Sotatercept for Connective Tissue Disease-Associated Pulmonary Arterial Hypertension with Concomitant Interstitial Lung Disease: Efficacy and Safety Insights
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASI | Activin Signaling Inhibitor |
| CTD | Connective Tissue Disease |
| CTD-PAH | Connective Tissue Disease-Associated Pulmonary Arterial Hypertension |
| DLCO | Diffusing Capacity for Carbon Monoxide |
| eRVSP | Estimated Right Ventricular Systolic Pressure |
| ERA | Endothelin Receptor Antagonist |
| FDA | Food and Drug Administration |
| FVC | Forced Vital Capacity |
| ILD | Interstitial Lung Disease |
| MCTD | Mixed Connective Tissue Disease |
| mPAP | Mean Pulmonary Artery Pressure |
| NSIP | Nonspecific Interstitial Pneumonia |
| NT-proBNP | N-terminal Prohormone Brain Natriuretic Peptide |
| PAH | Pulmonary Arterial Hypertension |
| PCWP | Pulmonary Capillary Wedge Pressure |
| PDE-5i | Phosphodiesterase-5 Inhibitor |
| PH | Pulmonary Hypertension |
| PH-ILD | Pulmonary Hypertension Associated with Interstitial Lung Disease |
| PFT | Pulmonary Function Test |
| PVR | Pulmonary Vascular Resistance |
| RHC | Right Heart Catheterization |
| RISE-IIP | Riociguat for Idiopathic Interstitial Pneumonia |
| RCT | Randomized Controlled Trial |
| sGC | Soluble Guanylate Cyclase |
| TAPSE | Tricuspid Annular Plane Systolic Excursion |
| V/Q | Ventilation-Perfusion |
| WHO | World Health Organization |
| WUs | Wood Units |
| 6MWD | Six-Minute Walk Distance |
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: Developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG). Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, P.M. Pulmonary arterial hypertension. N. Engl. J. Med. 2021, 385, 2361–2376. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Badesch, D.B.; Ghofrani, H.A.; Gibbs, J.S.R.; Gomberg-Maitland, M.; McLaughlin, V.V.; Preston, I.R.; Souza, R.; Waxman, A.B.; Grünig, E.; et al. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2023, 388, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- Launay, D.; Sitbon, O.; Hachulla, E.; Mouthon, L.; Gressin, V.; Rottat, L.; Clerson, P.; Cordier, J.F.; Simonneau, G.; Humbert, M. Survival in systemic sclerosis-associated pulmonary arterial hypertension in the modern management era. Ann. Rheum. Dis. 2013, 72, 1940–1946. [Google Scholar] [CrossRef] [PubMed]
- Rhee, R.L.; Gabler, N.B.; Sangani, S.; Praestgaard, A.; Merkel, P.A.; Kawut, S.M. Comparison of treatment response in idiopathic and connective tissue disease–associated pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2015, 192, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- The Idiopathic Pulmonary Fibrosis Clinical Research Network; Zisman, D.A.; Schwarz, M.; Anstrom, K.J.; Collard, H.R.; Flaherty, K.R.; Hunninghake, G.W. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N. Engl. J. Med. 2010, 363, 620–628. [Google Scholar] [PubMed]
- Raghu, G.; Million-Rousseau, R.; Morganti, A.; Perchenet, L.; Behr, J.; MUSIC Study Group. Macitentan for the treatment of idiopathic pulmonary fibrosis: The randomised controlled MUSIC trial. Eur. Respir. J. 2013, 42, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Nathan, S.D.; Behr, J.; Collard, H.R.; Cottin, V.; Hoeper, M.M.; Martinez, F.J.; Corte, T.J.; Keogh, A.M.; Leuchte, H.; Mogulkoc, N.; et al. Riociguat for idiopathic interstitial pneumonia-associated pulmonary hypertension (RISE-IIP): A randomised, placebo-controlled phase 2b study. Lancet Respir. Med. 2019, 7, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Shlobin, O.A.; Brown, A.W.; Nathan, S.D. Pulmonary hypertension in diffuse parenchymal lung diseases. Chest 2017, 151, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Nathan, S.D.; Tapson, V.F.; Elwing, J.; Rischard, F.; Mehta, J.; Shapiro, S.; Shen, E.; Deng, C.; Smith, P.; Waxman, A. Efficacy