External Validation of the JAKPOT Score for Diagnosing JAK2-Positive Erythrocytosis: A Retrospective Cohort Study †
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Population
2.2. Data Extraction and Laboratory Assays
2.3. Outcomes
2.4. Statistical Analysis
3. Results
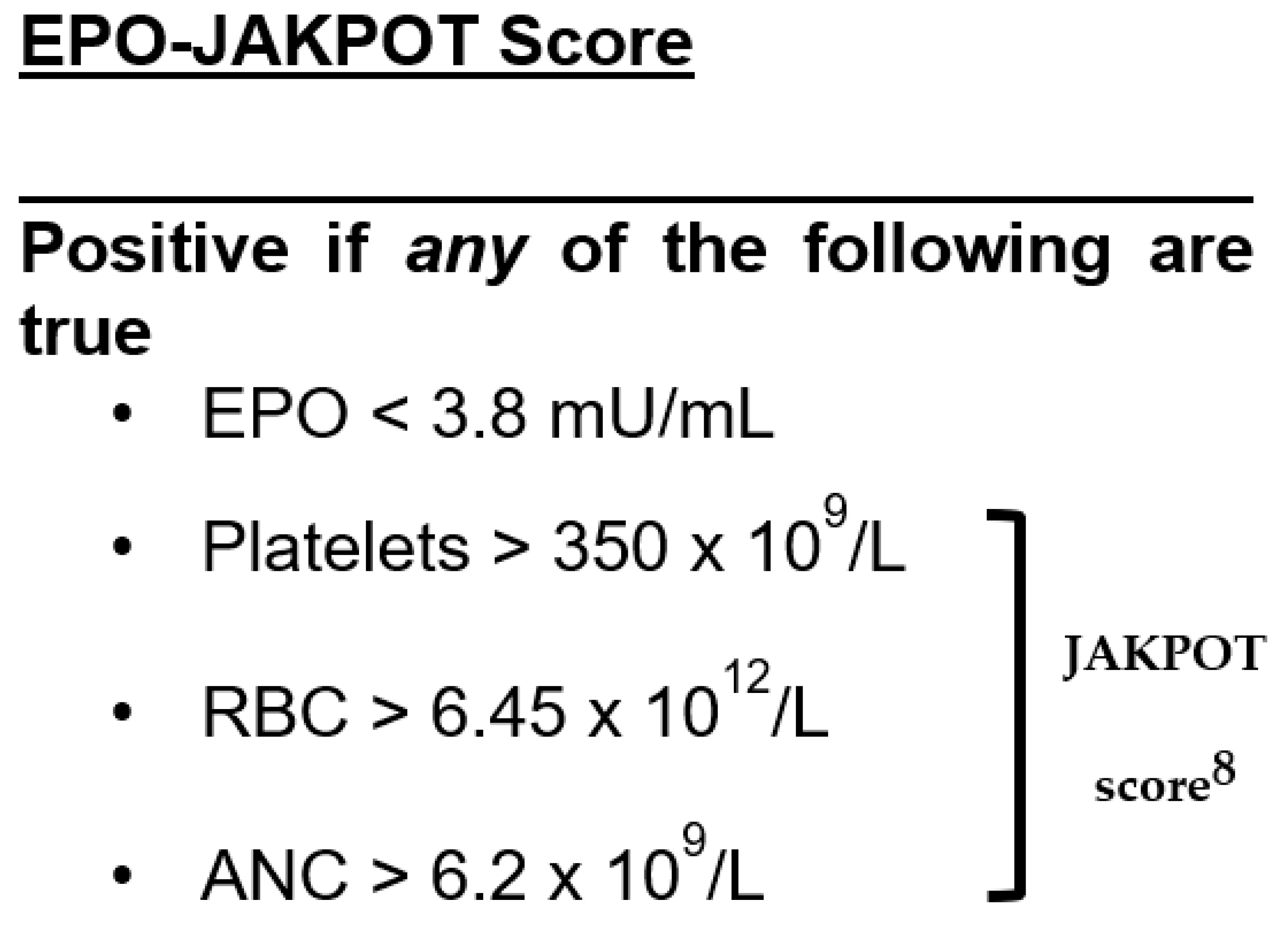
3.1. Performance of Low EPO or JAKPOT to Diagnose JAK2-Positive Erythrocytosis
3.2. Performance of EPO-JAKPOT to Diagnose JAK2-Positive Erythrocytosis
3.3. Univariate Predictors of JAK2-Positive Erythrocytosis
3.4. Adjusted (Multivariate) Predictors of JAK2-Positive Erythrocytosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institution Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baram, D.V.; Asaulenko, Z.P.; Spiridonov, I.N.; Krivolapov, Y.A. WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues, 2022 (5th Edition): Lymphoid Tumors. Arkh. Patol. 2023, 85, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Busque, L.; Porwit, A.; Day, R.; Olney, H.J.; Leber, B.; Ethier, V.; Sirhan, S.; Foltz, L.; Prchal, J.; Kamel-Reid, S.; et al. Laboratory Investigation of Myeloproliferative Neoplasms (MPNs) Recommendations of the Canadian MPN Group. Am. J. Clin. Pathol. Oct. 2016, 146, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P. Epidemiology of the Myeloproliferative Disorders Polycythemia Vera and Essential Thrombocythemia. Semin. Thromb. Hemost. 2006, 32, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Titmarsh, G.J.; Duncombe, A.S.; McMullin, M.F.; O’Rorke, M.; Mesa, R.; De Vocht, F.; Horan, S.; Fritschi, L.; Clarke, M.; Anderson, L.A. How Common Are Myeloproliferative Neoplasms? A Systematic Review and Meta-Analysis. Am. J. Hematol. 2014, 89, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Barbui, T.; Thiele, J.; Gisslinger, H.; Kvasnicka, H.M.; Vannucchi, A.M.; Guglielmelli, P.; Orazi, A.; Tefferi, A. The 2016 WHO Classification and Diagnostic Criteria for Myeloproliferative Neoplasms: Document Summary and in-Depth Discussion. Blood Cancer J. 2018, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Chin-Yee, B.; Cheong, I.; Matyashin, M.; Lazo-Langner, A.; Chin-Yee, I.; Bhayana, V.; Bhai, P.; Lin, H.; Sadikovic, B.; Hsia, C.C. Serum Erythropoietin Levels in 696 Patients Investigated for Erythrocytosis with JAK2 Mutation Analysis. Am. J. Hematol. 2022, 97, 1409–1412. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Rumi, E.; Finazzi, G.; Gisslinger, H.; Vannucchi, A.M.; Rodeghiero, F.; Randi, M.L.; Vaidya, R.; Cazzola, M.; Rambaldi, A.; et al. Survival and Prognosis among 1545 Patients with Contemporary Polycythemia Vera: An International Study. Leukemia 2013, 27, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Chin-Yee, B.; Bhai, P.; Cheong, I.; Matyashin, M.; Hsia, C.C.; Kawata, E.; Ho, J.M.; Levy, M.A.; Stuart, A.; Lin, H.; et al. A Rational Approach to JAK2 Mutation Testing in Patients with Elevated Hemoglobin: Results from the JAK2 Prediction Cohort (JAKPOT) Study. J. Gen. Intern. Med. 2023, 38, 1828–1833. