Why High-Volume Post-Dilution Hemodiafiltration Should Be the New Standard in Dialysis Care: A Comprehensive Review of Clinical Outcomes and Mechanisms
Abstract
1. Introduction
2. Materials and Methods
3. Short-Term Intermediate Outcomes
3.1. Enhanced Toxin Clearance
3.2. Improved Hemodynamic Stability
3.3. Reduction in Intradialytic Cramps
3.4. Improved Biocompatibility, Reduced Inflammation and Oxidative Stress
3.5. Improved Anemia Management
3.6. Preservation of Residual Kidney Function
3.7. Reduction in Skin Hyperpigmentation
4. Mid-Term Intermediate Outcomes
4.1. β2-Microglobulin Amyloidosis and Joint Symptoms Control
4.2. Improved Nutritional Status
4.3. Reduced Infection Risk
4.4. Cardiovascular Benefits
4.5. Peripheral Neuropathy Improvements
4.6. Cognitive and Quality-of-Life Benefits
5. Long-Term Outcomes
5.1. Randomized Controlled Trials
5.2. Meta-Analyses: Expanding the Case for HVHDF
5.3. Reinforcement from Real World Evidence and Dose–Response Across Observational Studies
6. Economic and Implementation Considerations
7. Conclusions
8. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- United States Renal Data System. 2024 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2024. [Google Scholar]
- Blankestijn, P.J.; Vernooij, R.W.M.; Hockham, C.; Strippoli, G.F.M.; Canaud, B.; Hegbrant, J.; Barth, C.; Covic, A.; Cromm, K.; Cucui, A.; et al. Effect of Hemodiafiltration or Hemodialysis on Mortality in Kidney Failure. N. Engl. J. Med. 2023, 389, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Vernooij, R.W.M.; Hockham, C.; Strippoli, G.; Green, S.; Hegbrant, J.; Davenport, A.; Barth, C.; Canaud, B.; Woodward, M.; Blankestijn, P.J.; et al. Haemodiafiltration versus haemodialysis for kidney failure: An individual patient data meta-analysis of randomised controlled trials. Lancet 2024, 404, 1742–1749. [Google Scholar] [CrossRef]
- Rose, M.; Fischer, F.H.; Liegl, G.; Strippoli, G.F.M.; Hockham, C.; Vernooij, R.W.M.; Barth, C.; Canaud, B.; Covic, A.; Cromm, K.; et al. The CONVINCE randomized trial found positive effects on quality of life for patients with chronic kidney disease treated with hemodiafiltration. Kidney Int. 2024, 106, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Strippoli, G.F.M.; Green, S.C. Actioning the findings of hard endpoint clinical trials as they emerge in the realm of chronic kidney disease care: A review and a call to action. Clin. Kidney J. 2024, 17, sfae035. [Google Scholar] [CrossRef]
- Peters, S.A.; Bots, M.L.; Canaud, B.; Davenport, A.; Grooteman, M.P.; Kircelli, F.; Locatelli, F.; Maduell, F.; Morena, M.; Nubé, M.J.; et al. Haemodiafiltration and mortality in end-stage kidney disease patients: A pooled individual participant data analysis from four randomized controlled trials. Nephrol. Dial. Transpl. 2016, 31, 978–984. [Google Scholar] [CrossRef]
- Battaglia, Y.; Shroff, R.; Meijers, B.; Nistor, I.; Alfano, G.; Franssen, C.; Luyckx, V.; Liakopoulos, V.; Mantovani, A.; Baciga, F.; et al. Haemodiafiltration versus high-flux haemodialysis—A Consensus Statement from the EuDial Working Group of the ERA. Nephrol. Dial. Transpl. 2025, gfaf024. [Google Scholar] [CrossRef]
- Canaud, B.; Blankestijn, P.J.; Grooteman, M.P.C.; Davenport, A. Why and how high volume hemodiafiltration may reduce cardiovascular mortality in stage 5 chronic kidney disease dialysis patients? A comprehensive literature review on mechanisms involved. Semin. Dial. 2022, 35, 117–128. [Google Scholar] [CrossRef]
- Lang, T.; Zawada, A.M.; Theis, L.; Braun, J.; Ottillinger, B.; Kopperschmidt, P.; Gagel, A.; Kotanko, P.; Stauss-Grabo, M.; Kennedy, J.P.; et al. Hemodiafiltration: Technical and Medical Insights. Bioengineering 2023, 10, 145. [Google Scholar] [CrossRef]
- Pedreros-Rosales, C.; Jara, A.; Lorca, E.; Mezzano, S.; Pecoits-Filho, R.; Herrera, P. Unveiling the Clinical Benefits of High-Volume Hemodiafiltration: Optimizing the Removal of Medium-Weight Uremic Toxins and Beyond. Toxins 2023, 15, 531. [Google Scholar] [CrossRef]
- Canaud, B.; Strippoli, G.; Davenport, A. High-Volume Hemodiafiltration Versus High-Flux Hemodialysis: A Narrative Review for the Clinician. J. Clin. Med. 2025, 14, 2614. [Google Scholar] [CrossRef]
- Stuard, S.; Maddux, F.W. High-Volume Hemodiafiltration: Expanding the Evidence Beyond Randomized Trials—A Critical Perspective on the 2025 EuDial Consensus. J. Clin. Med. 2025, 14, 3174. [Google Scholar] [PubMed]
- Schiffl, H. High-volume online haemodiafiltration treatment and outcome of end-stage renal disease patients: More than one mode. Int. Urol. Nephrol. 2020, 52, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C. Hemodiafiltration: Technical and Clinical Issues. Blood Purif. 2015, 40 (Suppl. S1), 2–11. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Hamano, T.; Wada, A.; Nakai, S.; Masakane, I. Predilution online hemodiafiltration is associated with improved survival compared with hemodialysis. Kidney Int. 2019, 95, 929–938. [Google Scholar] [CrossRef]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef]
- Neirynck, N.; Vanholder, R.; Schepers, E.; Eloot, S.; Pletinck, A.; Glorieux, G. An update on uremic toxins. Int. Urol. Nephrol. 2013, 45, 139–150. [Google Scholar] [CrossRef]
- Rosner, M.H.; Reis, T.; Husain-Syed, F.; Vanholder, R.; Hutchison, C.; Stenvinkel, P.; Blankestijn, P.J.; Cozzolino, M.; Juillard, L.; Kashani, K.; et al. Classification of Uremic Toxins and Their Role in Kidney Failure. Clin. J. Am. Soc. Nephrol. 2021, 16, 1918–1928. [Google Scholar] [CrossRef]
- Canaud, B.; Bosc, J.Y.; Leblanc, M.; Garred, L.J.; Vo, T.; Mion, C. Evaluation of high-flux hemodiafiltration efficiency using an on-line urea monitor. Am. J. Kidney Dis. 1998, 31, 74–80. [Google Scholar] [CrossRef]
- Canaud, B.; Bragg-Gresham, J.L.; Marshall, M.R.; Desmeules, S.; Gillespie, B.W.; Depner, T.; Klassen, P.; Port, F.K. Mortality risk for patients receiving hemodiafiltration versus hemodialysis: European results from the DOPPS. Kidney Int. 2006, 69, 2087–2093. [Google Scholar] [CrossRef]
- Pedrini, L.A.; De Cristofaro, V.; Comelli, M.; Casino, F.G.; Prencipe, M.; Baroni, A.; Campolo, G.; Manzoni, C.; Coli, L.; Ruggiero, P.; et al. Long-term effects of high-efficiency on-line haemodiafiltration on uraemic toxicity. A multicentre prospective randomized study. Nephrol. Dial. Transpl. 2011, 26, 2617–2624. [Google Scholar] [CrossRef]
- Shinzato, T.; Kobayakawa, H.; Maeda, K. Comparison of various treatment modes in terms of beta 2-microglobulin removal: Hemodialysis, hemofiltration, and push/pull HDF. Artif. Organs 1989, 13, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; Meert, N.; Schepers, E.; Glorieux, G. From uremic toxin retention to removal by convection: Do we know enough? Contrib. Nephrol. 2008, 161, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Ferramosca, E.; Mancini, E.; Monari, C.; Varasani, M.; Sereni, L.; Wratten, M. Reverse mid-dilution: New way to remove small and middle molecules as well as phosphate with high intrafilter convective clearance. Nephrol. Dial. Transpl. 2007, 22, 2000–2005. [Google Scholar] [CrossRef]
