Impact of Tumor Necrosis Factor Antagonist Therapy on Circulating Angiopoietin-like Protein 8 (ANGPTL8) Levels in Crohn’s Disease—A Prospective Multi-Center Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
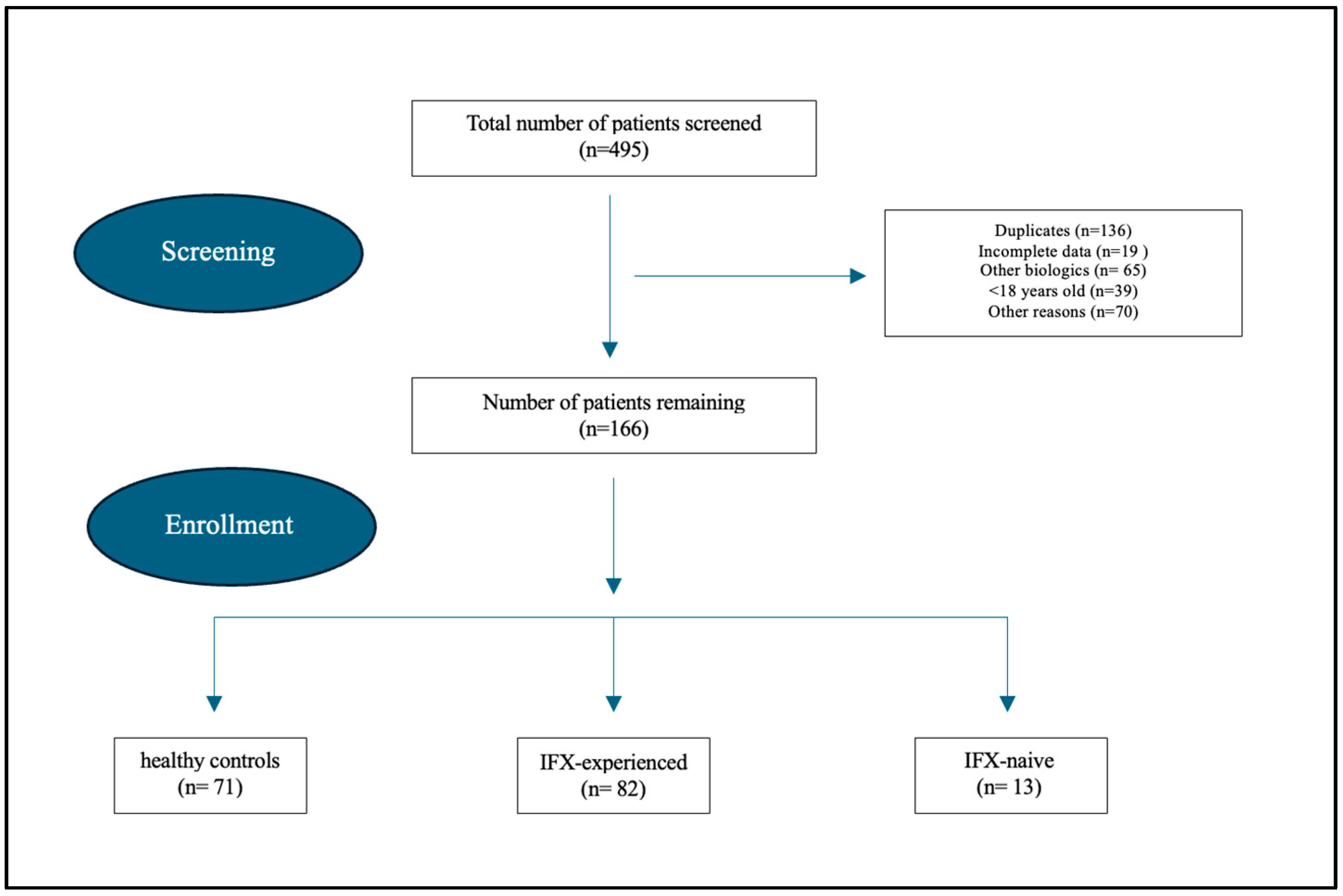
2.2. Study Population and Outcome Measures
2.3. Blood Processing
2.4. Measurement of Circulating Levels of ANGPTL8 by ELISA
2.5. Statistical Analysis
3. Results
3.1. Study Demographics
3.2. Main Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANGPTL8 | Angiopoietin-like protein 8 |
| Anti-TNFα | Tumor necrosis factor antagonist |
| CD | Crohn’s disease |
| CRP | C-reactive protein |
| IFX | Infliximab |
References
- Muzammil, M.A.; Fariha, F.; Patel, T.; Sohail, R.; Kumar, M.; Khan, E.; Khanam, B.; Kumar, S.; Khatri, M.; Varrassi, G.; et al. Advancements in Inflammatory Bowel Disease: A Narrative Review of Diagnostics, Management, Epidemiology, Prevalence, Patient Outcomes, Quality of Life, and Clinical Presentation. Cureus 2023, 15, e41120. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Cheifetz, A.S. Crohn Disease: Epidemiology, Diagnosis, and Management. Mayo Clin. Proc. 2017, 92, 1088–1103. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004, 126, 1504–1517. [Google Scholar] [CrossRef]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Hussain, F.; Yánez, V.V.; Yáne, M.D.; Dave, R.; Milena, R.; Al Hooti, J.; Ekasi, S.; Vargas, R.E.; Pamugo, C.; Moisés, J.; et al. Advances in the Diagnosis and Management of Inflammatory Bowel Disease (IBD). Adv. Res. Gastroenterol. Hepatol. 2024, 20, 556045. [Google Scholar]
- Ngo, B.; Farrell, C.P.; Barr, M.; Wolov, K.; Bailey, R.; Mullin, J.M.; Thornton, J.J. Tumor necrosis factor blockade for treatment of inflammatory bowel disease: Efficacy and safety. Curr. Mol. Pharmacol. 2010, 3, 145–152. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L. Anti-TNF therapy in inflammatory bowel diseases: A huge review. Minerva Gastroenterol. Dietol. 2010, 56, 233–243. [Google Scholar]
- Abu-Farha, M.; Madhu, D.; Hebbar, P.; Mohammad, A.; Channanath, A.; Kavalakatt, S.; Alam-Eldin, N.; Alterki, F.; Taher, I.; Alsmadi, O.; et al. The Proinflammatory Role of ANGPTL8 R59W Variant in Modulating Inflammation through NF-κB Signaling Pathway under TNFα Stimulation. Cells 2023, 12, 2563. [Google Scholar] [CrossRef]
- Li, Y.; Teng, C. Angiopoietin-like proteins 3, 4 and 8: Regulating lipid metabolism and providing new hope for metabolic syndrome. J. Drug Target. 2014, 22, 679–687. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Ghosh, A.; Al-Khairi, I.; Madiraju, S.M.; Abubaker, J.; Prentki, M. The multi-faces of Angptl8 in health and disease: Novel functions beyond lipoprotein lipase modulation. Prog. Lipid Res. 2020, 80, 101067. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Abubaker, J.; Tuomilehto, J. ANGPTL8 (betatrophin) role in diabetes and metabolic diseases. Diabetes Metab. Res. Rev. 2017, 33, e2919. [Google Scholar] [CrossRef] [PubMed]
