Cognitive Reserve and Its Associations with Pain, Anxiety, and Depression in Patients with Chronic Migraine: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Study Setting and Population
2.3. Clinical Assessment and Data Collection
2.4. Assessment Tools and Measurements
2.5. Sample Size Calculation and Power Analysis
2.6. Statistical Analysis
2.7. Use of GenAI in Writing
3. Results
3.1. Baseline Demographic and Clinical Data
3.2. Associations Among Clinical Parameters
3.3. Associations Between Clinical Variables and Cognitive Reserve
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAI | Beck Anxiety Inventory |
| BDI | Beck Depression Inventory |
| ICHD-3 | International Classification of Headache Disorders, Third Edition |
| MHD | monthly headache days |
| MIDAS | migraine disability assessment |
| MMSE | mini-mental state examination |
| NRS | numeric rating scale |
| PSQI | Pittsburgh Sleep Quality Index. |
References
- Burch, R.C.; Buse, D.C.; Lipton, R.B. Migraine: Epidemiology, Burden, and Comorbidity. Neurol. Clin. 2019, 37, 631–649. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd ed.; Cephalalgia; Sage: Thousand Oaks, CA, USA, 2018; Volume 38, pp. 1–211. [Google Scholar]
- Wang, Y.F.; Wang, S.-J.; Huang, Y.H.; Chen, Y.-T.; Yen, Y.-C.; Shia, B.-C.; Tsai, C.-W.; Chan, H.-F.; Panni, T.; Dell, G. Treatment pattern and health care resource utilization for Taiwanese patients with migraine: A population-based study. Front. Neurol. 2023, 14, 1222912. [Google Scholar] [CrossRef]
- Raggi, A.; Leonardi, M.; Arruda, M.; Caponnetto, V.; Castaldo, M.; Coppola, G.; Pietra, A.D.; Fan, X.; Garcia-Azorin, D.; Gazerani, P.; et al. Hallmarks of primary headache: Part 1—Migraine. J. Headache Pain 2024, 25, 189. [Google Scholar] [CrossRef]
- Cai, X.; Xu, X.; Zhang, A.; Lin, J.; Wang, X.; He, W.; Fang, Y. Cognitive Decline in Chronic Migraine with Nonsteroid Anti-inflammation Drug Overuse: A Cross-Sectional Study. Pain Res. Manag. 2019, 2019, 7307198. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, K.S.; Teixeira, C.T.; Cáfaro, C.; Oliver, G.Z.; Carvalho, G.L.P.; Carvalho, L.A.S.D.; Silva, B.G.; Haes, F.B.B.; Ciciarelli, M.C. Chronic migraine patients show cognitive impairment in an extended neuropsychological assessment. Arq. Neuropsiquiatr. 2018, 76, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Latysheva, N.; Filatova, E.; Osipova, D.; Danilov, A.B. Cognitive impairment in chronic migraine: A cross-sectional study in a clinic-based sample. Arq. Neuropsiquiatr. 2020, 78, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Dapkute, A.; Watson, E.; Kazaishvili, I.; Chądzyński, P.; Varanda, S.; Di Antonio, S.; Munday, V.; Maassen Van Den Brink, A.; Lampl, C.; et al. Migraine and cognitive dysfunction: A narrative review. J. Headache Pain 2024, 25, 221. [Google Scholar] [CrossRef]