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap Consortium: Building an International Community of Software Platform Partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.B. Probable Inference, the Law of Succession, and Statistical Inference. J. Am. Stat. Assoc. 1927, 22, 209–212. [Google Scholar] [CrossRef]
- White, I.R.; Royston, P.; Wood, A.M. Multiple Imputation Using Chained Equations: Issues and Guidance for Practice. Stat. Med. 2011, 30, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Messinezy, M.; Westwood, N.B.; El-Hemaidi, I.; Marsden, J.T.; Sherwood, R.S.; Pearson, T.C. Serum Erythropoietin Values in Erythrocytoses and in Primary Thrombocythaemia. Br. J. Haematol. 2002, 117, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Lupak, O.; Han, X.; Xie, P.; Mahmood, S.; Mohammed, H.; Donthireddy, V. The Role of a Low Erythropoietin Level for the Polycythemia Vera Diagnosis. Blood Cells Mol. Dis. 2020, 80, 102355. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, M.R.; Stein, B.L.; Boccia, R.V.; Oh, S.T.; Paranagama, D.; Parasuraman, S.; Colucci, P.; Mesa, R. Clinical and Disease Characteristics From REVEAL at Time of Enrollment (Baseline): Prospective Observational Study of Patients With Polycythemia Vera in the United States. Clin. Lymphoma Myeloma Leuk. 2018, 18, 788–795.e2. [Google Scholar] [CrossRef] [PubMed]
- Tukker, M.; Bruwiere, E.; Bos, S.; Caliskan, K. SGLT2 Inhibitor–Related Polycythemia in a Patient With Chronic Heart Failure: A Potential Severe Adverse Event. Circ. Heart Fail. 2023, 16. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gupta, A.; Shrikhande, M.; Tyagi, K.; Ghosh, A.; Misra, A. Marked Erythrocytosis during Treatment with Sodium Glucose Cotransporter-2 Inhibitors-Report of Two Cases. Diabetes Res. Clin. Pract. 2020, 162, 108127. [Google Scholar] [CrossRef] [PubMed]
- Gangat, N.; Szuber, N.; Alkhateeb, H.; Al-Kali, A.; Pardanani, A.; Tefferi, A. JAK2 Wild-Type Erythrocytosis Associated with Sodium-Glucose Cotransporter 2 Inhibitor Therapy. Blood 2021, 138, 2886–2889. [Google Scholar] [CrossRef] [PubMed]
- Mithoowani, S.; Laureano, M.; Crowther, M.; Hillis, C. Investigation and management of erythrocytosis. Can. Med. Assoc. J. 2020, 192, E913–E918. [Google Scholar] [CrossRef] [PubMed]
- Piris-Villaespesa, M.; Álvarez-Larrán, A.; Saez-Marín, A.; Nuñez-Torrón, C.; Muñoz-Martin, G.; Sánchez, R.; del Castillo, F.J.; Villarrubia, J.; Lopez-Jimenez, J.; Martinez-Lopez, J.; et al. Development and Validation of a Sequential Two-Step Algorithm for the Screening of Individuals with Potential Polycythaemia Vera. Sci. Rep. 2021, 11, 209. [Google Scholar] [CrossRef] [PubMed]