- Onalan, E.; Bozkurt, A.; Gursu, M.F.; Yakar, B.; Donder, E. Role of Betatrophin and Inflammation Markers in Type 2 Diabetes Mellitus, Prediabetes and Metabolic Syndrome. J. Coll. Physicians Surg. Pak. 2022, 32, 303–307. [Google Scholar] [PubMed]
- Kersten, S. New insights into angiopoietin-like proteins in lipid metabolism and cardiovascular disease risk. Curr. Opin. Lipidol. 2019, 30, 205–211. [Google Scholar] [CrossRef]
- Wang, Y.; Quagliarini, F.; Gusarova, V.; Gromada, J.; Valenzuela, D.M.; Cohen, J.C.; Hobbs, H.H. Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc. Natl. Acad. Sci. USA 2013, 110, 16109–16114. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Cuschieri, S. The STROBE guidelines. Saudi J. Anaesth. 2019, 13 (Suppl. S1), S31. [Google Scholar] [CrossRef]
- Daperno, M.; D’Haens, G.; Van Assche, G.; Baert, F.; Bulois, P.; Maunoury, V.; Sostegni, R.; Rocca, R.; Pera, A.; Gevers, A.; et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: The SES-CD. Gastrointest. Endosc. 2004, 60, 505–512. [Google Scholar] [CrossRef]
- Sylvers-Davie, K.L.; Davies, B.S.J. Regulation of lipoprotein metabolism by ANGPTL3, ANGPTL4, and ANGPTL8. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E493–E508. [Google Scholar] [CrossRef]
- Guo, Q.; Cao, S.; Wang, X. Betatrophin and Insulin Resistance. Metabolites 2022, 12, 925. [Google Scholar] [CrossRef]
- Pan, J.; Wang, X.; Zhang, Y.; Wang, T.; Lv, S.; Zhang, X.; Zhou, Y.; Peng, T.; Song, Y. Associations Between APOC3 and ANGPTL8 Gene Polymorphisms With MASLD Risk and the Mediation Effect of Triglyceride on MASLD in the Chinese Population. J. Cell. Mol. Med. 2025, 29, e70542. [Google Scholar] [CrossRef]
- Abdelhameed, F.; Lagojda, L.; Kite, C.; Dallaway, A.; Mustafa, A.; Ni Than, N.; Kassi, E.; Randeva, H.S.; Kyrou, I. Circulating angiopoietin-like protein 8 (ANGPTL8) and steatotic liver disease related to metabolic dysfunction: An updated systematic review and meta-analysis. Front. Endocrinol. 2025, 16, 1574842. [Google Scholar] [CrossRef] [PubMed]
- Mohammedsaeed, W.; Binjawhar, D. Dyslipidemia and ANGPTL8 evaluation in young females with Type 1 diabetes mellitus. Endocrine 2024, 86, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Ding, X.; Peng, L.; Hou, Y.; Ling, Y.; Gu, M.; Wang, Y.; Peng, Y.; Sun, H. Increased Serum ANGPTL8 Concentrations in Patients with Prediabetes and Type 2 Diabetes. J. Diabetes Res. 2017, 2017, 8293207. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Sun, W.; Yu, S.; Hong, X.; Qian, W.; Tang, B.; Wang, D.; Yang, L.; Wang, J.; Mao, C.; et al. Increased circulating levels of betatrophin in newly diagnosed type 2 diabetic patients. Diabetes Care 2014, 37, 2718–2722. [Google Scholar] [CrossRef]