- Canaud, B.; Gagel, A.; Peters, A.; Maierhofer, A.; Stuard, S. Does online high-volume hemodiafiltration offer greater efficiency and sustainability compared with high-flux hemodialysis? A detailed simulation analysis anchored in real-world data. Clin. Kidney J. 2024, 17, sfae147. [Google Scholar] [CrossRef]
- Canaud, B. The early years of on-line HDF: How did it all start? How did we get here? Contrib. Nephrol. 2011, 175, 93–109. [Google Scholar] [CrossRef]
- Lornoy, W.; De Meester, J.; Becaus, I.; Billiouw, J.M.; Van Malderen, P.A.; Van Pottelberge, M. Impact of convective flow on phosphorus removal in maintenance hemodialysis patients. J. Ren. Nutr. 2006, 16, 47–53. [Google Scholar] [CrossRef]
- Zehnder, C.; Gutzwiller, J.P.; Renggli, K. Hemodiafiltration—A new treatment option for hyperphosphatemia in hemodialysis patients. Clin. Nephrol. 1999, 52, 152–159. [Google Scholar]
- Penne, E.L.; van der Weerd, N.C.; van den Dorpel, M.A.; Grooteman, M.P.; Levesque, R.; Nube, M.J.; Bots, M.L.; Blankestijn, P.J.; ter Wee, P.M.; Investigators, C. Short-term effects of online hemodiafiltration on phosphate control: A result from the randomized controlled Convective Transport Study (CONTRAST). Am. J. Kidney Dis. 2010, 55, 77–87. [Google Scholar] [CrossRef]
- Davenport, A.; Gardner, C.; Delaney, M.; Pan Thames Renal Audit, G. The effect of dialysis modality on phosphate control: Haemodialysis compared to haemodiafiltration. The Pan Thames Renal Audit. Nephrol. Dial. Transpl. 2010, 25, 897–901. [Google Scholar] [CrossRef]
- Daugirdas, J.T. Comparison of measured vs. kinetic-model predicted phosphate removal during hemodialysis and hemodiafiltration. Nephrol. Dial. Transpl. 2022, 37, 2522–2527. [Google Scholar] [CrossRef]
- Movilli, E.; Camerini, C.; Gaggia, P.; Poiatti, P.; Pola, A.; Viola, B.F.; Zubani, R.; Jeannin, G.; Cancarini, G. Effect of post-dilutional on-line haemodiafiltration on serum calcium, phosphate and parathyroid hormone concentrations in uraemic patients. Nephrol. Dial. Transpl. 2011, 26, 4032–4037. [Google Scholar] [CrossRef]
- Ok, E.; Asci, G.; Toz, H.; Ok, E.S.; Kircelli, F.; Yilmaz, M.; Hur, E.; Demirci, M.S.; Demirci, C.; Duman, S.; et al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: Results from the Turkish OL-HDF Study. Nephrol. Dial. Transpl. 2013, 28, 192–202. [Google Scholar] [CrossRef]
- Maduell, F.; Moreso, F.; Pons, M.; Ramos, R.; Mora-Macia, J.; Carreras, J.; Soler, J.; Torres, F.; Campistol, J.M.; Martinez-Castelao, A.; et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J. Am. Soc. Nephrol. 2013, 24, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.J.; Lee, J.E.; Kim, J.K.; Yoon, S.Y.; Kang, S.W.; Choi, K.H.; Ha, S.K.; Park, H.C. The relationship between hemodialysis modality and insulin resistance in non-diabetic hemodialysis patients. Blood Purif. 2015, 39, 224–229. [Google Scholar] [CrossRef]
- Chen, H.; Han, X.; Cui, Y.; Ye, Y.; Purrunsing, Y.; Wang, N. Parathyroid Hormone Fragments: New Targets for the Diagnosis and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder. Biomed. Res. Int. 2018, 2018, 9619253. [Google Scholar] [CrossRef]
- Stein, G.; Franke, S.; Mahiout, A.; Schneider, S.; Sperschneider, H.; Borst, S.; Vienken, J. Influence of dialysis modalities on serum AGE levels in end-stage renal disease patients. Nephrol. Dial. Transpl. 2001, 16, 999–1008. [Google Scholar] [CrossRef]
- Jørstad, S.; Smeby, L.C.; Balstad, T.; Widerøe, T.E. Generation and removal of anaphylatoxins during hemofiltration with five different membranes. Blood Purif. 1988, 6, 325–335. [Google Scholar] [CrossRef]
- Padrini, R.; Canova, C.; Conz, P.; Mancini, E.; Rizzioli, E.; Santoro, A. Convective and adsorptive removal of beta2-microglobulin during predilutional and postdilutional hemofiltration. Kidney Int. 2005, 68, 2331–2337. [Google Scholar] [CrossRef]
- Lornoy, W.; Becaus, I.; Billiouw, J.M.; Sierens, L.; Van Malderen, P.; D’Haenens, P. On-line haemodiafiltration. Remarkable removal of beta2-microglobulin. Long-term clinical observations. Nephrol. Dial. Transpl. 2000, 15 (Suppl. S1), 49–54. [Google Scholar] [CrossRef]
- Maduell, F.; del Pozo, C.; Garcia, H.; Sanchez, L.; Hdez-Jaras, J.; Albero, M.D.; Calvo, C.; Torregrosa, I.; Navarro, V. Change from conventional haemodiafiltration to on-line haemodiafiltration. Nephrol. Dial. Transpl. 1999, 14, 1202–1207. [Google Scholar] [CrossRef]
- Roumelioti, M.E.; Trietley, G.; Nolin, T.D.; Ng, Y.H.; Xu, Z.; Alaini, A.; Figueroa, R.; Unruh, M.L.; Argyropoulos, C.P. Beta-2 microglobulin clearance in high-flux dialysis and convective dialysis modalities: A meta-analysis of published studies. Nephrol. Dial. Transpl. 2018, 33, 542. [Google Scholar] [CrossRef]
- Locatelli, F.; Mastrangelo, F.; Redaelli, B.; Ronco, C.; Marcelli, D.; La Greca, G.; Orlandini, G. Effects of different membranes and dialysis technologies on patient treatment tolerance and nutritional parameters. The Italian Cooperative Dialysis Study Group. Kidney Int. 1996, 50, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Susantitaphong, P.; Tiranathanagul, K.; Katavetin, P.; Townamchai, N.; Praditpornsilpa, K.; Tungsanga, K.; Eiam-Ong, S. Efficacy of convective-controlled double high-flux hemodiafiltration versus on-line hemodiafiltration: 1-year prospective study. Blood Purif. 2010, 29, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.A.; Greene, T.; Hartmann, B.; Samtleben, W. Resistance to intercompartmental mass transfer limits beta2-microglobulin removal by post-dilution hemodiafiltration. Kidney Int. 2006, 69, 1431–1437. [Google Scholar] [CrossRef]
- Guedes, M.; Vernooij, R.W.M.; Davenport, A.; Kuhlmann, M.K.; Aregger, F.; Pecoits-Filho, R. Clinical performance, intermediate and long-term outcomes of high-volume hemodiafiltration in patients with kidney failure. Semin. Dial. 2022, 35, 420–426. [Google Scholar] [CrossRef]
- Widjaja, A.; Kielstein, J.T.; Horn, R.; von zur Muhlen, A.; Kliem, V.; Brabant, G. Free serum leptin but not bound leptin concentrations are elevated in patients with end-stage renal disease. Nephrol. Dial. Transpl. 2000, 15, 846–850. [Google Scholar] [CrossRef]
- Kim, S.; Oh, K.H.; Chin, H.J.; Na, K.Y.; Kim, Y.S.; Chae, D.W.; Ahn, C.; Han, J.S.; Kim, S.; Joo, K.W. Effective removal of leptin via hemodiafiltration with on-line endogenous reinfusion therapy. Clin. Nephrol. 2009, 72, 442–448. [Google Scholar] [CrossRef]
- Kuo, H.L.; Chou, C.Y.; Liu, Y.L.; Yang, Y.F.; Huang, C.C.; Lin, H.H. Reduction of pro-inflammatory cytokines through hemodiafiltration. Ren. Fail. 2008, 30, 796–800. [Google Scholar] [CrossRef]
- Morena, M.; Creput, C.; Bouzernidj, M.; Rodriguez, A.; Chalabi, L.; Seigneuric, B.; Lauret, C.; Bargnoux, A.S.; Dupuy, A.M.; Cristol, J.P. Randomised trial on clinical performances and biocompatibility of four high-flux hemodialyzers in two mode treatments: Hemodialysis vs. post dilution hemodiafiltration. Sci. Rep. 2019, 9, 18265. [Google Scholar] [CrossRef]