- Bartol, T. Thoughtful use of diagnostic testing: Making practical sense of sensitivity, specificity, and predictive value. Nurse Pract. 2015, 40, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Senecal, J.; Madan, Y.; Rajkumar, S.; Tahir, R.; Crowther, M.A.; Mithoowani, S. Diagnostic Accuracy of Erythropoietin and Jakpot in Predicting JAK2-Positive Erythrocytosis: A Retrospective Cohort Study. Blood 2024, 144, 5017. [Google Scholar] [CrossRef]

| All Patients (n = 213) | JAK2 Positive (n = 40) | JAK2 Negative (n = 173) | p Value (Nominal) | p Value (Corrected *) | |
|---|---|---|---|---|---|
| Age, years (mean [SD]) | 57.7 (15.2) | 66.1 (11.4) | 54 (15.7) | <0.01 | <0.01 |
| Male (N [%]) | 163 (69%) | 22 (55%) | 123 (71%) | 0.08 | 1.0 |
| BMI, kg/m2 (mean [SD]) | 33.0 (7.9) | 28.4 (4.6) | 32.9 (8.5) | <0.01 | 0.09 |
| Weight, kg (mean [SD]) | 91.4 (19.5) | 84.1 (15.1) | 93.2 (20.2) | 0.02 | 0.42 |
| Comorbidities | |||||
| OSA (N [%]) | 45 (21%) | 3 (8%) | 42 (24%) | 0.02 | 0.34 |
| COPD (N [%]) | 18 (8%) | 3 (8%) | 15 (9%) | 1.00 | 1.0 |
| Current smoking (N [%]) | 65 (30%) | 4 (10%) | 61 (35%) | <0.01 | 0.03 |
| Prior history of smoking (N [%]) | 52 (23%) | 10 (25%) | 42 (24%) | 1.0 | 1.0 |
| Any VTE (N [%]) | 17 (8%) | 6 (16%) | 11 (6%) | 0.10 | 1.0 |
| Acute coronary syndrome (N [%]) | 16 (8%) | 4 (10%) | 12 (7%) | 0.51 | 1.0 |
| Ischemic stroke (N [%]) | 5 (2%) | 2 (5%) | 3 (2%) | 0.24 | 1.0 |
| Peripheral arterial disease (N [%]) | 3 (1%) | 1 (3%) | 2 (1%) | 0.47 | 1.0 |
| Chronic kidney disease (N [%]) | 3 (1%) | 1 (3%) | 2 (1%) | 1.00 | 1.0 |
| Cancer (N [%]) ** | 1 (0.5%) | 1 (3%) | 0 (0%) | 0.19 | 1.0 |
| Medications | |||||
| Any diuretic (N [%]) | 42 (20%) | 4 (10%) | 38 (22%) | 0.12 | 1.0 |
| SGLT2 Inhibitor (N [%]) | 10 (5%) | 0 (0%) | 10 (6%) | 0.21 | 1.0 |
| Testosterone (N [%]) | 21 (9%) | 1 (2.5%) | 20 (12%) | 0.14 | 1.0 |
| Any antiplatelet (N [%]) | 57 (27%) | 14 (35%) | 43 (25%) | 0.23 | 1.0 |
| Any anticoagulant (N [%]) | 23 (11%) | 6 (15%) | 17 (10%) | 0.40 | 1.0 |
| Laboratory parameters | |||||
| EPO, mU/mL (mean [SD]), reference interval 3.8–16.9 | 10.7 (11.1) | 4.4 (1.4) | 12.3 (12.3) | <0.01 | <0.01 |
| Hemoglobin, g/L (mean [SD]), reference interval: 115–180 | 174 (16.3) | 179 (20.1) | 172 (14.7) | 0.05 | 0.83 |
| Hematocrit, L/L (mean [SD]), reference interval 0.37–0.54 | 0.52 (0.05) | 0.56 (0.06) | 0.51 (0.05) | <0.01 | <0.01 |
| Absolute red blood cells, 109 cells/L (mean [SD]), reference interval 3.8–6.5 | 5.8 (0.74) | 6.4 (0.98) | 5.7 (0.60) | <0.01 | <0.01 |
| Platelets, 109 cells/L (mean [SD]), reference interval 150–400 | 279.1 (158.1) | 461.0 (217.4) | 236.9 (103.0) | <0.01 | <0.01 |
