sialolithiasis: A Prospective Cohort Study. Otolaryngol.-Head Neck Surg. 2021, 164, 595–601. [Google Scholar] [CrossRef] [PubMed]
- de Paiva Leite, S.H.; Morton, R.P.; Ahmad, Z.; Marchal, F. Do Postoperative Oral Corticosteroids Improve Results After Sialendoscopy for Ductal Stenosis? Laryngoscope 2021, 131, E1503–E1509. [Google Scholar] [CrossRef] [PubMed]
- Hackett, A.M.; Baranano, C.F.; Reed, M.; Duvvuri, U.; Smith, R.J.; Mehta, D. Sialoendoscopy for the treatment of pediatric salivary gland disorders. Arch. Otolaryngol.-Head Neck Surg. 2012, 138, 912–915. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ardekian, L.; Klein, H.; Al Abri, R.; Marchal, F. Sialendoscopy for the diagnosis and treatment of juvenile recurrent parotitis. Rev. Stomatol. Chir. Maxillofac. Chir. Orale 2014, 115, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Quenin, S.; Plouin-Gaudon, I.; Marchal, F.; Froehlich, P.; Disant, F.; Faure, F. Juvenile recurrent parotitis: Sialendoscopic Approach. Arch Otolaryngol. Head Neck Surg. 2008, 134, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Roby, B.B.; Mattingly, J.; Jensen, E.L.; Gao, D.; Chan, K.H. Treatment of juvenile recurrent parotitis of childhood: An analysis of effectiveness. JAMA Otolaryngol.-Head Neck Surg. 2015, 141, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Prendes, B.L.; Orloff, L.A.; Eisele, D.W. Therapeutic sialendoscopy for the management of radioiodine sialadenitis. Arch. Otolaryngol.-Head Neck Surg. 2012, 138, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Han, G.S.; Lee, S.H.; Lee, D.Y.; Kim, Y.M. Sialoendoscopic treatment for radioiodine induced sialadenitis. Laryngoscope 2007, 117, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Bulut, O.C.; Haufe, S.; Hohenberger, R.; Hein, M.; Kratochwil, C.; Rathke, H.; Plinkert, P.K.; Baumann, I.; Holland-Letz, T.; Haberkorn, U.; et al. Impact of sialendoscopy on improving health related quality of life in patients suffering from radioiodineinduced xerostomia. Nukl. Med. 2018, 57, 160–167. [Google Scholar] [CrossRef]
- Delagnes, E.A.; Aubin-Pouliot, A.; Zheng, M.; Chang, J.L.; Ryan, W.R. Sialadenitis without sialolithiasis: Prospective outcomes after sialendoscopy-assisted salivary duct surgery. Laryngoscope 2017, 127, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Brookes, S.M.; Cohen, S.B.A.; Price, E.J.; Webb, L.M.C.; Feldmann, M.; Maini, R.N.; Venables, P.J.W. T cell clones from a Sjögren’s syndrome salivary gland biopsy produce high levels of IL-10. Clin. Exp. Immunol. 1996, 103, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Capaccio, P.; Canzi, P.; Torretta, S.; Rossi, V.; Benazzo, M.; Bossi, A.; Vitali, C.; Cavagna, L.; Pignataro, L. Combined interventional sialendoscopy and intraductal steroid therapy for recurrent sialadenitis in Sjögren’s syndrome. Results of a pilot monocentric trial. Clin. Otolaryngol. 2018, 43, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Karagozoglu, K.H.; Vissink, A.; Forouzanfar, T.; De Visscher, J.G.A.M.; Maarse, F.; Brand, H.S.; Van De Ven, P.M.; Jager, D.H.J. Sialendoscopy increases saliva secretion and reduces xerostomia up to 60 weeks in Sjögren’s syndrome patients: A randomized controlled study. Rheumatology 2021, 60, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Mahalakshmi, S.; Kandula, S.; Shilpa, P.; Kokila, G. Chronic Recurrent Non-specific Parotitis: A Case Report and Review. Ethiop. J. Health Sci. 2017, 27, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Aničin, A.; Jerman, A.; Urbančič, J.; Pušnik, L. Sialendoscopy-Based Analysis of Submandibular Duct Papillae with a Proposal for Classification. J. Clin. Med. 2023, 12, 1129. [Google Scholar] [CrossRef] [PubMed]
- Luers, J.C.; Vent, J.; Beutner, D. Methylene blue for easy and safe detection of salivary duct papilla in sialendoscopy. Otolaryngol.-Head Neck Surg. 2008, 139, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Faure, F.; Querin, S.; Dulguerov, P.; Froehlich, P.; Disant, F.; Marchal, F. Pediatric salivary gland obstructive swelling: Sialendoscopic approach. Laryngoscope 2007, 117, 1364–1367. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsikopoulos, A.; Garefis, K.; Sidiropoulos, K.; Triaridis, S.; Nikolaidis, V.; Konstantinidis, I. Efficacy of Sialendoscopy with Steroid Irrigation for Non-Lithiasic Chronic Sialadenitis: A Systematic Review and Proportional Meta-Analysis. J. Clin. Med. 2025, 14, 5202. https://doi.org/10.3390/jcm14155202
Tsikopoulos A, Garefis K, Sidiropoulos K, Triaridis S, Nikolaidis V, Konstantinidis I. Efficacy of Sialendoscopy with Steroid Irrigation for Non-Lithiasic Chronic Sialadenitis: A Systematic Review and Proportional Meta-Analysis. Journal of Clinical Medicine. 2025; 14(15):5202. https://doi.org/10.3390/jcm14155202
Chicago/Turabian StyleTsikopoulos, Alexios, Konstantinos Garefis, Konstantinos Sidiropoulos, Stefanos Triaridis, Vasileios Nikolaidis, and Iordanis Konstantinidis. 2025. "Efficacy of Sialendoscopy with Steroid Irrigation for Non-Lithiasic Chronic Sialadenitis: A Systematic Review and Proportional Meta-Analysis" Journal of Clinical Medicine 14, no. 15: 5202. https://doi.org/10.3390/jcm14155202
APA StyleTsikopoulos, A., Garefis, K., Sidiropoulos, K., Triaridis, S., Nikolaidis, V., & Konstantinidis, I. (2025). Efficacy of Sialendoscopy with Steroid Irrigation for Non-Lithiasic Chronic Sialadenitis: A Systematic Review and Proportional Meta-Analysis. Journal of Clinical Medicine, 14(15), 5202. https://doi.org/10.3390/jcm14155202