- Suzuki, S.; Moriyama, K.; Hara, Y.; Hinoue, T.; Kato, Y.; Hasegawa, D.; Kuriyama, N.; Nakamura, T.; Komatsu, S.; Yamashita, C.; et al. Comparison of myoglobin clearance in three types of blood purification modalities. Ther. Apher. Dial. 2021, 25, 401–406. [Google Scholar] [CrossRef]
- Canaud, B.; Wizemann, V.; Pizzarelli, F.; Greenwood, R.; Schultze, G.; Weber, C.; Falkenhagen, D. Cellular interleukin-1 receptor antagonist production in patients receiving on-line haemodiafiltration therapy. Nephrol. Dial. Transpl. 2001, 16, 2181–2187. [Google Scholar] [CrossRef]
- Krieter, D.H.; Falkenhain, S.; Chalabi, L.; Collins, G.; Lemke, H.D.; Canaud, B. Clinical cross-over comparison of mid-dilution hemodiafiltration using a novel dialyzer concept and post-dilution hemodiafiltration. Kidney Int. 2005, 67, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Bourguignon, C.; Chenine, L.; Bargnoux, A.S.; Leray-Moragues, H.; Canaud, B.; Cristol, J.P.; Morena, M. Hemodiafiltration improves free light chain removal and normalizes kappa/lambda ratio in hemodialysis patients. J. Nephrol. 2016, 29, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Lukkanalikitkul, E.; Kidkaem, H.; Phonrat, M.; Prathompong, P.; Anutrakulchai, S. A randomized trial comparing medium cut-off membrane dialyzers with online hemodiafiltration for uremic toxins clearance in hemodialysis patients. Sci. Rep. 2025, 15, 5467. [Google Scholar] [CrossRef]
- Ward, R.A.; Schmidt, B.; Hullin, J.; Hillebrand, G.F.; Samtleben, W. A comparison of on-line hemodiafiltration and high-flux hemodialysis: A prospective clinical study. J. Am. Soc. Nephrol. 2000, 11, 2344–2350. [Google Scholar] [CrossRef]
- Stefánsson, B.V.; Abramson, M.; Nilsson, U.; Haraldsson, B. Hemodiafiltration improves plasma 25-hepcidin levels: A prospective, randomized, blinded, cross-over study comparing hemodialysis and hemodiafiltration. Nephron Extra. 2012, 2, 55–65. [Google Scholar] [CrossRef]
- Meijers, B.K.; Van Kerckhoven, S.; Verbeke, K.; Dehaen, W.; Vanrenterghem, Y.; Hoylaerts, M.F.; Evenepoel, P. The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am. J. Kidney Dis. 2009, 54, 891–901. [Google Scholar] [CrossRef]
- Sakurai, K.; Saito, T.; Hosoya, H.; Kurihara, Y.; Yamauchi, F. Therapeutic effect of high-efficiency online hemodiafiltration for recurrent restless legs syndrome in dialysis patients. J. Artif. Organs 2020, 23, 296–301. [Google Scholar] [CrossRef]
- den Hoedt, C.H.; Bots, M.L.; Grooteman, M.P.; van der Weerd, N.C.; Mazairac, A.H.; Penne, E.L.; Levesque, R.; ter Wee, P.M.; Nube, M.J.; Blankestijn, P.J.; et al. Online hemodiafiltration reduces systemic inflammation compared to low-flux hemodialysis. Kidney Int. 2014, 86, 423–432. [Google Scholar] [CrossRef]
- Patrier, L.; Dupuy, A.M.; Granger Vallee, A.; Chalabi, L.; Morena, M.; Canaud, B.; Cristol, J.P. FGF-23 removal is improved by on-line high-efficiency hemodiafiltration compared to conventional high flux hemodialysis. J. Nephrol. 2013, 26, 342–349. [Google Scholar] [CrossRef]
- Nishizawa, Y.; Hosoda, Y.; Horimoto, A.; Omae, K.; Ito, K.; Higuchi, C.; Sakura, H.; Nitta, K.; Ogawa, T. Fibroblast growth factor 23 (FGF23) level is associated with ultrafiltration rate in patients on hemodialysis. Heart Vessel. 2021, 36, 414–423. [Google Scholar] [CrossRef]
- Abad, S.; Vega, A.; Quiroga, B.; Arroyo, D.; Panizo, N.; Reque, J.E.; Lopez-Gomez, J.M. Protein-bound toxins: Added value in their removal with high convective volumes. Nefrologia 2016, 36, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Thammathiwat, T.; Tiranathanagul, K.; Limjariyakul, M.; Chariyavilaskul, P.; Takkavatakarn, K.; Susantitaphong, P.; Meesangnin, S.; Wittayalertpanya, S.; Praditpornsilpa, K.; Eiam-Ong, S. Super high-flux hemodialysis provides comparable effectiveness with high-volume postdilution online hemodiafiltration in removing protein-bound and middle-molecule uremic toxins: A prospective cross-over randomized controlled trial. Ther. Apher. Dial. 2021, 25, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.D.; Guedes, M.; Rodrigues, S.D.; Florido, A.C.S.; Moreno-Amaral, A.N.; Barra, A.B.; Canziani, M.E.; Cuvello-Neto, A.; Poli-de-Figueiredo, C.E.; Pecoits-Filho, R.; et al. High-volume hemodiafiltration decreases the pre-dialysis concentrations of indoxyl sulfate and p-cresyl sulfate compared to hemodialysis: A post-hoc analysis from the HDFit randomized controlled trial. J. Nephrol. 2022, 35, 1449–1456. [Google Scholar] [CrossRef]
- Stuard, S.; Ridel, C.; Cioffi, M.; Trost-Rupnik, A.; Gurevich, K.; Bojic, M.; Karibayev, Y.; Mohebbi, N.; Marcinkowski, W.; Kupres, V. Hemodialysis Procedures for Stable Incident and Prevalent Patients Optimize Hemodynamic Stability, Dialysis Dose, Electrolytes, and Fluid Balance. J. Clin. Med. 2024, 13, 3211. [Google Scholar]
- Bleyer, A.J.; Russell, G.B.; Satko, S.G. Sudden and cardiac death rates in hemodialysis patients. Kidney Int. 1999, 55, 1553–1559. [Google Scholar] [CrossRef]
- Flythe, J.E.; Xue, H.; Lynch, K.E.; Curhan, G.C.; Brunelli, S.M. Association of mortality risk with various definitions of intradialytic hypotension. J. Am. Soc. Nephrol. 2015, 26, 724–734. [Google Scholar] [CrossRef]
- Burton, J.O.; Jefferies, H.J.; Selby, N.M.; McIntyre, C.W. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin. J. Am. Soc. Nephrol. 2009, 4, 1925–1931. [Google Scholar] [CrossRef]
- Locatelli, F.; Altieri, P.; Andrulli, S.; Bolasco, P.; Sau, G.; Pedrini, L.A.; Basile, C.; David, S.; Feriani, M.; Montagna, G.; et al. Hemofiltration and hemodiafiltration reduce intradialytic hypotension in ESRD. J. Am. Soc. Nephrol. 2010, 21, 1798–1807. [Google Scholar] [CrossRef]
- Donauer, J.; Schweiger, C.; Rumberger, B.; Krumme, B.; Bohler, J. Reduction of hypotensive side effects during online-haemodiafiltration and low temperature haemodialysis. Nephrol. Dial. Transpl. 2003, 18, 1616–1622. [Google Scholar] [CrossRef]
- Sande, F.M.V.; Kooman, J.P.; Konings, C.J.; Leunissen, K.M.L. Thermal effects and blood pressure response during postdilution hemodiafiltration and hemodialysis: The effect of amount of replacement fluid and dialysate temperature. J. Am. Soc. Nephrol. 2001, 12, 1916–1920. [Google Scholar] [CrossRef] [PubMed]
- Morena, M.; Jaussent, A.; Chalabi, L.; Leray-Moragues, H.; Chenine, L.; Debure, A.; Thibaudin, D.; Azzouz, L.; Patrier, L.; Maurice, F.; et al. Treatment tolerance and patient-reported outcomes favor online hemodiafiltration compared to high-flux hemodialysis in the elderly. Kidney Int. 2017, 91, 1495–1509. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Ninomiya, T.; Al-Kahwa, A.; Perkovic, V.; Gallagher, M.P.; Hawley, C.; Jardine, M.J. Effect of hemodiafiltration or hemofiltration compared with hemodialysis on mortality and cardiovascular disease in chronic kidney failure: A systematic review and meta-analysis of randomized trials. Am. J. Kidney Dis. 2014, 63, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, H. Is There Enough Evidence to Prove That Hemodiafiltration Is Superior? Blood Purif. 2018, 46, 3–6. [Google Scholar] [CrossRef]