- Biagianti, B.; Grazzi, L.; Gambini, O.; Usai, S.; Muffatti, R.; Scarone, S.; Bussone, G. Orbitofrontal dysfunction and medication overuse in patients with migraine. Headache 2012, 52, 1511–1519. [Google Scholar] [CrossRef]
- Biagianti, B.; Grazzi, L.; Gambini, O.; Usai, S.; Muffatti, R.; Scarone, S.; Bussone, G. Decision-making deficit in chronic migraine patients with medication overuse. Neurol. Sci. 2012, 33 (Suppl. S1), S151–S155. [Google Scholar] [CrossRef]
- Galvez-Sánchez, C.M.; Duschek, S.; del Paso, G.A.R. Psychological impact of fibromyalgia: Current perspectives. Psychol. Res. Behav. Manag. 2019, 12, 117–127. [Google Scholar] [CrossRef]
- Galvez-Sánchez, C.M.; de Guevara, C.M.L.; Montoro, C.I.; Fernández-Serrano, M.J.; Duschek, S.; Del Paso, G.A.R. Cognitive deficits in fibromyalgia syndrome are associated with pain responses to low intensity pressure stimulation. PLoS ONE 2018, 13, e0201488. [Google Scholar] [CrossRef]
- Wu, Y.L.; Huang, C.-J.; Fang, S.-C.; Ko, L.-H.; Tsai, P.-S. Cognitive Impairment in Fibromyalgia: A Meta-Analysis of Case-Control Studies. Psychosom. Med. 2018, 80, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Karimi, L.; Wijeratne, T.; Crewther, S.G.; Evans, A.E.; Ebaid, D.; Khalil, H. The Migraine-Anxiety Comorbidity Among Migraineurs: A Systematic Review. Front. Neurol. 2020, 11, 613372. [Google Scholar] [CrossRef] [PubMed]
- Seng, E.K.; Buse, D.C.; Klepper, J.E.; Mayson, S.J.; Grinberg, A.S.; Grosberg, B.M.; Pavlovic, J.M.; Robbins, M.S.; Vollbracht, S.E.; Lipton, R.B. Psychological Factors Associated with Chronic Migraine and Severe Migraine-Related Disability: An Observational Study in a Tertiary Headache Center. Headache 2017, 57, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.T. Cognitive Reserve and the Prevention of Dementia: The Role of Physical and Cognitive Activities. Curr. Psychiatry Rep. 2016, 18, 85. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012, 11, 1006–1012. [Google Scholar] [CrossRef]
- Stern, Y.; Arenaza-Urquijo, E.M.; Bartrés-Faz, D.; Belleville, S.; Cantilon, M.; Chetelat, G.; Ewers, M.; Franzmeier, N.; Kempermann, G.; Kremen, W.S.; et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2020, 16, 1305–1311. [Google Scholar] [CrossRef]
- Katzman, R.; Zhang, M.Y.; Ouang-Ya-Qu; Wang, Z.Y.; Liu, W.T.; Yu, E.; Wong, S.C.; Salmon, D.P.; Grant, I. A Chinese version of the Mini-Mental State Examination; impact of illiteracy in a Shanghai dementia survey. J. Clin. Epidemiol. 1988, 41, 971–978. [Google Scholar] [CrossRef]
- Garrigós-Pedrón, M.; Segura-Ortí, E.; Gracia-Naya, M.; La Touche, R. Predictive factors of sleep quality in patients with chronic migraine. Neurologia 2022, 37, 101–109. [Google Scholar] [CrossRef]
- Chen, Y.M.; Wang, J.-H.; Liang, C.-S.; Lin, Y.-K.; Yang, F.-C. Clinical and psychological predictors of sleep quality in chronic migraine: A preliminary retrospective analysis study. BMC Neurol. 2025, 25, 156. [Google Scholar] [CrossRef]