of inhaled treprostinil on multiple disease progression events in patients with pulmonary hypertension due to parenchymal lung disease in the INCREASE trial. Am. J. Respir. Crit. Care Med. 2022, 205, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Szturmowicz, M.; Kacprzak, A.; Kuś, J. Pulmonary hypertension in diffuse parenchymal lung diseases—Is there any benefit of PAH-specific therapy? Adv. Respir. Med. 2017, 85, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Sitbon, O.; Chaouat, A.; Bertocchi, M.; Habib, G.; Gressin, V.; Yaici, A.; Weitzenblum, E.; Cordier, J.F.; Chabot, F.; et al. Pulmonary arterial hypertension in France: Results from a national registry. Am. J. Respir. Crit. Care Med. 2006, 173, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Badesch, D.B.; Raskob, G.E.; Elliott, C.G.; Krichman, A.M.; Farber, H.W.; Frost, A.E.; Barst, R.J.; Benza, R.L.; Liou, T.G.; Turner, M.; et al. Pulmonary arterial hypertension: Baseline characteristics from the REVEAL Registry. Chest 2010, 137, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Nathan, S.D.; Hassoun, P.M. Pulmonary hypertension due to lung disease and/or hypoxia. Clin. Chest Med. 2013, 34, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, C.J.; Nathan, S.D.; Browning, R.F.; Barnett, S.D.; Ahmad, S.; Shorr, A.F. The distance-saturation product predicts mortality in idiopathic pulmonary fibrosis. Respir. Med. 2006, 100, 1734–1741. [Google Scholar] [CrossRef] [PubMed]
- Seeger, W.; Adir, Y.; Barberà, J.A.; Champion, H.; Coghlan, J.G.; Cottin, V.; Marco, T.D.; Galiè, N.; Ghio, S.; Gibbs, S.; et al. Pulmonary hypertension in chronic lung diseases. TURK Kardiyol. Dern. ARSIVI. 2014, 42, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; McLaughlin, V.; Gibbs, J.S.R.; Gomberg-Maitland, M.; Hoeper, M.M.; Preston, I.R.; Souza, R.; Waxman, A.; Escribano Subias, P.; Feldman, J.; et al. Sotatercept for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2021, 384, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; McLaughlin, V.; Gibbs, J.S.R.; Gomberg-Maitland, M.; Hoeper, M.M.; Preston, I.R.; Souza, R.; Waxman, A.B.; Ghofrani, H.A.; Escribano Subias, P.; et al. Sotatercept for the treatment of pulmonary arterial hypertension: PULSAR open-label extension. Eur. Respir. J. 2023, 61, 2201347. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.; Badesch, D.B.; Ghofrani, H.A.; Gibbs, J.S.R.; Gomberg-Maitland, M.; McLaughlin, V.V.; Preston, I.R.; Waxman, A.B.; Grünig, E.; Kopeć, G.; et al. Effects of sotatercept on haemodynamics and right heart function: Analysis of the STELLAR trial. Eur. Respir. J. 2023, 62, 2301107. [Google Scholar] [CrossRef] [PubMed]
- Gomberg-Maitland, M.; McLaughlin, V.V.; Badesch, D.B.; Ghofrani, H.A.; Hoeper, M.M.; Humbert, M.; Preston, I.R.; Souza, R.; Waxman, A.B.; de Oliveira, P.J.; et al. Long-term effects of sotatercept on right ventricular function: Results from the PULSAR study. Heart Fail. 2023, 11, 1457–1459. [Google Scholar]
- Preston, I.R.; Badesch, D.; Ghofrani, H.A.; Gibbs, J.S.R.; Gomberg-Maitland, M.; Hoeper, M.M.; Humbert, M.; McLaughlin, V.V.; Waxman, A.B.; Manimaran, S.; et al. Late Breaking Abstract-A long-term follow-up (LTFU) study of sotatercept for pulmonary arterial hypertension (PAH). Eur. Respir. Soc. 2023. [Google Scholar] [CrossRef]
- Co, M. A Phase 2, Double-Blind, Randomized, Placebo-Controlled Study to Assess the Efficacy and Safety of Sotatercept in Adults With Combined Postcapillary and Precapillary Pulmonary Hypertension (Cpc-PH) Due to Heart Failure With Preserved Ejection Fraction (HFpEF) (CADENCE Trial). 2024. Available online: https://clinicaltrials.gov/study/NCT04945460 (accessed on 30 June 2025).