- Sars, B.; van der Sande, F.M.; Kooman, J.P. Intradialytic Hypotension: Mechanisms and Outcome. Blood Purif. 2020, 49, 158–167. [Google Scholar] [CrossRef]
- Gross, M.; Gagel, A.; Maierhofer, A. The Donnan equilibrium is still valid in high-volume HDF. Int. J. Artif. Organs 2024, 47, 867–875. [Google Scholar] [CrossRef]
- Waniewski, J.; Pietribiasi, M.; Pstras, L. Calculation of the Gibbs-Donnan factors for multi-ion solutions with non-permeating charge on both sides of a permselective membrane. Sci. Rep. 2021, 11, 22150. [Google Scholar] [CrossRef]
- Rodriguez, A.; Morena, M.; Bargnoux, A.S.; Chenine, L.; Leray-Moragues, H.; Cristol, J.P.; Canaud, B. Quantitative assessment of sodium mass removal using ionic dialysance and sodium gradient as a proxy tool: Comparison of high-flux hemodialysis versus online hemodiafiltration. Artif. Organs 2021, 45, E280–E292. [Google Scholar] [CrossRef]
- Chazot, C.; Deleuze, S.; Fadel, B.; Hebibi, H.; Jean, G.; Levannier, M.; Puyoo, O.; Attaf, D.; Stuard, S.; Canaud, B. Is high-volume post-dilution haemodiafiltration associated with risk of fluid volume imbalance? A national multicentre cross-sectional cohort study. Nephrol. Dial. Transpl. 2019, 34, 2089–2095. [Google Scholar] [CrossRef]
- La Milia, V.; Ravasi, C.; Carfagna, F.; Alberghini, E.; Baragetti, I.; Buzzi, L.; Ferrario, F.; Furiani, S.; Barbone, G.S.; Pontoriero, G. Sodium removal and plasma tonicity balance are not different in hemodialysis and hemodiafiltration using high-flux membranes. J. Nephrol. 2019, 32, 461–469. [Google Scholar] [CrossRef]
- Myburgh, J.A.; Mythen, M.G. Resuscitation fluids. N. Engl. J. Med. 2013, 369, 2462–2463. [Google Scholar] [CrossRef] [PubMed]
- Oberleithner, H. Vascular endothelium: A vulnerable transit zone for merciless sodium. Nephrol. Dial. Transpl. 2014, 29, 240–246. [Google Scholar] [CrossRef]
- Daugirdas, J.T. Lower cardiovascular mortality with high-volume hemodiafiltration: A cool effect? Nephrol. Dial. Transpl. 2016, 31, 853–856. [Google Scholar] [CrossRef]
- Maggiore, Q.; Pizzarelli, F.; Sisca, S.; Zoccali, C.; Parlongo, S.; Nicolo, F.; Creazzo, G. Blood temperature and vascular stability during hemodialysis and hemofiltration. Trans. Am. Soc. Artif. Intern. Organs 1982, 28, 523–527. [Google Scholar]
- Ağbaş, A.; Canpolat, N.; Çalışkan, S.; Yılmaz, A.; Ekmekçi, H.; Mayes, M.; Aitkenhead, H.; Schaefer, F.; Sever, L.; Shroff, R. Hemodiafiltration is associated with reduced inflammation, oxidative stress and improved endothelial risk profile compared to high-flux hemodialysis in children. PLoS ONE 2018, 13, e0198320. [Google Scholar] [CrossRef]
- Filiopoulos, V.; Hadjiyannakos, D.; Metaxaki, P.; Sideris, V.; Takouli, L.; Anogiati, A.; Vlassopoulos, D. Inflammation and oxidative stress in patients on hemodiafiltration. Am. J. Nephrol. 2008, 28, 949–957. [Google Scholar] [CrossRef]
- Marcelli, D.; Bayh, I.; Merello, J.I.; Ponce, P.; Heaton, A.; Kircelli, F.; Chazot, C.; Di Benedetto, A.; Marelli, C.; Ladanyi, E.; et al. Dynamics of the erythropoiesis stimulating agent resistance index in incident hemodiafiltration and high-flux hemodialysis patients. Kidney Int. 2016, 90, 192–202. [Google Scholar] [CrossRef]
- Panichi, V.; Scatena, A.; Rosati, A.; Giusti, R.; Ferro, G.; Malagnino, E.; Capitanini, A.; Piluso, A.; Conti, P.; Bernabini, G.; et al. High-volume online haemodiafiltration improves erythropoiesis-stimulating agent (ESA) resistance in comparison with low-flux bicarbonate dialysis: Results of the REDERT study. Nephrol. Dial. Transpl. 2015, 30, 682–689. [Google Scholar] [CrossRef]
- Molina, P.; Vizcaino, B.; Molina, M.D.; Beltran, S.; Gonzalez-Moya, M.; Mora, A.; Castro-Alonso, C.; Kanter, J.; Avila, A.I.; Gorriz, J.L.; et al. The effect of high-volume online haemodiafiltration on nutritional status and body composition: The ProtEin Stores prEservaTion (PESET) study. Nephrol. Dial. Transpl. 2018, 33, 1223–1235. [Google Scholar] [CrossRef]
- Aichi, M.; Kuragano, T.; Iwasaki, T.; Ookawa, S.; Masumoto, M.; Mizusaki, K.; Yahiro, M.; Kida, A.; Nanami, M. Hemodiafiltration Improves Low Levels of Health-Related Quality Of Life (Qol) and Nutritional Conditions of Hemodialysis Patients. ASAIO J. 2022, 68, 297–302. [Google Scholar] [CrossRef]
- Pecoits-Filho, R.; Larkin, J.; Poli-de-Figueiredo, C.E.; Cuvello-Neto, A.L.; Barra, A.B.L.; Goncalves, P.B.; Sheth, S.; Guedes, M.; Han, M.; Calice-Silva, V.; et al. Effect of hemodiafiltration on measured physical activity: Primary results of the HDFIT randomized controlled trial. Nephrol. Dial. Transpl. 2021, 36, 1057–1070. [Google Scholar] [CrossRef]
- Karkar, A.; Abdelrahman, M.; Locatelli, F. A Randomized Trial on Health-Related Patient Satisfaction Level with High-Efficiency Online Hemodiafiltration versus High-Flux Dialysis. Blood Purif. 2015, 40, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Hazim, A.; Adarmouch, L.; Eloury, A.; Aasfara, J.; Asly, M.; Slassi, I. Hemodialysis-related headache: Still a challenge in 2020? Effect of conventional versus online hemodiafiltration from a study in Casablanca, Morocco. Artif. Organs 2021, 45, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Kantartzi, K.; Panagoutsos, S.; Mourvati, E.; Roumeliotis, A.; Leivaditis, K.; Devetzis, V.; Passadakis, P.; Vargemezis, V. Can dialysis modality influence quality of life in chronic hemodialysis patients? Low-flux hemodialysis versus high-flux hemodiafiltration: A cross-over study. Ren. Fail. 2013, 35, 216–221. [Google Scholar] [CrossRef]
- Vilar, E.; Fry, A.C.; Wellsted, D.; Tattersall, J.E.; Greenwood, R.N.; Farrington, K. Long-term outcomes in online hemodiafiltration and high-flux hemodialysis: A comparative analysis. Clin. J. Am. Soc. Nephrol. 2009, 4, 1944–1953. [Google Scholar] [CrossRef]
- Zoccali, C.; Tripepi, G.; Carioni, P.; Fu, E.L.; Dekker, F.; Stel, V.; Jager, K.J.; Mallamaci, F.; Hymes, J.L.; Maddux, F.W.; et al. Antihypertensive Drug Treatment and the Risk for Intrahemodialysis Hypotension. Clin. J. Am. Soc. Nephrol. 2024, 19, 1310–1318. [Google Scholar] [CrossRef]
- Rootjes, P.A.; Chaara, S.; de Roij van Zuijdewijn, C.L.M.; Nubé, M.J.; Wijngaarden, G.; Grooteman, M.P.C. High-Volume Hemodiafiltration and Cool Hemodialysis Have a Beneficial Effect on Intradialytic Hemodynamics: A Randomized Cross-Over Trial of Four Intermittent Dialysis Strategies. Kidney Int. Rep. 2022, 7, 1980–1990. [Google Scholar] [CrossRef]
- Neal, C.R.; Resnikoff, E.; Unger, A.M. Treatment of dialysis-related muscle cramps with hypertonic dextrose. Arch. Intern. Med. 1981, 141, 171–173. [Google Scholar]