- Jensen, M.P.; Karoly, P.; Braver, S. The measurement of clinical pain intensity: A comparison of six methods. Pain 1986, 27, 117–126. [Google Scholar] [CrossRef]
- Stewart, W.F.; Lipton, R.B.; Kolodner, K.B.; Sawyer, J.; Lee, C.; Liberman, J.N. Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain 2000, 88, 41–52. [Google Scholar] [CrossRef]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef]
- Emmons, C.A.; Fetting, J.H.; Zonderman, A.B. A comparison of the symptoms of medical and psychiatric patients matched on the Beck Depression Inventory. Gen. Hosp. Psychiatry 1987, 9, 398–404. [Google Scholar] [CrossRef]
- Tsai, P.S.; Wang, S.-Y.; Wang, M.-Y.; Su, C.-T.; Yang, T.-T.; Huang, C.-J.; Fang, S.-C. Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects. Qual. Life Res. 2005, 14, 1943–1952. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Green, S.B. How Many Subjects Does It Take to Do a Regression Analysis. Multivar. Behav. Res. 1991, 26, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi. Med. J. 2012, 24, 69–71. [Google Scholar] [PubMed]
- Vatcheva, K.P.; Lee, M.; McCormick, J.B.; Rahbar, M.H. Multicollinearity in Regression Analyses Conducted in Epidemiologic Studies. Epidemiology 2016, 6, 227. [Google Scholar] [CrossRef]
- Gómez-Beldarrain, M.; Anton-Ladislao, A.; Aguirre-Larracoechea, U.; Oroz, I.; García-Moncó, J.C. Low cognitive reserve is associated with chronic migraine with medication overuse and poor quality of life. Cephalalgia 2015, 35, 683–691. [Google Scholar] [CrossRef]
- Lozano-Soto, E.; Cruz-Gómez, Á.J.; Rashid-López, R.; Sanmartino, F.; Espinosa-Rosso, R.; Forero, L.; González-Rosa, J.J. Neuropsychological and Neuropsychiatric Features of Chronic Migraine Patients during the Interictal Phase. J. Clin. Med. 2023, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Minen, M.T.; De Dhaem, O.B.; Van Diest, A.K.; Powers, S.; Schwedt, T.J.; Lipton, R.; Silbersweig, D. Migraine and its psychiatric comorbidities. J. Neurol. Neurosurg. Psychiatry 2016, 87, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Seng, E.K.; Seng, C.D. Understanding migraine and psychiatric comorbidity. Curr. Opin. Neurol. 2016, 29, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Vuralli, D.; Ayata, C.; Bolay, H. Cognitive dysfunction and migraine. J. Headache Pain 2018, 19, 109. [Google Scholar] [CrossRef]
- Gómez-Beldarrain, M.; Carrasco, M.; Bilbao, A.; García-Moncó, J.C. Orbitofrontal dysfunction predicts poor prognosis in chronic migraine with medication overuse. J. Headache Pain 2011, 12, 459–466. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, Y.; Song, M.; Lee, J.J.; Sohn, J.-H. Differences in Frontal Lobe Dysfunction in Patients with Episodic and Chronic Migraine. J. Clin. Med. 2021, 10, 2779. [Google Scholar] [CrossRef]