- King, T.E., Jr.; Behr, J.; Brown, K.K.; du Bois, R.M.; Lancaster, L.; de Andrade, J.A.; Stähler, G.; Leconte, I.; Roux, S.; Raghu, G. BUILD-1: A randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2008, 177, 75–81. [Google Scholar] [CrossRef] [PubMed]
- King, T.E., Jr.; Brown, K.K.; Raghu, G.; du Bois, R.M.; Lynch, D.A.; Martinez, F.; Valeyre, D.; Leconte, I.; Morganti, A.; Roux, S.; et al. BUILD-3: A randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2011, 184, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Behr, J.; Brown, K.K.; Egan, J.J.; Kawut, S.M.; Flaherty, K.R.; Martinez, F.J.; Nathan, S.D.; Wells, A.U.; Collard, H.R.; et al. Treatment of idiopathic pulmonary fibrosis with ambrisentan: A parallel, randomized trial. Ann. Intern. Med. 2013, 158, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V.; Price, L.C.; Valenzuela, C. The unmet medical need of pulmonary hypertension in idiopathic pulmonary fibrosis. Eur. Respir. Soc. 2018, 51, 170596. [Google Scholar] [CrossRef] [PubMed]
- Corte, T.J.; Keir, G.J.; Dimopoulos, K.; Howard, L.; Corris, P.A.; Parfitt, L.; Foley, C.; Yanez-Lopez, M.; Babalis, D.; Marino, P.; et al. Bosentan in pulmonary hypertension associated with fibrotic idiopathic interstitial pneumonia. Am. J. Respir. Crit. Care Med. 2014, 190, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Waxman, A.; Restrepo-Jaramillo, R.; Thenappan, T.; Ravichandran, A.; Engel, P.; Bajwa, A.; Allen, R.; Feldman, J.; Argula, R.; Smith, P.; et al. Inhaled treprostinil in pulmonary hypertension due to interstitial lung disease. N. Engl. J. Med. 2021, 384, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Group GBC. Gossamer Bio and Chiesi Group Announce Global Collaboration on Seralutinib for PAH and PH-ILD; Group GBC: Etobicoke, ON, Canada, 2024. [Google Scholar]
- Pulmovant Inc. A Phase 2, Randomized, Placebo-Controlled Trial to Assess the Efficacy And Safety of Mosliciguat In Participants with Pulmonary Hypertension Associated with Interstitial Lung Disease; Springer: Heidelberg, Germany, 2024. [Google Scholar]