- Chen, X.; Lu, P.; He, W.; Zhang, J.; Liu, L.; Yang, Y.; Liu, Z.; Xie, J.; Shao, S.; Du, T.; et al. Circulating betatrophin levels are increased in patients with type 2 diabetes and associated with insulin resistance. J. Clin. Endocrinol. Metab. 2015, 100, E96–E100. [Google Scholar] [CrossRef]
- Ke, Y.; Liu, S.; Zhang, Z.; Hu, J. Circulating angiopoietin-like proteins in metabolic-associated fatty liver disease: A systematic review and meta-analysis. Lipids Health Dis. 2021, 20, 55. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Sriraman, D.; Cherian, P.; AlKhairi, I.; Elkum, N.; Behbehani, K.; Abubaker, J. Circulating ANGPTL8/Betatrophin Is Increased in Obesity and Reduced after Exercise Training. PLoS ONE 2016, 11, e0147367. [Google Scholar] [CrossRef]
- Pu, D.; Li, L.; Yin, J.; Liu, R.; Yang, G.; Liao, Y.; Wu, Q. Circulating ANGPTL8 Is Associated with the Presence of Metabolic Syndrome and Insulin Resistance in Polycystic Ovary Syndrome Young Women. Mediat. Inflamm. 2019, 2019, 6321427. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Q.; Kang, C.; Cui, W.; Xu, Z.; Zhong, F.; Gao, X. Serum and urine ANGPTL8 expression levels are associated with hyperlipidemia and proteinuria in primary nephrotic syndrome. BMC Nephrol. 2021, 22, 130. [Google Scholar] [CrossRef]
- AlMajed, H.T.; Abu-Farha, M.; Alshawaf, E.; Devarajan, S.; Alsairafi, Z.; Elhelaly, A.; Cherian, P.; Al-Khairi, I.; Ali, H.; Jose, R.M.; et al. Increased Levels of Circulating IGFBP4 and ANGPTL8 with a Prospective Role in Diabetic Nephropathy. Int. J. Mol. Sci. 2023, 24, 14244. [Google Scholar] [CrossRef]
- Fadaei, R.; Shateri, H.; DiStefano, J.K.; Moradi, N.; Mohammadi, M.; Emami, F.; Aghajani, H.; Ziamajidi, N. Higher circulating levels of ANGPTL8 are associated with body mass index, triglycerides, and endothelial dysfunction in patients with coronary artery disease. Mol. Cell. Biochem. 2020, 469, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Thorin, E.; Labbé, P.; Lambert, M.; Mury, P.; Dagher, O.; Miquel, G.; Thorin-Trescases, N. Angiopoietin-Like Proteins: Cardiovascular Biology and Therapeutic Targeting for the Prevention of Cardiovascular Diseases. Can. J. Cardiol. 2023, 39, 1736–1756. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, J.; Kashiwabara, K.; Torigoe, D.; Okadome, Y.; Aizawa, K.; Uemura, K.; Kurashima, A.; Matsunaga, E.; Fukami, H.; Horiguchi, H.; et al. Plasma ANGPTL8 Levels and Risk for Secondary Cardiovascular Events in Japanese Patients With Stable Coronary Artery Disease Receiving Statin Therapy. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; He, J.; Yang, Y.; Yang, S.; Li, J.; Qin, Y. Associations between circulating full-length angiopoietin-like protein 8 levels and severity of coronary artery disease in Chinese non-diabetic patients: A case–control study. Cardiovasc. Diabetol. 2018, 17, 92. [Google Scholar] [CrossRef]
- Hu, L.; Wei, J.; Zhang, Y.; Wang, Z.; Tang, J.; Tang, J.; Gao, Y.; Zhang, X.; Li, Y.; Liu, Y.; et al. ANGPTL8 is a negative regulator in pathological cardiac hypertrophy. Cell Death Dis. 2022, 13, 621. [Google Scholar] [CrossRef]