- Zucca, M.; Rubino, E.; Vacca, A.; De Martino, P.; Roveta, F.; Govone, F.; Gai, A.; Caglio, M.; Gentile, S.; Giordana, M.T.; et al. Metacognitive impairment in patients with episodic and chronic migraine. J. Clin. Neurosci. 2020, 72, 119–123. [Google Scholar] [CrossRef]
- Migliore, S.; D’Aurizio, G.; Altamura, C.; Brunelli, N.; Costa, C.; Curcio, G.; Vernieri, F. Task-switching abilities in episodic and chronic migraine. Neurol. Sci. 2022, 43, 3803–3810. [Google Scholar] [CrossRef]
- de Tommaso, M.; Valeriani, M.; Guido, M.; Libro, G.; Specchio, L.M.; Tonali, P.; Puca, F. Abnormal brain processing of cutaneous pain in patients with chronic migraine. Pain 2003, 101, 25–32. [Google Scholar] [CrossRef]
- Mathur, V.A.; Khan, S.A.; Keaser, M.L.; Hubbard, C.S.; Goyal, M.; Seminowicz, D.A. Altered cognition-related brain activity and interactions with acute pain in migraine. Neuroimage Clin. 2015, 7, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Beldarrain, M.; Oroz, I.; Zapirain, B.G.; Ruanova, B.F.; Fernandez, Y.G.; Cabrera, A.; Anton-Ladislao, A.; Aguirre-Larracoechea, U.; Garcıa-Monco, J.C. Right fronto-insular white matter tracts link cognitive reserve and pain in migraine patients. J. Headache Pain 2015, 17, 4. [Google Scholar]
- Caetano, A.; Ladeira, F.; Mendonça, M.; Barbosa, R.; Pinto, M.; Pelejão, M.R.; Viana-Baptista, M. Underuse of Prophylactic Treatment Among Portuguese Patients with Primary Headache: A Retrospective Observational Study. J. Oral Facial Pain Headache 2019, 33, 331–336. [Google Scholar] [CrossRef]
- Pascual, J.; Panni, T.; Dell, G.; Gonderten, S.; Novick, D.; Evers, S. Preventive treatment patterns and treatment satisfaction in migraine: Results of the OVERCOME (EU) study. J. Headache Pain 2023, 24, 88. [Google Scholar] [CrossRef]
- Russo, M.; De Rosa, M.A.; Calisi, D.; Consoli, S.; Evangelista, G.; Dono, F.; Santilli, M.; Granzotto, A.; Onofrj, M.; Sensi, S.L. Migraine Pharmacological Treatment and Cognitive Impairment: Risks and Benefits. Int. J. Mol. Sci. 2022, 23, 11418. [Google Scholar] [CrossRef]
- Baars, M.A.; van Boxtel, M.P.J.; Jolles, J. Migraine does not affect cognitive decline: Results from the Maastricht aging study. Headache 2010, 50, 176–184. [Google Scholar] [CrossRef]
- Rist, P.M.; Dufouil, C.; Glymour, M.M.; Tzourio, C.; Kurth, T. Migraine and cognitive decline in the population-based EVA study. Cephalalgia 2011, 31, 1291–1300. [Google Scholar] [CrossRef]

| Item | Objective Cognitive Impairment | p-Value | ||
|---|---|---|---|---|
| No | Yes | Total | ||
| N | 44 | 6 | 50 | |
| Age (years) | 42.50 ± 13.50 | 42.33 ± 14.54 | 42.48 ± 13.47 | 0.978 |
| Sex | 0.576 | |||
| Women | 37 (84.1%) | 6 (100.0%) | 43 (86.0%) | |
| Men | 7 (15.9%) | 0 (0.0%) | 7 (14.0%) | |
| Regular exercise (%) | 16 (36.4%) | 1 (16.7%) | 17 (34.0%) | 0.650 |
| Weekly exercise duration (mins) | 96.86 ± 234.70 | 23.33 ± 57.15 | 88.04 ± 221.93 | 0.452 |