- McGill, R.L.; Weiner, D.E. Dialysate Composition for Hemodialysis: Changes and Changing Risk. Semin. Dial. 2017, 30, 112–120. [Google Scholar] [CrossRef]
- Flythe, J.E.; Hilliard, T.; Castillo, G.; Ikeler, K.; Orazi, J.; Abdel-Rahman, E.; Pai, A.B.; Rivara, M.B.; St Peter, W.L.; Weisbord, S.D.; et al. Symptom Prioritization among Adults Receiving In-Center Hemodialysis: A Mixed Methods Study. Clin. J. Am. Soc. Nephrol. 2018, 13, 735–745. [Google Scholar] [CrossRef]
- Punj, S.; Enaam, A.; Marquez, A.; Atkinson, A.J., Jr.; Batlle, D. A Survey on Dialysis-Related Muscle Cramping and a Hypothesis of Angiotensin II on Its Pathophysiology. Kidney Int. Rep. 2020, 5, 924–926. [Google Scholar] [CrossRef]
- Chillar, R.K.; Desforges, J.F. Muscular cramps during maintenance haemodialysis. Lancet 1972, 2, 285. [Google Scholar] [CrossRef]
- Kolb, J.; Kitzler, T.M.; Tauber, T.; Morris, N.; Skrabal, F.; Kotanko, P. Proto-dialytic cardiac function relates to intra-dialytic morbid events. Nephrol. Dial. Transpl. 2011, 26, 1645–1651. [Google Scholar] [CrossRef]
- Basile, C.; Lomonte, C. A neglected issue in dialysis practice: Haemodialysate. Clin. Kidney J. 2015, 8, 393–399. [Google Scholar] [CrossRef]
- Beladi Mousavi, S.S.; Zeraati, A.; Moradi, S.; Mousavi, M.B. The effect of gabapentin on muscle cramps during hemodialysis: A double-blind clinical trial. Saudi J. Kidney Dis. Transpl. 2015, 26, 1142–1148. [Google Scholar] [CrossRef]
- Noordzij, M.; Boeschoten, E.W.; Bos, W.J.; Dekker, F.W.; Bossuyt, P.M.; Krediet, R.T.; Korevaar, J.C.; Group, N.S. Disturbed mineral metabolism is associated with muscle and skin complaints in a prospective cohort of dialysis patients. Nephrol. Dial. Transpl. 2007, 22, 2944–2949. [Google Scholar] [CrossRef]
- Campo, S.; Lacquaniti, A.; Trombetta, D.; Smeriglio, A.; Monardo, P. Immune System Dysfunction and Inflammation in Hemodialysis Patients: Two Sides of the Same Coin. J. Clin. Med. 2022, 11, 3759. [Google Scholar] [CrossRef]
- Kokubo, K.; Kurihara, Y.; Kobayashi, K.; Tsukao, H.; Kobayashi, H. Evaluation of the Biocompatibility of Dialysis Membranes. Blood Purif. 2015, 40, 293–297. [Google Scholar] [CrossRef]
- Ojeda, R.; Arias-Guillén, M.; Gómez, M.; Vera, M.; Fontseré, N.; Rodas, L.; Filella, X.; Reverter, J.C.; Lozano, F.; Villamor, N.; et al. Study of Biocompatibility of Membranes in Online Hemodiafiltration. Blood Purif. 2020, 49, 400–408. [Google Scholar] [CrossRef]
- Rangel, A.V.; Kim, J.C.; Kaushik, M.; Garzotto, F.; Neri, M.; Cruz, D.N.; Ronco, C. Backfiltration: Past, present and future. Contrib. Nephrol. 2011, 175, 35–45. [Google Scholar] [CrossRef]
- Catapano, G.; Morrone, G.; Hu, L.; Fragomeni, G.; Buscaroli, A. Endotoxin-Retentive Filters for the Online Preparation of Ultrapure Dialysis Fluid and Non-Pyrogenic Substitution Fluid: A Critical Review and Reference Guide. Membranes 2025, 15, 51. [Google Scholar]
- Furuya, R.; Kumagai, H.; Takahashi, M.; Sano, K.; Hishida, A. Ultrapure dialysate reduces plasma levels of beta2-microglobulin and pentosidine in hemodialysis patients. Blood Purif. 2005, 23, 311–316. [Google Scholar] [CrossRef]
- Gazenfield-Gazit, E.; Eliahou, H.E. Endotoxin antibodies in patients on maintenance hemodialysis. Isr. J. Med. Sci. 1969, 5, 1032–1036. [Google Scholar]
- Fischer, D.C.; Smith, C.; De Zan, F.; Bacchetta, J.; Bakkaloglu, S.A.; Agbas, A.; Anarat, A.; Aoun, B.; Askiti, V.; Azukaitis, K.; et al. Hemodiafiltration Is Associated With Reduced Inflammation and Increased Bone Formation Compared With Conventional Hemodialysis in Children: The HDF, Hearts and Heights (3H) Study. Kidney Int. Rep. 2021, 6, 2358–2370. [Google Scholar] [CrossRef]
- Santoro, A.; Mancini, E. Is hemodiafiltration the technical solution to chronic inflammation affecting hemodialysis patients? Kidney Int. 2014, 86, 235–237. [Google Scholar] [CrossRef]
- Cavallari, C.; Dellepiane, S.; Fonsato, V.; Medica, D.; Marengo, M.; Migliori, M.; Quercia, A.D.; Pitino, A.; Formica, M.; Panichi, V.; et al. Online Hemodiafiltration Inhibits Inflammation-Related Endothelial Dysfunction and Vascular Calcification of Uremic Patients Modulating miR-223 Expression in Plasma Extracellular Vesicles. J. Immunol. 2019, 202, 2372–2383. [Google Scholar] [CrossRef]
- Carracedo, J.; Merino, A.; Nogueras, S.; Carretero, D.; Berdud, I.; Ramírez, R.; Tetta, C.; Rodríguez, M.; Martín-Malo, A.; Aljama, P. On-line hemodiafiltration reduces the proinflammatory CD14+CD16+ monocyte-derived dendritic cells: A prospective, crossover study. J. Am. Soc. Nephrol. 2006, 17, 2315–2321. [Google Scholar] [CrossRef]
- Rama, I.; Llaudo, I.; Fontova, P.; Cerezo, G.; Soto, C.; Javierre, C.; Hueso, M.; Montero, N.; Martinez-Castelao, A.; Torras, J.; et al. Online Haemodiafiltration Improves Inflammatory State in Dialysis Patients: A Longitudinal Study. PLoS ONE 2016, 11, e0164969. [Google Scholar] [CrossRef]
- Ramírez, R.; Martín-Malo, A.; Aljama, P. Evolution of the concept of biocompatibility and the cardioprotective effect of on-line hemodiafiltration. Contrib. Nephrol. 2011, 175, 110–116. [Google Scholar] [CrossRef]
- Panichi, V.; Tetta, C. The biological response to online hemodiafiltration. Contrib. Nephrol. 2007, 158, 194–200. [Google Scholar] [CrossRef]
- Canaud, B.; Chenine, L.; Henriet, D.; Leray, H. Online hemodiafiltration: A multipurpose therapy for improving quality of renal replacement therapy. Contrib. Nephrol. 2008, 161, 191–198. [Google Scholar] [CrossRef]
- Bowry, S.K.; Gatti, E. Impact of hemodialysis therapy on anemia of chronic kidney disease: The potential mechanisms. Blood Purif. 2011, 32, 210–219. [Google Scholar] [CrossRef]
- Bonforte, G.; Grillo, P.; Zerbi, S.; Surian, M. Improvement of anemia in hemodialysis patients treated by hemodiafiltration with high-volume on-line-prepared substitution fluid. Blood Purif. 2002, 20, 357–363. [Google Scholar] [CrossRef]
- Pedrini, L.A.; Comelli, M.; Ruggiero, P.; Feliciani, A.; Manfrini, V.; Cozzi, G.; Castellano, A.; Pezzotta, M.; Gatti, G.; Arazzi, M.; et al. Mixed hemodiafiltration reduces erythropoiesis stimulating agents requirement in dialysis patients: A prospective randomized study. J. Nephrol. 2020, 33, 1037–1048. [Google Scholar] [CrossRef]
- Lin, C.L.; Huang, C.C.; Yu, C.C.; Wu, C.H.; Chang, C.T.; Hsu, H.H.; Hsu, P.Y.; Yang, C.W. Improved iron utilization and reduced erythropoietin resistance by on-line hemodiafiltration. Blood Purif. 2002, 20, 349–356. [Google Scholar] [CrossRef]
- Macdougall, I.C. Role of uremic toxins in exacerbating anemia in renal failure. Kidney Int. 2001, 59 (Suppl. S78), S67–S72. [Google Scholar] [CrossRef]
- Yamada, S.; Kataoka, H.; Kobayashi, H.; Ono, T.; Minakuchi, J.; Kawano, Y. Identification of an erythropoietic inhibitor from the dialysate collected in the hemodialysis with PMMA membrane (BK-F). Contrib. Nephrol. 1999, 125, 159–172. [Google Scholar] [CrossRef]
- Ayli, D.; Ayli, M.; Azak, A.; Yuksel, C.; Kosmaz, G.P.; Atilgan, G.; Dede, F.; Abayli, E.; Camlibel, M. The effect of high-flux hemodialysis on renal anemia. J. Nephrol. 2004, 17, 701–706. [Google Scholar]