- Jiao, X.; Yu, H.; Du, Z.; Li, L.; Hu, C.; Du, Y.; Zhang, J.; Zhang, X.; Lv, Q.; Li, F.; et al. Vascular smooth muscle cells specific deletion of angiopoietin-like protein 8 prevents angiotensin II-promoted hypertension and cardiovascular hypertrophy. Cardiovasc. Res. 2023, 119, 1856–1868. [Google Scholar] [CrossRef]
- Dang, F.; Wu, R.; Wang, P.; Wu, Y.; Azam, S.; Xu, Q.; Chen, Y.; Liu, Y. Fasting and Feeding Signals Control the Oscillatory Expression of Angptl8 to Modulate Lipid Metabolism. Sci. Rep. 2016, 6, 36926. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, X.; Yan, W.; Chen, Y.; Ke, M.; Cheng, C.; Zhu, X.; Xue, W.; Zhou, Q.; Zheng, L.; et al. ANGPTL8 negatively regulates NF-κB activation by facilitating selective autophagic degradation of IKKγ. Nat. Commun. 2017, 8, 2164. [Google Scholar] [CrossRef]
- Li, D.P.; Huang, L.; Kan, R.R.; Meng, X.Y.; Wang, S.Y.; Zou, H.J.; Guo, Y.M.; Luo, P.Q.; Pan, L.M.; Xiang, Y.X.; et al. LILRB2/PirB mediates macrophage recruitment in fibrogenesis of nonalcoholic steatohepatitis. Nat. Commun. 2023, 14, 4436. [Google Scholar] [CrossRef]
- Vatner, D.F.; Goedeke, L.; Camporez, J.G.; Lyu, K.; Nasiri, A.R.; Zhang, D.; Bhanot, S.; Murray, S.F.; Still, C.D.; Gerhard, G.S.; et al. Angptl8 antisense oligonucleotide improves adipose lipid metabolism and prevents diet-induced NAFLD and hepatic insulin resistance in rodents. Diabetologia 2018, 61, 1435–1446. [Google Scholar] [CrossRef]
- Paschou, S.A.; Kothonas, F.; Lafkas, A.; Myroforidis, A.; Loi, V.; Terzi, T.; Karagianni, O.; Poulou, A.; Goumas, K.; Vryonidou, A. Favorable Effect of Anti-TNF Therapy on Insulin Sensitivity in Nonobese, Nondiabetic Patients with Inflammatory Bowel Disease. Int. J. Endocrinol. 2018, 2018, 6712901. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Peng, D. ANGPTL8: An Important Regulator in Metabolic Disorders. Front. Endocrinol. 2018, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Abu-Farha, M.; Abubaker, J.; Noronha, F.; Al-Khairi, I.; Cherian, P.; Alarouj, M.; Bennakhi, A.; Elkum, N. Lack of associations between betatrophin/ANGPTL8 level and C-peptide in type 2 diabetic subjects. Cardiovasc. Diabetol. 2015, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Saghafi, S.; Chamani, E.; Salmani, F.; Fadaei, R.; Shafiei, E.; Moradi, N.; Tavakoli, T. Genetic predisposition to nonalcoholic fatty liver disease: Insights from ANGPTL8 gene variants in Iranian adults. Lipids Health Dis. 2023, 22, 147. [Google Scholar] [CrossRef]


| Variables | IFX-Experienced (n = 82) | IFX-Naïve Week 0 | Healthy | ǂ p-Value | Multiple Comparisons with Post Hoc Bonferroni (p-Value) | ||
|---|---|---|---|---|---|---|---|
| (n = 13) | (n = 71) | IFX-Experienced vs. IFX-Naïve | IFX-Experienced vs. Healthy | IFX-Naïve vs. Healthy | |||
| Age, mean ± SEM | 29.76 ± 1.4 | 27.92 ± 3.82 | 33.66 ± 1.55 | 0.113 | 0.104 | 0.051 | 0.063 |