| PSQI score | 11.41 ± 5.26 | 15.17 ± 6.55 | 11.86 ± 5.50 | 0.117 |
| Insomnia(PSQI ≥ 6) (%) | 36 (81.8%) | 5 (83.3%) | 41 (82.0%) | 1.000 |
| Preventive medication use (%) | 11 (25.0%) | 1 (16.7%) | 12 (24.0%) | 1.000 |
| Preventive medication category | 1.000 | |||
| Used preventive medications | 11 (25.0%) | 1 (16.7%) | 12 (24.0%) | |
| Used medications potentially affecting cognition | 6 (13.6%) | 0 (0.0%) | 6 (12.0%) | |
| Used medications with less cognitive impact | 5 (11.4%) | 1 (16.7%) | 6 (12.0%) | |
| Did not use preventive medications | 33 (75.0%) | 5 (83.3%) | 38 (76.0%) | |
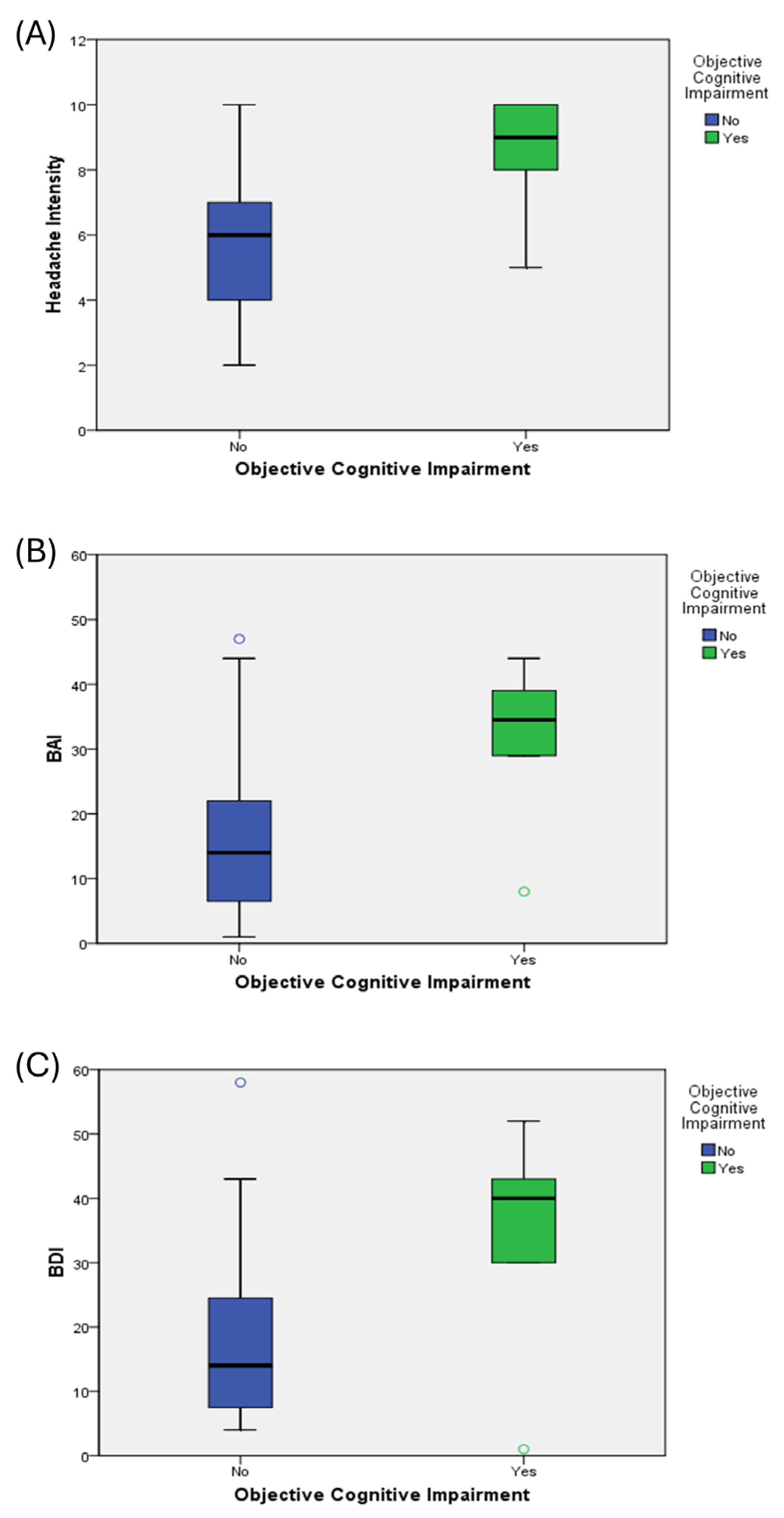
| Headache intensity (NRS) | 5.84 ± 1.75 | 8.50 ± 1.87 | 6.16 ± 1.95 | 0.001 * |
| Monthly headache days | 22.70 ± 6.77 | 25.83 ± 6.65 | 23.08 ± 6.77 | 0.293 |
| Headache days over past 3 months | 70.95 ± 23.72 | 89.17 ± 2.04 | 73.14 ± 23.02 | 0.069 |
| MIDAS score | 52.89 ± 54.54 | 85.17 ± 77.46 | 56.76 ± 57.74 | 0.202 |
| BAI score | 15.95 ± 11.84 | 31.50 ± 12.57 | 17.82 ± 12.85 | 0.004 * |
| BDI score | 17.16 ± 11.93 | 34.33 ± 17.83 | 19.22 ± 13.75 | 0.003 * |
| MMSE score (observed) | 28.43 ± 1.50 | 21.17 ± 1.72 | 27.56 ± 2.82 | <0.001 * |
| Correlation | Age | MHD | NRS | MIDAS | Weekly Exercise Duration (min) | BAI | BDI | MMSE (Observed) | PSQI |
|---|---|---|---|---|---|---|---|---|---|
| Age | 1 | 0.051 | −0.012 | −0.060 | 0.039 | −0.284 * | −0.298 * | −0.242 | −0.147 |
| MHD | 1 | 0.374 ** | 0.306 * | −0.011 | 0.128 | 0.107 | −0.248 | 0.180 | |
| NRS | 1 | 0.382 ** | −0.256 | 0.299 * | 0.373 ** | −0.424 ** | 0.282 * | ||
| MIDAS | 1 | −0.094 | 0.248 | 0.192 | −0.207 | 0.243 | |||
| Weekly exercise duration (min) | 1 | −0.039 | −0.011 | 0.035 | 0.043 | ||||
| BAI | 1 | 0.830 ** | −0.336 * | 0.532 ** | |||||
| BDI | 1 | −0.343 * | 0.492 ** | ||||||
| MMSE (observed) | 1 | −0.174 | |||||||
| PSQI | 1 |
| Crude | Adjusted (Model 1), R2 = 0.38 | Adjusted (Model 2), R2 = 0.37 | ||||||
|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p-Value | β (95% CI) | p-Value | VIF | β (95% CI) | p-Value | VIF | |
| Age | −0.03 (−0.08, 0.02) | 0.277 | −0.06 (−0.11, −0.01) | 0.026 * | 1.178 | −0.06 (−0.11, −0.01) | 0.026 * | 1.193 |
| Sex (man vs. woman) | 0.82 (−1.29, 2.94) | 0.438 | −0.27 (−2.16, 1.62) | 0.772 | 1.132 | −0.26 (−2.16, 1.64) | 0.785 | 1.132 |
| Preventive medication usage (yes vs. no) | 0.26 (−1.47, 1.99) | 0.765 | 0.84 (−0.64, 2.32) | 0.261 | 1.053 | 0.76 (−0.73, 2.25) | 0.308 | 1.052 |
| MHD | −0.10 (−0.20, 0.01) | 0.075 | ||||||
| NRS | −0.60 (−0.94, −0.26) | 0.001 * | −0.47 (−0.84, −0.10) | 0.015 * | 1.345 | −0.41 (−0.79, −0.02) | 0.038 * | 1.435 |
| MIDAS | −0.01 (−0.02, 0.001) | 0.081 | −0.001 (−0.013, 0.01) | 0.807 | 1.225 | −0.003 (−0.015, 0.009) | 0.585 | 1.201 |
| BAI | −0.08 (−0.14, −0.03) | 0.003 * | −0.08 (−0.13, −0.02) | 0.005 * | 1.230 | |||