- Aucella, F.; Scalzulli, R.P.; Vigilante, M.; Stallone, C. [The hemodiafiltration with endogenous reinfusion reduces the erythroid progenitor inhibition by uremic serum]. G. Ital. Nefrol. 2004, 21 (Suppl. S30), S128–S132. [Google Scholar]
- Allen, D.A.; Breen, C.; Yaqoob, M.M.; Macdougall, I.C. Inhibition of CFU-E colony formation in uremic patients with inflammatory disease: Role of IFN-gamma and TNF-alpha. J. Investig. Med. 1999, 47, 204–211. [Google Scholar]
- Panichi, V.; Rizza, G.M.; Paoletti, S.; Bigazzi, R.; Aloisi, M.; Barsotti, G.; Rindi, P.; Donati, G.; Antonelli, A.; Panicucci, E.; et al. Chronic inflammation and mortality in haemodialysis: Effect of different renal replacement therapies. Results from the RISCAVID study. Nephrol. Dial. Transpl. 2008, 23, 2337–2343. [Google Scholar] [CrossRef]
- Malyszko, J.; Malyszko, J.S.; Kozminski, P.; Mysliwiec, M. Type of renal replacement therapy and residual renal function may affect prohepcidin and hepcidin. Ren. Fail. 2009, 31, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Bolasco, P.G.; Ghezzi, P.M.; Serra, A.; Corazza, L.; Murtas, S.; Mascia, M.; Cossu, M.; Ferrara, R.; Cogoni, G.; Cadinu, F.; et al. Hemodiafiltration with endogenous reinfusion with and without acetate-free dialysis solutions: Effect on ESA requirement. Blood Purif. 2011, 31, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Lutton, J.D.; Solangi, K.B.; Ibraham, N.G.; Goodman, A.I.; Levere, R.D. Inhibition of erythropoiesis in chronic renal failure: The role of parathyroid hormone. Am. J. Kidney Dis. 1984, 3, 380–384. [Google Scholar] [CrossRef]
- Horl, W.H. The clinical consequences of secondary hyperparathyroidism: Focus on clinical outcomes. Nephrol. Dial. Transpl. 2004, 19 (Suppl. S5), V2–V8. [Google Scholar] [CrossRef]
- Vilar, E.; Farrington, K. Emerging importance of residual renal function in end-stage renal failure. Semin. Dial. 2011, 24, 487–494. [Google Scholar] [CrossRef]
- Lin, C.L.; Huang, C.C.; Chang, C.T.; Wu, M.S.; Hung, C.C.; Chien, C.C.; Yang, C.W. Clinical improvement by increased frequency of on-line hemodialfiltration. Ren. Fail. 2001, 23, 193–206. [Google Scholar] [CrossRef]
- Moon, S.J.; Kim, D.K.; Chang, J.H.; Kim, C.H.; Kim, H.W.; Park, S.Y.; Han, S.H.; Lee, J.E.; Yoo, T.H.; Han, D.S.; et al. The impact of dialysis modality on skin hyperpigmentation in haemodialysis patients. Nephrol. Dial. Transpl. 2009, 24, 2803–2809. [Google Scholar] [CrossRef]
- Shibata, M.; Nagai, K.; Usami, K.; Tawada, H.; Taniguchi, S. The quantitative evaluation of online haemodiafiltration effect on skin hyperpigmentation. Nephrol. Dial. Transpl. 2011, 26, 988–992. [Google Scholar] [CrossRef]
- Locatelli, F.; Marcelli, D.; Conte, F.; Limido, A.; Malberti, F.; Spotti, D. Comparison of mortality in ESRD patients on convective and diffusive extracorporeal treatments. The Registro Lombardo Dialisi E Trapianto. Kidney Int. 1999, 55, 286–293. [Google Scholar] [CrossRef]
- Cornelis, T.; van der Sande, F.M.; Eloot, S.; Cardinaels, E.; Bekers, O.; Damoiseaux, J.; Leunissen, K.M.; Kooman, J.P. Acute hemodynamic response and uremic toxin removal in conventional and extended hemodialysis and hemodiafiltration: A randomized crossover study. Am. J. Kidney Dis. 2014, 64, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Gal, R.; Korzets, A.; Schwartz, A.; Rath-Wolfson, L.; Gafter, U. Systemic distribution of beta 2-microglobulin-derived amyloidosis in patients who undergo long-term hemodialysis. Report of seven cases and review of the literature. Arch. Pathol. Lab. Med. 1994, 118, 718–721. [Google Scholar] [PubMed]
- Takayama, F.; Miyazaki, S.; Morita, T.; Hirasawa, Y.; Niwa, T. Dialysis-related amyloidosis of the heart in long-term hemodialysis patients. Kidney Int. 2001, 59 (Suppl. S78), S172–S176. [Google Scholar] [CrossRef]
- Schiffl, H.; Lang, S.M.; Fischer, R. Ultrapure dialysis fluid slows loss of residual renal function in new dialysis patients. Nephrol. Dial. Transpl. 2002, 17, 1814–1818. [Google Scholar] [CrossRef]
- Maeda, K.; Kobayakawa, H.; Fujita, Y.; Takai, I.; Morita, H.; Emoto, Y.; Miyazaki, T.; Shinzato, T. Effectiveness of push/pull hemodiafiltration using large-pore membrane for shoulder joint pain in long-term dialysis patients. Artif. Organs 1990, 14, 321–327. [Google Scholar] [CrossRef]
- Paglialonga, F.; Monzani, A.; Prodam, F.; Smith, C.; De Zan, F.; Canpolat, N.; Agbas, A.; Bayazit, A.; Anarat, A.; Bakkaloglu, S.A.; et al. Nutritional and Anthropometric Indices in Children Receiving Haemodiafiltration vs. Conventional Haemodialysis—The HDF, Heart and Height (3H) Study. J. Ren. Nutr. 2023, 33, 17–28. [Google Scholar] [CrossRef]
- Mandolfo, S.; Borlandelli, S.; Imbasciati, E. Leptin and beta2-microglobulin kinetics with three different dialysis modalities. Int. J. Artif. Organs 2006, 29, 949–955. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kopple, J.D.; Humphreys, M.H.; Block, G. Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrol. Dial. Transpl. 2004, 19, 1507–1519. [Google Scholar] [CrossRef]
- Wolley, M.; Jardine, M.; Hutchison, C.A. Exploring the Clinical Relevance of Providing Increased Removal of Large Middle Molecules. Clin. J. Am. Soc. Nephrol. 2018, 13, 805–814. [Google Scholar] [CrossRef]
- Haag-Weber, M.; Cohen, G.; Horl, W.H. Clinical significance of granulocyte-inhibiting proteins. Nephrol. Dial. Transpl. 2000, 15 (Suppl. S1), 15–16. [Google Scholar] [CrossRef]
- Murtas, S.; Aquilani, R.; Iadarola, P.; Deiana, M.L.; Secci, R.; Cadeddu, M.; Bolasco, P. Differences and Effects of Metabolic Fate of Individual Amino Acid Loss in High-Efficiency Hemodialysis and Hemodiafiltration. J. Ren. Nutr. 2020, 30, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Bevier, A.; Novel-Catin, E.; Blond, E.; Pelletier, S.; Parant, F.; Koppe, L.; Fouque, D. Water-Soluble Vitamins and Trace Elements Losses during On-Line Hemodiafiltration. Nutrients 2022, 14, 3454. [Google Scholar] [CrossRef]
- Johansen, K.L.; Gilbertson, D.T.; Li, S.; Li, S.; Liu, J.; Roetker, N.S.; Ku, E.; Schulman, I.H.; Greer, R.C.; Chan, K.; et al. US Renal Data System 2023 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2024, 83, A8–A13. [Google Scholar] [CrossRef] [PubMed]
- Silberzweig, J.I. Reducing Infections in Outpatient Hemodialysis: The Impact of Human Factors. Am. J. Kidney Dis. 2024, 84, 4–5. [Google Scholar] [CrossRef]
- Kato, S.; Chmielewski, M.; Honda, H.; Pecoits-Filho, R.; Matsuo, S.; Yuzawa, Y.; Tranaeus, A.; Stenvinkel, P.; Lindholm, B. Aspects of immune dysfunction in end-stage renal disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1526–1533. [Google Scholar] [CrossRef]
- Vanholder, R.; Ringoir, S. Infectious morbidity and defects of phagocytic function in end-stage renal disease: A review. J. Am. Soc. Nephrol. 1993, 3, 1541–1554. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Pahl, M.V.; Crum, A.; Norris, K. Effect of uremia on structure and function of immune system. J. Ren. Nutr. 2012, 22, 149–156. [Google Scholar] [CrossRef]
