The Pre-Catching Sperm Technique Increases the Efficiency of the Intracytoplasmic Sperm Injection Method by Improving Fertilization and Blastocyst Formation Rates
Abstract
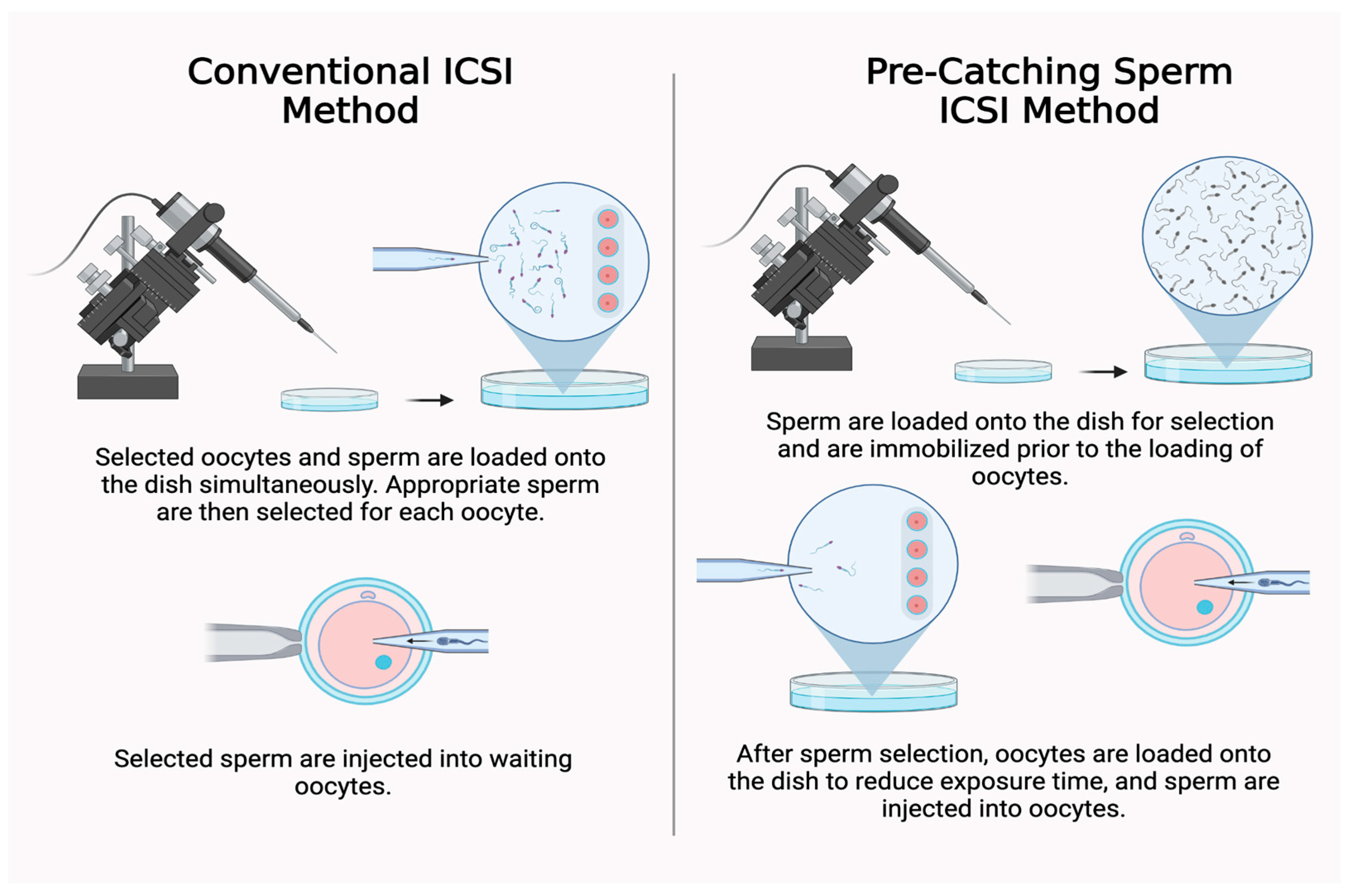
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, D.; Nguyen, Q.N.; Li, R.; Dang, V.Q. Is Intracytoplasmic Sperm Injection the Solution for all in Unexplained Infertility? Semin. Reprod. Med. 2020, 38, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.D.; O’neill, C.L.; Chow, S.; Cheung, S.; Parrella, A.; Pereira, N.; Rosenwaks, Z. Intracytoplasmic sperm injection: State of the art in humans. Reprod. Camb. Engl. 2017, 154, F93–F110. [Google Scholar] [CrossRef]
- Korakaki, D.; Mouroutsos, S.; Tripsianis, G.; Nikolettos, N.; Asimakopoulos, B. Temperature Decline in Embryological Culture Dishes outside Incubator. Int. J. Fertil. Steril. 2020, 14, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Lavi, R.; Ankri, R.; Sinyakov, M.; Eichler, M.; Friedmann, H.; Shainberg, A.; Breitbart, H.; Lubart, R. The plasma membrane is involved in the visible light-tissue interaction. Photomed. Laser Surg. 2012, 30, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Gong, S.P.; Lee, S.T.; Lee, E.J.; Lim, J.M. Light intensity and wavelength during embryo manipulation are important factors for maintaining viability of preimplantation embryos in vitro. Fertil Steril. 2007, 88 (Suppl. S4), 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A.M.; Cissen, M.; Brandes, M.; Smeenk, J.M.J.; de Bruin, J.P.; Kremer, J.A.M.; Nelen, W.L.D.M.; Hamilton, C.J.C.M. Total motile sperm count: A better indicator for the severity of male factor infertility than the WHO sperm classification system. Hum. Reprod. 2015, 30, 1110–1121. [Google Scholar] [PubMed]
- Liu, L.; Jiang, X.; Liu, Z.; Chen, J.; Yang, C.; Chen, K.; Yang, X.; Cai, J.; Ren, J. Oocyte degeneration in a cohort adversely affects clinical outcomes in conventional IVF cycles: A propensity score matching study. Front. Endocrinol. 2023, 14, 1164371. [Google Scholar] [CrossRef]
- Crean, A.J.; Immler, S. Evolutionary consequences of environmental effects on gamete performance. Philos. Trans. R Soc. Lond. B Biol. Sci. 2021, 376, 20200122. [Google Scholar] [CrossRef]
- Gardner, D.K.; Schoolcraft, W.B. In vitro culture of human blastocysts. Towards Reprod. Certain. Fertil. Genet. Beyond 1999, 1999, 378–388. [Google Scholar]
- Hu, X.; Liu, Y.; Zhang, X.; Lee, P.; Wen, Y.; Ding, C.; Zhou, C.; Xu, Y. Oocyte Degeneration After ICSI Is Not an Indicator of Live Birth in Young Women. Front. Endocrinol. 2021, 12, 705733. [Google Scholar] [CrossRef]
- Xie, P.; Cheung, S.; Kocur, O.; Ng, L.; De Jesus, A.; Rosenwaks, Z.; Palermo, G.D.; Aitken, R.J.; Schlegel, P.N. Intracytoplasmic sperm injection is still the best management of male factor infertility. Fertil. Steril. 2024, 121, 563–575. [Google Scholar] [CrossRef] [PubMed]