| Parameter | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 |
|---|---|---|---|---|---|---|---|
| Age (years) | 55 | 42 | 57 | 71 | 73 | 39 | 62 |
| Gender | F | F | M | F | M | F | F |
| CTD Type | Scleroderma | Scleroderma | MCTD | MCTD | Scleroderma | Scleroderma | Scleroderma |
| CTD Duration (years) | 3 | 2 | 4 | 4 | 1 | 4 | 2 |
| PAH Duration (years) | 2 | 1 | 2 | 3 | 1 | 2 | 1 |
| PAH Treatment | Riociguat, macitentan, selexipag | Tadalafil, macitentan, inhaled treprostinil | Tadalafil, macitentan, IV treprostinil | Tadalafil, macitentan, inhaled treprostinil | Tadalafil, macitentan, inhaled treprostinil | Tadalafil, macitentan, selexipag | Tadalafil, macitentan, inhaled treprostinil |
| ILD Type | NSIP | NSIP | NSIP | NSIP | NSIP | NSIP | NSIP |
| ILD Treatment | None | Nintedanib | Nintedanib | None | None | None | None |
| CTD Treatment | Mycophenolate mofetil Hydroxychloroquine | Mycophenolate mofetil | Mycophenolate mofetil Hydroxychloroquine | Hydroxychloroquine | Hydroxychloroquine | Mycophenolate mofetil | Mycophenolate mofetil |