| BMI | 24.22 ± 0.60 | 24.06 ± 1.31 | 29.53 ± 0.83 | <0.001 | 1.000 | <0.001 | <0.001 |
| Male | 51 (62.2%) | 9 (69.2%) | 22 (31.0%) | <0.001 | |||
| Female | 31 (37.8%) | 4 (30.8%) | 49 (69.0%) | ||||
| Smoking | 23 (28.0%) | 4 (30.8%) | 13 (18.3%) | 0.308 | |||
| CDAI score, mean ± SD | 201 ± 11 | 441 ± 12 | 138 ± 7 | <0.001 | <0.001 | <0.001 | <0.001 |
| Fecal Cal, μg/mg | 121 | 493 | 49 | ||||
| CRP, mg/L | 12.3 | 31 | 7 | ||||
| Albumin, g/L | 49 | 55 | 33 | ||||
| Azathioprine | 26 (31.7%) | 10 (76.9%) | 0 (0.0) | ||||
| Methotrexate | 4 (4.9%) | 0 (0.0) | 0 (0.0) | ||||
| Corticosteroids | 5 (6.1%) | 5 (38.5%) | 0 (0.0) | ||||
| ANGPTL8, pmol | 135.8 ± 7.5 | 168.8 ± 10.9 | 102.5 ± 6.0 | <0.001 | <0.001 | <0.001 | <0.001 |
| Predictor | Beta | SE | Wald χ² | p-Value | 95% CI for Beta |
|---|---|---|---|---|---|
| Intercept | 4.824 | 0.064 | 27.88 | <0.001 | [3.699–4.949] |
| Age | 0.032 | 0.001 | 2.039 | 0.153 | [0.020–0.047] |
| BMI | 0.024 | 0.002 | 5.010 | 0.025 * | [0.011–0.043] |
| Gender (Male vs. Female) | −0.173 | 0.020 | 9.600 | <0.001 * | [−0.212–−0.135] |
| Use of Corticosteroid (Yes vs. No) | −0.229 | 0.040 | 6.924 | <0.001 * | [−0.308–−0.150] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shehab, M.; Al-Fajri, S.; Alanqar, A.; Alborom, M.; Alrashed, F.; Alshammaa, F.; Alfadhli, A.; Devarajan, S.; Alkhairi, I.; Cherian, P.; et al. Impact of Tumor Necrosis Factor Antagonist Therapy on Circulating Angiopoietin-like Protein 8 (ANGPTL8) Levels in Crohn’s Disease—A Prospective Multi-Center Study. J. Clin. Med. 2025, 14, 5006. https://doi.org/10.3390/jcm14145006
Shehab M, Al-Fajri S, Alanqar A, Alborom M, Alrashed F, Alshammaa F, Alfadhli A, Devarajan S, Alkhairi I, Cherian P, et al. Impact of Tumor Necrosis Factor Antagonist Therapy on Circulating Angiopoietin-like Protein 8 (ANGPTL8) Levels in Crohn’s Disease—A Prospective Multi-Center Study. Journal of Clinical Medicine. 2025; 14(14):5006. https://doi.org/10.3390/jcm14145006
Chicago/Turabian StyleShehab, Mohammad, Sharifa Al-Fajri, Ahmed Alanqar, Mohammad Alborom, Fatema Alrashed, Fatemah Alshammaa, Ahmad Alfadhli, Sriraman Devarajan, Irina Alkhairi, Preethi Cherian, and et al. 2025. "Impact of Tumor Necrosis Factor Antagonist Therapy on Circulating Angiopoietin-like Protein 8 (ANGPTL8) Levels in Crohn’s Disease—A Prospective Multi-Center Study" Journal of Clinical Medicine 14, no. 14: 5006. https://doi.org/10.3390/jcm14145006
APA StyleShehab, M., Al-Fajri, S., Alanqar, A., Alborom, M., Alrashed, F., Alshammaa, F., Alfadhli, A., Devarajan, S., Alkhairi, I., Cherian, P., Abubaker, J., Abu-Farha, M., & Al-Mulla, F. (2025). Impact of Tumor Necrosis Factor Antagonist Therapy on Circulating Angiopoietin-like Protein 8 (ANGPTL8) Levels in Crohn’s Disease—A Prospective Multi-Center Study. Journal of Clinical Medicine, 14(14), 5006. https://doi.org/10.3390/jcm14145006