- den Hoedt, C.H.; Grooteman, M.P.; Bots, M.L.; Blankestijn, P.J.; van der Tweel, I.; van der Weerd, N.C.; Penne, E.L.; Mazairac, A.H.; Levesque, R.; ter Wee, P.M.; et al. The Effect of Online Hemodiafiltration on Infections: Results from the CONvective TRAnsport STudy. PLoS ONE 2015, 10, e0135908. [Google Scholar] [CrossRef]
- Allon, M.; Radeva, M.; Bailey, J.; Beddhu, S.; Butterly, D.; Coyne, D.W.; Depner, T.A.; Gassman, J.J.; Kaufman, A.M.; Kaysen, G.A.; et al. The spectrum of infection-related morbidity in hospitalized haemodialysis patients. Nephrol. Dial. Transpl. 2005, 20, 1180–1186. [Google Scholar] [CrossRef]
- Kaplowitz, L.G.; Comstock, J.A.; Landwehr, D.M.; Dalton, H.P.; Mayhall, C.G. A prospective study of infections in hemodialysis patients: Patient hygiene and other risk factors for infection. Infect. Control Hosp. Epidemiol. 1988, 9, 534–541. [Google Scholar] [CrossRef]
- Akmal, M. Hemodialysis in diabetic patients. Am. J. Kidney Dis. 2001, 38, S195–S199. [Google Scholar] [CrossRef] [PubMed]
- Hoen, B.; Paul-Dauphin, A.; Hestin, D.; Kessler, M. EPIBACDIAL: A multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J. Am. Soc. Nephrol. 1998, 9, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Schild, A.F.; Perez, E.; Gillaspie, E.; Seaver, C.; Livingstone, J.; Thibonnier, A. Arteriovenous fistulae vs. arteriovenous grafts: A retrospective review of 1,700 consecutive vascular access cases. J. Vasc. Access 2008, 9, 231–235. [Google Scholar] [PubMed]
- Canaud, B.; Popa, C.; Leray-Moragues, H.; Morena-Carrere, M.; Cristol, J.P. Can Gut Instinct Guide the Detection of Intestinal Bacterial Translocation in Dialysis Patients? Kidney Int. Rep. 2025, 10, 12–16. [Google Scholar] [CrossRef]
- Rootjes, P.A.; Grooteman, M.P.C.; Budding, A.E.; Bontkes, H.J.; Wijngaarden, G.; Nube, M.J.; de Roij van Zuijdewijn, C.L.M. Randomized Trial Demonstrating No Translocation of Intact Intestinal Bacteria During Hemodialysis or Hemodiafiltration. Kidney Int. Rep. 2025, 10, 109–119. [Google Scholar] [CrossRef]
- Nongnuch, A.; Ngampongpan, W.; Srichatrapimuk, S.; Wongsa, A.; Thongpraphai, S.; Boonarkart, C.; Sanmeema, N.; Chittaganpitch, M.; Auewarakul, P.; Tassaneetrithep, B.; et al. Immune response to influenza vaccination in ESRD patients undergoing hemodialysis vs. hemodiafiltration. PLoS ONE 2020, 15, e0227719. [Google Scholar] [CrossRef]
- Chuva, T.; Santos, T.; Goncalves, F.; Costa, L.; Alves, E.; Neves, I.; Paiva, A.; Carvalho, B.; Sousa, T.; Ramalheiro, A.; et al. Humoral immunity against Covid-19 six months after the Pfizer BNT162b2 vaccine in hemodialysis patients: Data from five dialysis units. Is there a protective role for hemodiafiltration in the Covid-19 pandemic? J. Nephrol. 2022, 35, 1543–1545. [Google Scholar] [CrossRef]
- Lioulios, G.; Fylaktou, A.; Asouchidou, D.; Xochelli, A.; Nikolaidou, V.; Stai, S.; Christodoulou, M.; Giamalis, P.; Tsouchnikas, I.; Papagianni, A.; et al. Effect of Lymphocyte Phenotypic Alterations on the Humoral Response to Vaccination Against SARS-COV-2 in Dialysis Patients. Ann. Lab. Med. 2023, 43, 451–460. [Google Scholar] [CrossRef]
- Hebibi, H.; Edeas, M.; Cornillac, L.; Beaudreuil, S.; Achiche, J.; Attaf, D.; Saibi, S.; Chazot, C.; Ouaaz, F.; Canaud, B. SARS-CoV-2 mRNA Vaccine Immunogenicity in Hemodialysis Patients: Promising Vaccine Protection That May Be Hindered by Fluid Overload. Kidney Dial. 2022, 2, 44–56. [Google Scholar]
- Carrera, F.; Jacobson, S.H.; Costa, J.; Marques, M.; Ferrer, F. Better Anti-Spike IgG Antibody Response to SARS-CoV-2 Vaccine in Patients on Haemodiafiltration than on Haemodialysis. Blood Purif. 2023, 52, 600–608. [Google Scholar] [CrossRef]
- Czifra, A.; Pall, A.; Kulcsar, J.; Barta, K.; Kertesz, A.; Paragh, G.; Lorincz, I.; Jenei, Z.; Agarwal, A.; Zarjou, A.; et al. Hemodialysis and hemodiafiltration differently modulate left ventricular diastolic function. BMC Nephrol. 2013, 14, 76. [Google Scholar] [CrossRef]
- Mostovaya, I.M.; Blankestijn, P.J.; Bots, M.L.; Covic, A.; Davenport, A.; Grooteman, M.P.; Hegbrant, J.; Locatelli, F.; Vanholder, R.; Nube, M.J.; et al. Clinical evidence on hemodiafiltration: A systematic review and a meta-analysis. Semin. Dial. 2014, 27, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Ohtake, T.; Oka, M.; Ishioka, K.; Honda, K.; Mochida, Y.; Maesato, K.; Moriya, H.; Hidaka, S.; Kobayashi, S. Cardiovascular protective effects of on-line hemodiafiltration: Comparison with conventional hemodialysis. Ther. Apher. Dial. 2012, 16, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Charitaki, E.; Davenport, A. Does hemodiafiltration reduce vascular stiffness measured by aortic pulse wave velocity compared with high-flux hemodialysis? Hemodialysis international. Int. Symp. Home Hemodial. 2014, 18, 391–395. [Google Scholar] [CrossRef]
- Shroff, R.; Smith, C.; Ranchin, B.; Bayazit, A.K.; Stefanidis, C.J.; Askiti, V.; Azukaitis, K.; Canpolat, N.; Agbas, A.; Aitkenhead, H.; et al. Effects of Hemodiafiltration versus Conventional Hemodialysis in Children with ESKD: The HDF, Heart and Height Study. J. Am. Soc. Nephrol. 2019, 30, 678–691. [Google Scholar] [CrossRef]
- Chang, J.W.; Yang, W.S.; Seo, J.W.; Lee, J.S.; Lee, S.K.; Park, S.K. Continuous venovenous hemodiafiltration versus hemodialysis as renal replacement therapy in patients with acute renal failure in the intensive care unit. Scand. J. Urol. Nephrol. 2004, 38, 417–421. [Google Scholar] [CrossRef]
- Nistor, I.; Palmer, S.C.; Craig, J.C.; Saglimbene, V.; Vecchio, M.; Covic, A.; Strippoli, G.F. Convective versus diffusive dialysis therapies for chronic kidney failure: An updated systematic review of randomized controlled trials. Am. J. Kidney Dis. 2014, 63, 954–967. [Google Scholar] [CrossRef]
- Lakshman, S.G.; Ravikumar, P.; Kar, G.; Das, D.; Bhattacharjee, K.; Bhattacharjee, P. A Comparative Study of Neurological Complications in Chronic Kidney Disease with Special Reference to its Stages and Haemodialysis Status. J. Clin. Diagn. Res. 2016, 10, OC01–OC04. [Google Scholar] [CrossRef]
- Arnold, R.; Issar, T.; Krishnan, A.V.; Pussell, B.A. Neurological complications in chronic kidney disease. JRSM Cardiovasc. Dis. 2016, 5, 2048004016677687. [Google Scholar] [CrossRef]
- Chillon, J.M.; Massy, Z.A.; Stengel, B. Neurological complications in chronic kidney disease patients. Nephrol. Dial. Transpl. 2016, 31, 1606–1614. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Kiernan, M.C. Neurological complications of chronic kidney disease. Nat. Rev. Neurol. 2009, 5, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Phoon, R.K.; Pussell, B.A.; Charlesworth, J.A.; Kiernan, M.C. Sensory nerve excitability and neuropathy in end stage kidney disease. J. Neurol. Neurosurg. Psychiatry 2006, 77, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Lin, C.S.; Kiernan, M.C. Nerve excitability properties in lower-limb motor axons: Evidence for a length-dependent gradient. Muscle Nerve 2004, 29, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.R.S.; Schoueri, J.H.M.; Alves, B.; Veiga, G.; Fonseca, F.L.A.; Bacci, M.R. Uremic neuropathy: An overview of the current literature. Rev. Assoc. Med. Bras. 2019, 65, 469–474. [Google Scholar] [CrossRef]