| Predicted FVC (%)—Baseline | 62 | 69 | 71 | 60 | 55 | 59 | 58 |
| Predicted DLCO (%)—Baseline | 39 | 41 | 33 | 37 | 49 | 31 | 44 |
| Parameter | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 |
|---|---|---|---|---|---|---|---|
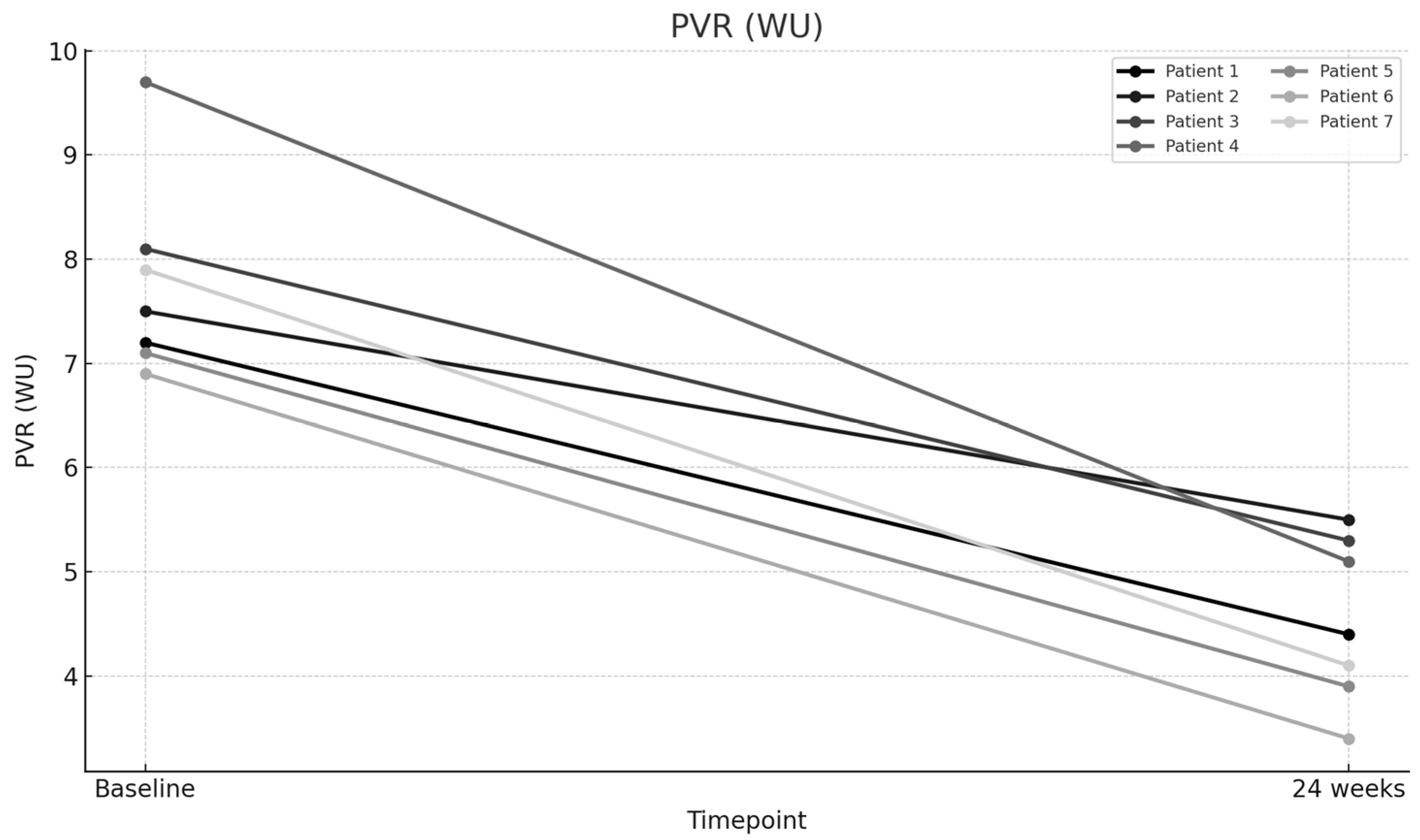
| PVR (WU)—Baseline | 7.2 | 7.5 | 8.1 | 9.7 | 7.1 | 6.9 | 7.9 |
| PVR (WU)—24 weeks | 4.4 | 5.5 | 5.3 | 5.1 | 3.9 | 3.4 | 4.1 |
| WHO Functional Class—Baseline | 3 | 4 | 4 | 4 | 3 | 3 | 3 |
| WHO Functional Class—24 weeks | 2 | 3 | 3 | 2 | 1 | 1 | 1 |
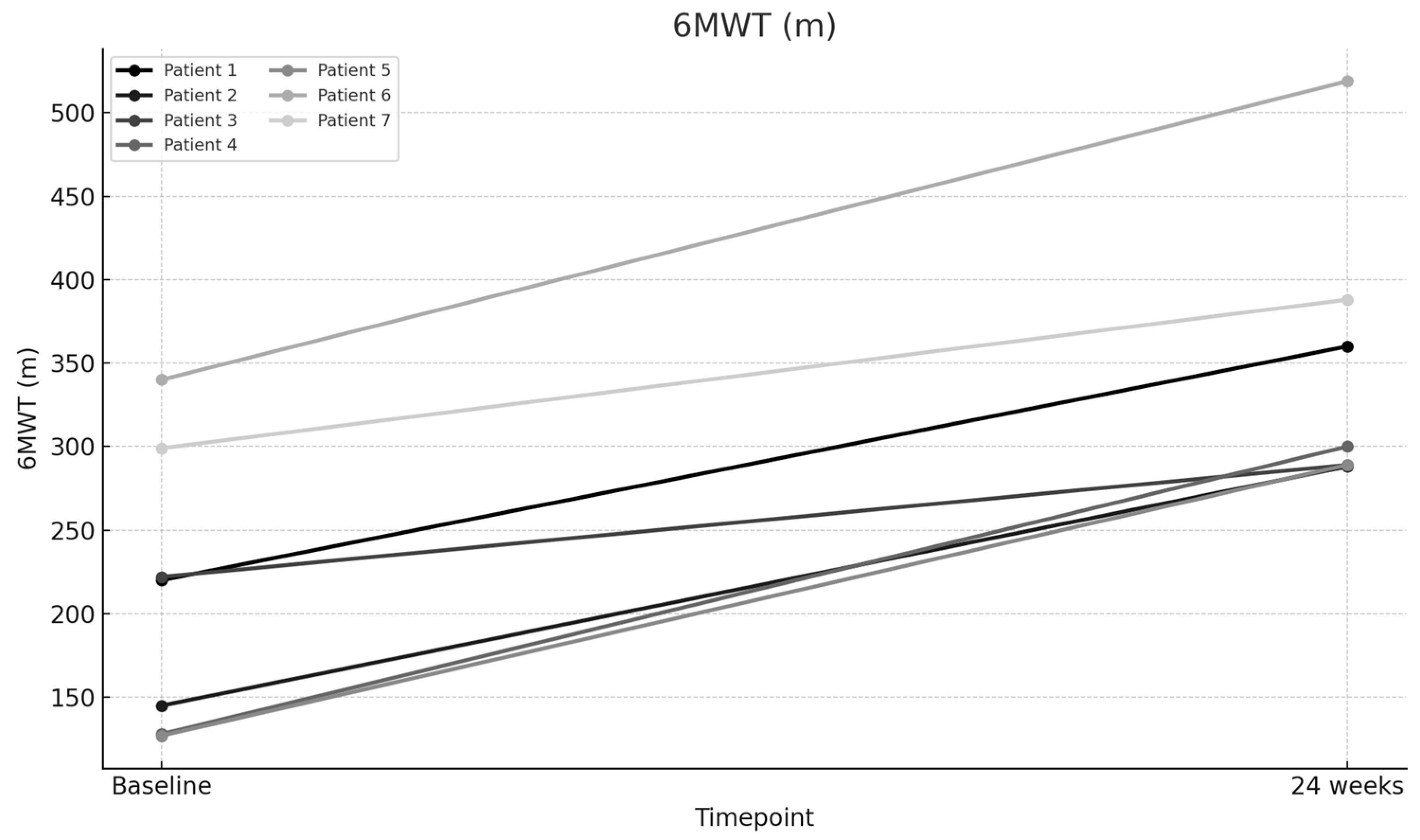
| 6-Min Walk Test (m)—Baseline | 220 | 145 | 222 | 128 | 127 | 340 | 299 |
| 6-Min Walk Test (m)—24 weeks | 360 | 288 | 289 | 300 | 289 | 519 | 388 |
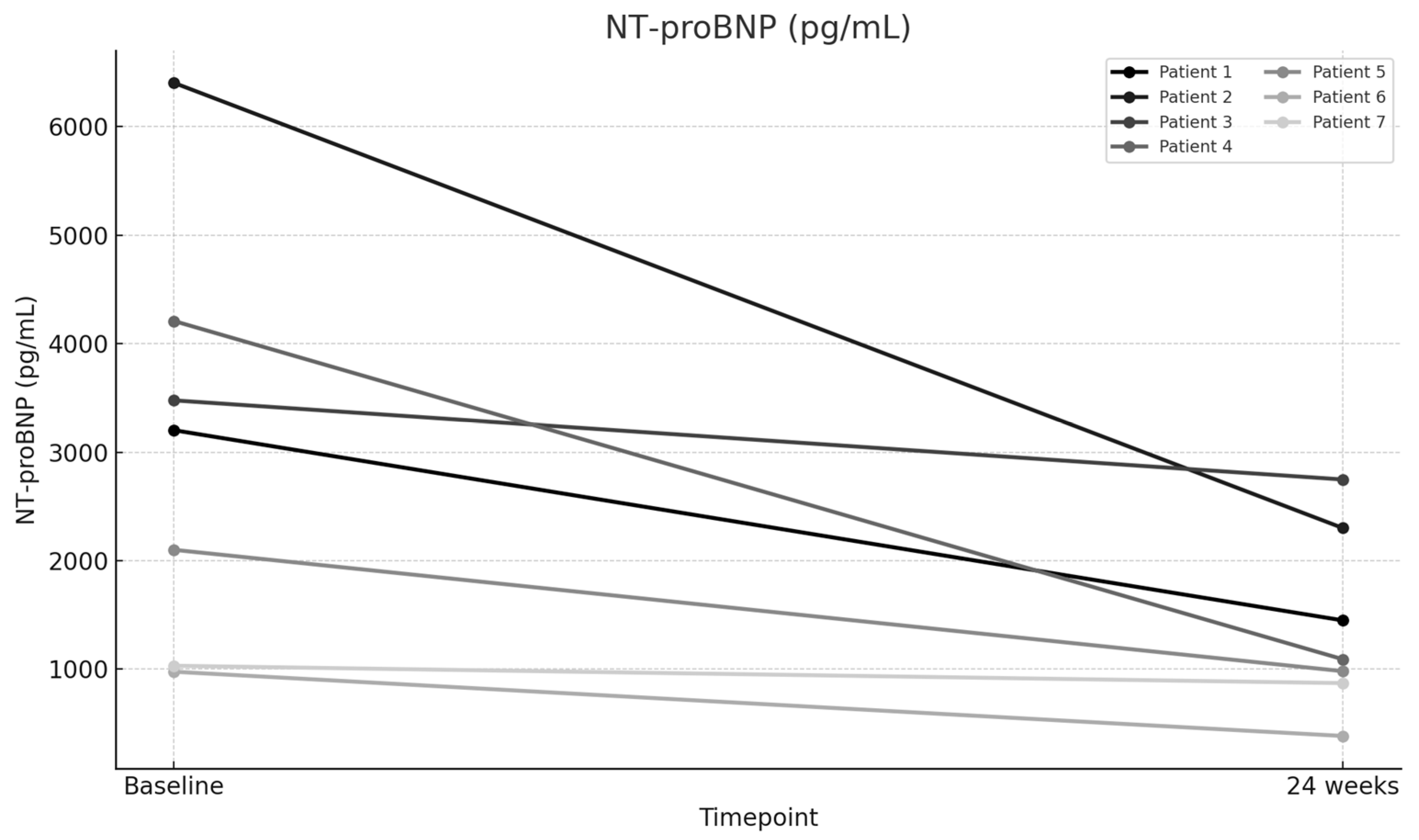
| NT-proBNP (pg/mL)—Baseline | 3202 | 6402 | 3477 | 4208 | 2100 | 977 | 1032 |
| NT-proBNP (pg/mL)—24 weeks | 1450 | 2301 | 2748 | 1092 | 982 | 385 | 872 |
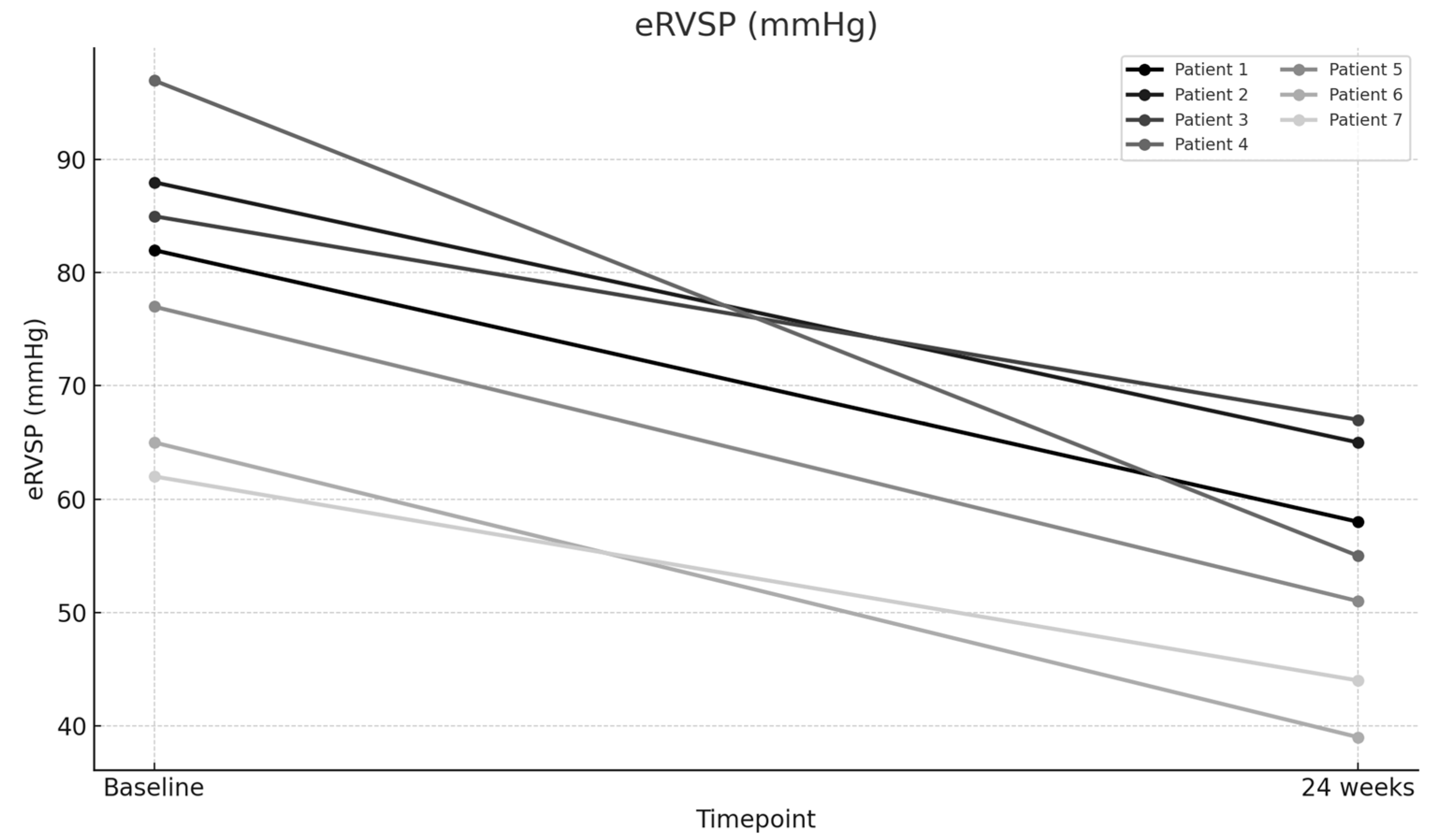
| eRVSP (mmHg)—Baseline | 82 | 88 | 85 | 97 | 77 | 65 | 62 |