- The Practice Committees of the American Society for Reproductive Medicine and Society for Assisted Reproductive Technology. Practice Committee of American Society for Reproductive Medicine. Intracytoplasmic sperm injection (ICSI). Fertil. Steril. 2008, 90 (Suppl. S5), S187. [Google Scholar] [CrossRef]
- Bori, L.; Meseguer, F.; Valera, M.A.; Galan, A.; Remohi, J.; Meseguer, M. The higher the score, the better the clinical outcome: Retrospective evaluation of automatic embryo grading as a support tool for embryo selection in IVF laboratories. Hum. Reprod. Oxf. Engl. 2022, 37, 1148–1160. [Google Scholar] [CrossRef]
- Liu, L.; Cai, J.; Li, P.; Jiang, X.; Ren, J. Clinical outcome of cycles with oocyte degeneration after intracytoplasmic sperm injection. Syst. Biol. Reprod. Med. 2017, 63, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Endo, Y.; Mitsuhata, S.; Hayashi, M.; Motoyama, H. Evaluation of the effect of piezo-intracytoplasmic sperm injection on the laboratory, clinical, and neonatal outcomes. Reprod. Med. Biol. 2020, 19, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, K.; Kitamura, S. Clinical efficiency of Piezo-ICSI using micropipettes with a wall thickness of 0.625 μm. J. Assist. Reprod. Genet. 2015, 32, 1827–1833. [Google Scholar] [CrossRef] [PubMed]
- Zander-Fox, D.; Green, M.; Watson, K.; Turner, R.; Bakos, H.W.; Foo, J.; Pacella-Ince, L.; Caddy, M.; McPherson, N.O.; Rombauts, L. Improved fertilization, degeneration, and embryo quality rates with PIEZO–intracytoplasmic sperm injection compared with conventional intracytoplasmic sperm injection: A sibling oocyte split multicenter trial. Fertil. Steril. 2024, 121, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Nakagawa, K. O-184 Piezo-ICSI reduces oocyte degeneration, improves ICSI success rates and has shown less dependence on the experience level of embryologists: Cumulative data with 65,033 MII oocytes. Hum. Reprod. 2024, 39 (Suppl. S1), deae108.217. [Google Scholar] [CrossRef]
- Caddy, M.; Popkiss, S.; Weston, G.; Vollenhoven, B.; Rombauts, L.; Green, M.; Zander-Fox, D. PIEZO-ICSI increases fertilization rates compared with conventional ICSI in patients with poor prognosis. J. Assist. Reprod. Genet. 2023, 40, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Manvelyan, E.; Haering, C.; Coyne, K.; Palavos, L.; Hamrick, J.; Findley, J.; Weinerman, R.; Flyckt, R.; Kim, S.T. Efficiency in intracytoplasmic sperm injection method improves fertilization and blastocyst formation rates. In Proceedings of the Annual Congress of Pacific Coast Reproductive Society, Indian Wells, CA, USA, 20–23 March 2024. [Google Scholar]
| Stimulation Protocol | PCS (n = 330) | Conventional (n = 287) |