| BDI | −0.08 (−0.13, −0.03) | 0.002 * | −0.07 (−0.12, −0.02) | 0.008 * | 1.294 | |||
| Study (Year) | Sample (CM) | Cognitive Findings | Headache Intensity/Disability | Psychological Factors (Anxiety/Depression) |
|---|---|---|---|---|
| Present study, 2025 | N = 50 CM (86% women); MMSE used for cognition | 12% met criteria for objective cognitive impairment (MMSE < cutoff) ↑ BAI/BDI scores in cognitively impaired group | ↑ headache intensity correlated with ↓ CR | ↑ anxiety and depression associated with ↓ CR |
| Gómez-Beldarrain et al., 2015 [32] | N = 18 CM–MOH; 90.9% women; CR index used for cognition | ↓ CR in CM–MOH group; ↓ CR associated with ↓ QoL | – association with headache intensity not analyzed | ↑ anxiety and depression in CM–MOH; ↑ CR associated with ↓ anxiety and depression |
| Ferreira et al., 2018 [6] | N = 30 CM vs. 30 controls; comprehensive neuropsychological battery (MoCA, Stroop, etc.) | ↓ cognition in CM; CM = independent risk factor | – association with headache intensity not analyzed | – cognitive deficits occurred regardless of depression/anxiety or preventive medication use |
| Latysheva et al., 2020 [7] | N = 144 CM vs. 44 EM; neuropsychological tests (MoCA, RAVLT, etc.) and HADS for mood | ↑ cognitive deficits in CM vs. EM; predictors: CM diagnosis, low education | – CM (i.e., ↑ frequency) linked to ↓ cognitive performance; headache intensity not analyzed | – no independent effect of anxiety/depression on cognition after adjustment |
| Lozano-Soto et al., 2023 [33] | N = 39 CM vs. 20 controls; full neuropsychological tests during interictal period | 54% had neuropsychological impairment; | ↑ headache intensity/disability severity predicted ↓ global cognitive performance | – no independent effect of anxiety/depression on cognition after adjustment |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-M.; Wang, J.-H. Cognitive Reserve and Its Associations with Pain, Anxiety, and Depression in Patients with Chronic Migraine: A Retrospective Study. J. Clin. Med. 2025, 14, 5193. https://doi.org/10.3390/jcm14155193
Chen Y-M, Wang J-H. Cognitive Reserve and Its Associations with Pain, Anxiety, and Depression in Patients with Chronic Migraine: A Retrospective Study. Journal of Clinical Medicine. 2025; 14(15):5193. https://doi.org/10.3390/jcm14155193
Chicago/Turabian StyleChen, Yu-Ming, and Jen-Hung Wang. 2025. "Cognitive Reserve and Its Associations with Pain, Anxiety, and Depression in Patients with Chronic Migraine: A Retrospective Study" Journal of Clinical Medicine 14, no. 15: 5193. https://doi.org/10.3390/jcm14155193
APA StyleChen, Y.-M., & Wang, J.-H. (2025). Cognitive Reserve and Its Associations with Pain, Anxiety, and Depression in Patients with Chronic Migraine: A Retrospective Study. Journal of Clinical Medicine, 14(15), 5193. https://doi.org/10.3390/jcm14155193