| eRVSP (mmHg)—24 weeks | 58 | 65 | 67 | 55 | 51 | 39 | 44 |
| Supplemental O2 Therapy (L/min)—Baseline | 4 | 6 | 4 | 3 | 2 | 0 | 2 |
| Supplemental O2 Therapy (L/min)—24 weeks | 2 | 4 | 2 | 0 | 0 | 0 | 0 |
| Parameter | Baseline Mean | Mean After 24 Weeks | Difference | Percentage Change (%) | T Score | p Value |
|---|---|---|---|---|---|---|
| PVR (WU) | 7.77 | 4.53 | 3.24 | 41.7 | 10.308 | 0.000049 |
| 6MWD (m) | 211.57 | 347.57 | 136 | 64.28 | 8.459 | 0.00015 |
| NT-proBNP (pg/mL) | 3056.86 | 1404.29 | 1652.57 | 54.06 | 3.013 | 0.02362 |
| eRVSP (mmHg) | 79.43 | 54.14 | 25.29 | 31.84 | 8.262 | 0.00017 |
| Supplemental O2 (L/min) | 3 | 1.14 | 1.86 | 62 | 5.461 | 0.00157 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dagher, C.; Akiki, M.; Swanson, K.; Carollo, B.; Fiscus, G.; Farber, H.W.; Parikh, R. Sotatercept for Connective Tissue Disease-Associated Pulmonary Arterial Hypertension with Concomitant Interstitial Lung Disease: Efficacy and Safety Insights. J. Clin. Med. 2025, 14, 5177. https://doi.org/10.3390/jcm14155177
Dagher C, Akiki M, Swanson K, Carollo B, Fiscus G, Farber HW, Parikh R. Sotatercept for Connective Tissue Disease-Associated Pulmonary Arterial Hypertension with Concomitant Interstitial Lung Disease: Efficacy and Safety Insights. Journal of Clinical Medicine. 2025; 14(15):5177. https://doi.org/10.3390/jcm14155177
Chicago/Turabian StyleDagher, Chebly, Maria Akiki, Kristin Swanson, Brett Carollo, Garett Fiscus, Harrison W. Farber, and Raj Parikh. 2025. "Sotatercept for Connective Tissue Disease-Associated Pulmonary Arterial Hypertension with Concomitant Interstitial Lung Disease: Efficacy and Safety Insights" Journal of Clinical Medicine 14, no. 15: 5177. https://doi.org/10.3390/jcm14155177
APA StyleDagher, C., Akiki, M., Swanson, K., Carollo, B., Fiscus, G., Farber, H. W., & Parikh, R. (2025). Sotatercept for Connective Tissue Disease-Associated Pulmonary Arterial Hypertension with Concomitant Interstitial Lung Disease: Efficacy and Safety Insights. Journal of Clinical Medicine, 14(15), 5177. https://doi.org/10.3390/jcm14155177