|---|---|---|
| Agonist Flare | 52 (15.8%) | 45 (15.7%) |
| Agonist suppression | 40 (12.1%) | 25 (8.7%) |
| Antagonist suppression | 238 (72.1%) | 217 (75.6%) |
| Demographic Variables | PCS (n = 330) | Conventional (n = 287) | p-Value |
|---|---|---|---|
| Age (yr) | 35 [32, 38] | 35 [32, 38] | ns * |
| BMI (kg/m2) * | 27 [23, 33] | 26 [22, 30] | ns * |
| AMH (ng/mL) * | 2.7 [1.6, 4.9] | 3.0 [1.8, 4.6] | ns * |
| Baseline E2 (pg/mL) * | 24 [20, 43] | 15 [0, 29] | 0.002 |
| Peak E2 (pg/mL) * | 2766 [1975, 3897] | 2414 [1703, 3397] | <0.001 |
| PCS (n = 330) | Conventional (n = 287) | p-Value | |
|---|---|---|---|
| No. of retrieved mature oocytes | 3840 | 3387 | ns * |
| Fertilized oocytes (%) | 84.0 | 79.3 | <0.001 |
| Degenerated oocytes (%) | 1.4 | 3.5 | <0.001 |
| Abnormal fertilization (%) | 1.4 | 1.8 | 0.1601 |
| Good-quality blastocyst (%) | 54.9 | 48.0 | <0.001 |
| PCS (n = 434) | Conventional (n = 616) | p-Value | |
|---|---|---|---|
| Average age | 35.4 | 35.6 | ns * |
| Avg. number transferred embryo | 1.1 | 1.2 | ns * |
| Pregnancy rate (%) | 78.8 | 75.8 | 0.2646 |
| Clinical pregnancy (%) | 69.6 | 66.9 | 0.3826 |
| Ongoing/live birth (%) | 57.6 | 55.7 | 0.5695 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haering, C.; Manvelyan, E.; Coyne, K.; Hyams, L.A.; Hamrick, J.; Findley, J.; Weinerman, R.; Flyckt, R.; Kim, S.T. The Pre-Catching Sperm Technique Increases the Efficiency of the Intracytoplasmic Sperm Injection Method by Improving Fertilization and Blastocyst Formation Rates. J. Clin. Med. 2025, 14, 4872. https://doi.org/10.3390/jcm14144872
Haering C, Manvelyan E, Coyne K, Hyams LA, Hamrick J, Findley J, Weinerman R, Flyckt R, Kim ST. The Pre-Catching Sperm Technique Increases the Efficiency of the Intracytoplasmic Sperm Injection Method by Improving Fertilization and Blastocyst Formation Rates. Journal of Clinical Medicine. 2025; 14(14):4872. https://doi.org/10.3390/jcm14144872
Chicago/Turabian StyleHaering, Catherine, Evelina Manvelyan, Kathryn Coyne, Lauren Alexis Hyams, James Hamrick, Joseph Findley, Rachel Weinerman, Rebecca Flyckt, and Sung Tae Kim. 2025. "The Pre-Catching Sperm Technique Increases the Efficiency of the Intracytoplasmic Sperm Injection Method by Improving Fertilization and Blastocyst Formation Rates" Journal of Clinical Medicine 14, no. 14: 4872. https://doi.org/10.3390/jcm14144872
APA StyleHaering, C., Manvelyan, E., Coyne, K., Hyams, L. A., Hamrick, J., Findley, J., Weinerman, R., Flyckt, R., & Kim, S. T. (2025). The Pre-Catching Sperm Technique Increases the Efficiency of the Intracytoplasmic Sperm Injection Method by Improving Fertilization and Blastocyst Formation Rates. Journal of Clinical Medicine, 14(14), 4872. https://doi.org/10.3390/jcm14144872