| Absolute neutrophils, 109 cells/L (mean [SD]), reference interval 2.0–7.5 | 6.0 (4.5) | 8.5 (8.0) | 5.5 (2.9) | 0.02 | 0.42 |
| Ferritin, μg/L (mean [SD]), reference interval 30–100 | 151.3 (181.7) | 63.1 (75.9) | 170.6 (192.2) | <0.01 | <0.01 |
| Imaging studies | |||||
| Spleen size, cm (mean [SD]) 71 patients | 12.0 (2.5) | 13.7 (3.0) | 11.4 (2.1) | 0.01 | 0.19 |
| Liver size, cm (mean [SD]) 71 patients | 16.7 (2.3) | 16.1 (2.9) | 16.9 (2.2) | 0.37 | 1.0 |
| Number of Patients (N [%]) | JAK2+ (N [%]) | Sensitivity (95% CI) | Specificity (95% CI) | Negative Likelihood Ratio (95% CI) | Positive Likelihood Ratio (95% CI) | |
| EPO | ||||||
| Low (<3.8 mU/mL) | 35 (16%) | 31 (88%) | 0.77 (0.62–0.87) | 0.98 (0.94–0.99) | 0.23 (0.13–0.41) | 33 (12.5–89.6) |
| Normal (3.8–16.9 mU/mL) | 150 (70%) | 9 (6%) | ||||
| High (>16.9 mU/mL) | 28 (13%) | 0 (0%) | ||||
| JAKPOT * | ||||||
| Positive | 95 (45%) | 35 (36%) | 0.88 (0.73–0.94) | 0.65 (0.57–0.72) | 0.19 (0.08–0.44) | 2.5 (2.0–3.2) |
| Negative | 118 (55%) | 5 (4%) | ||||
| EPO-JAKPOT ** | ||||||
| Positive | 98 (46%) | 38 (39%) | 0.95 (0.83–0.98) | 0.66 (0.58–0.72) | 0.07 (0.02–0.30) | 2.7 (2.2–3.4) |
| Negative | 115 (54%) | 2 (2%) | ||||
| Odds for JAK2-Positive Erythrocytosis (95% CI) | p Value | |
|---|---|---|
| Age (years) | 1.10 (1.04–1.16) | <0.01 |
| Male sex | 1.12 (0.28–4.37) | 0.88 |
| EPO (mU/mL) | 0.50 (0.32–0.76) | <0.01 |
| ANC (109 cells/L) | 1.06 (0.84–1.33) | 0.63 |
| RBC (109 cells/L) | 1.69 (0.61–4.63) | 0.31 |
| Platelets (109 cells/L) | 1.01 (1.00–1.01) | <0.01 |
| Ferritin (μg/L) * | 0.99 (0.98–1.00) | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senecal, J.B.; Madan, Y.; Tahir, R.; Rajkumar, S.; Lim, W.; Crowther, M.; Mithoowani, S. External Validation of the JAKPOT Score for Diagnosing JAK2-Positive Erythrocytosis: A Retrospective Cohort Study. J. Clin. Med. 2025, 14, 5173. https://doi.org/10.3390/jcm14155173
Senecal JB, Madan Y, Tahir R, Rajkumar S, Lim W, Crowther M, Mithoowani S. External Validation of the JAKPOT Score for Diagnosing JAK2-Positive Erythrocytosis: A Retrospective Cohort Study. Journal of Clinical Medicine. 2025; 14(15):5173. https://doi.org/10.3390/jcm14155173
Chicago/Turabian StyleSenecal, Justin Bruni, Yasmine Madan, Rabia Tahir, Sabina Rajkumar, Wendy Lim, Mark Crowther, and Siraj Mithoowani. 2025. "External Validation of the JAKPOT Score for Diagnosing JAK2-Positive Erythrocytosis: A Retrospective Cohort Study" Journal of Clinical Medicine 14, no. 15: 5173. https://doi.org/10.3390/jcm14155173
APA StyleSenecal, J. B., Madan, Y., Tahir, R., Rajkumar, S., Lim, W., Crowther, M., & Mithoowani, S. (2025). External Validation of the JAKPOT Score for Diagnosing JAK2-Positive Erythrocytosis: A Retrospective Cohort Study. Journal of Clinical Medicine, 14(15), 5173. https://doi.org/10.3390/jcm14155173