- Arnold, R.; Pussell, B.A.; Pianta, T.J.; Grinius, V.; Lin, C.S.; Kiernan, M.C.; Howells, J.; Jardine, M.J.; Krishnan, A.V. Effects of hemodiafiltration and high flux hemodialysis on nerve excitability in end-stage kidney disease. PLoS ONE 2013, 8, e59055. [Google Scholar] [CrossRef]
- Jiang, X.; Ji, F.; Chen, Z.W.; Huang, Q.L. Comparison of high-flux hemodialysis with hemodialysis filtration in treatment of uraemic pruritus: A randomized controlled trial. Int. Urol. Nephrol. 2016, 48, 1533–1541. [Google Scholar] [CrossRef]
- Arzhan, S.; Roumelioti, M.E.; Unruh, M.L. Itch and Ache on Dialysis: New Approaches to Manage Uremic Pruritus and Restless Legs. Blood Purif. 2020, 49, 222–227. [Google Scholar] [CrossRef]
- Kang, A.; Arnold, R.; Gallagher, M.; Snelling, P.; Green, J.; Fernando, M.; Kiernan, M.C.; Hand, S.; Grimley, K.; Burman, J.; et al. Effect of Hemodiafiltration on the Progression of Neuropathy with Kidney Failure: A Randomized Controlled Trial. Clin. J. Am. Soc. Nephrol. 2021, 16, 1365–1375. [Google Scholar] [CrossRef]
- Sehgal, A.R.; Grey, S.F.; DeOreo, P.B.; Whitehouse, P.J. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am. J. Kidney Dis. 1997, 30, 41–49. [Google Scholar] [CrossRef]
- Kurella Tamura, M.; Yaffe, K. Dementia and cognitive impairment in ESRD: Diagnostic and therapeutic strategies. Kidney Int. 2011, 79, 14–22. [Google Scholar] [CrossRef]
- Murray, A.M. Cognitive Impairment in the Aging Dialysis and Chronic Kidney Disease Populations: An Occult Burden. Adv. Chronic Kidney Dis. 2008, 15, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.A.; Weiner, D.E.; Tighiouart, H.; Duncan, S.; Gupta, A.; Scott, T.; Sarnak, M.J. Cognitive Decline and Its Risk Factors in Prevalent Hemodialysis Patients. Am. J. Kidney Dis. 2017, 69, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Van Campenhout, A.; Golledge, J. Osteoprotegerin, vascular calcification and atherosclerosis. Atherosclerosis 2009, 204, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.S.; Lin, L.F.; Chen, V.C.; Hsieh, C.W.; Hsiao, H.P.; McIntyre, R.S.; Iacobucci, M.; Coles, A.S.; Tsai, D.J.; Weng, J.C.; et al. Effects of Lower Past-Year Serum Sodium and Hyponatremia on Depression Symptoms and Cognitive Impairments in Patients With Hemodialysis. Ther. Apher. Dial. 2020, 24, 169–177. [Google Scholar] [CrossRef]
- Olczyk, P.; Kusztal, M.; Golebiowski, T.; Letachowicz, K.; Krajewska, M. Cognitive Impairment in End Stage Renal Disease Patients Undergoing Hemodialysis: Markers and Risk Factors. Int. J. Environ. Res. Public Health 2022, 19, 2389. [Google Scholar] [CrossRef]
- Hsieh, H.L.; Yang, C.M. Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed. Res. Int. 2013, 2013, 484613. [Google Scholar] [CrossRef]
- Kurella Tamura, M.; Wadley, V.; Yaffe, K.; McClure, L.A.; Howard, G.; Go, R.; Allman, R.M.; Warnock, D.G.; McClellan, W. Kidney function and cognitive impairment in US adults: The Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am. J. Kidney Dis. 2008, 52, 227–234. [Google Scholar] [CrossRef]
- Murray, A.M.; Tupper, D.E.; Knopman, D.S.; Gilbertson, D.T.; Pederson, S.L.; Li, S.; Smith, G.E.; Hochhalter, A.K.; Collins, A.J.; Kane, R.L. Cognitive impairment in hemodialysis patients is common. Neurology 2006, 67, 216–223. [Google Scholar] [CrossRef]
- Cervellati, C.; Romani, A.; Seripa, D.; Cremonini, E.; Bosi, C.; Magon, S.; Passaro, A.; Bergamini, C.M.; Pilotto, A.; Zuliani, G. Oxidative balance, homocysteine, and uric acid levels in older patients with Late Onset Alzheimer’s Disease or Vascular Dementia. J. Neurol. Sci. 2014, 337, 156–161. [Google Scholar] [CrossRef]
- MacEwen, C.; Sutherland, S.; Daly, J.; Pugh, C.; Tarassenko, L. Relationship between Hypotension and Cerebral Ischemia during Hemodialysis. J. Am. Soc. Nephrol. 2017, 28, 2511–2520. [Google Scholar] [CrossRef]
- Casserly, I.; Topol, E.J. Convergence of atherosclerosis and Alzheimer’s disease: Inflammation, cholesterol, and misfolded proteins. Lancet 2004, 363, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Breteler, M.M.; van Amerongen, N.M.; van Swieten, J.C.; Claus, J.J.; Grobbee, D.E.; van Gijn, J.; Hofman, A.; van Harskamp, F. Cognitive correlates of ventricular enlargement and cerebral white matter lesions on magnetic resonance imaging. The Rotterdam Study. Stroke 1994, 25, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.; Wang, H.; Chu, Z.; Li, J.; Qian, T.; Mark Haacke, E.; Xia, S.; Shen, W. Reduced regional cerebral venous oxygen saturation is a risk factor for the cognitive impairment in hemodialysis patients: A quantitative susceptibility mapping study. Brain Imaging Behav. 2020, 14, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Arieff, A.I. Aluminum and the Pathogenesis of Dialysis Encephalopathy. Am. J. Kidney Dis. 1985, 6, 317–321. [Google Scholar] [CrossRef]
- Han, M.; Guedes, M.; Larkin, J.; Raimann, J.G.; Lesqueves Barra, A.B.; Canziani, M.E.F.; Cuvello Neto, A.L.; Poli-de-Figueiredo, C.E.; Kotanko, P.; Pecoits-Filho, R. Effect of Hemodiafiltration on Self-Reported Sleep Duration: Results from a Randomized Controlled Trial. Blood Purif. 2020, 49, 168–177. [Google Scholar] [CrossRef]
- Ahlmann, C.; Stronach, L.; Waters, K.; Walker, K.; Oh, J.; Schmitt, C.P.; Ranchin, B.; Shroff, R. Hemodiafiltration for children with stage 5 chronic kidney disease: Technical aspects and outcomes. Pediatr. Nephrol. 2024, 39, 2611–2626. [Google Scholar] [CrossRef]
- Nofal, E.; Farag, F.; Nofal, A.; Eldesouky, F.; Alkot, R.; Abdelkhalik, Z. Gabapentin: A promising therapy for uremic pruritus in hemodialysis patients: A randomized-controlled trial and review of literature. J. Dermatol. Treat. 2016, 27, 515–519. [Google Scholar] [CrossRef]
- Jing, Y.; Zhao, H.; Ge, Y.; Jia, F.; He, Q.; Wang, S.; Meng, J. Application of total parathyroidectomy with auto-transplantation for uremia secondary hyperparathyroidism treatment. Int. J. Clin. Exp. Med. 2015, 8, 11188–11194. [Google Scholar]
- Pisoni, R.L.; Wikstrom, B.; Elder, S.J.; Akizawa, T.; Asano, Y.; Keen, M.L.; Saran, R.; Mendelssohn, D.C.; Young, E.W.; Port, F.K. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol. Dial. Transpl. 2006, 21, 3495–3505. [Google Scholar] [CrossRef]
- van der Willik, E.M.; Lengton, R.; Hemmelder, M.H.; Hoogeveen, E.K.; Bart, H.A.J.; van Ittersum, F.J.; Ten Dam, M.; Bos, W.J.W.; Dekker, F.W.; Meuleman, Y. Itching in dialysis patients: Impact on health-related quality of life and interactions with sleep problems and psychological symptoms-results from the RENINE/PROMs registry. Nephrol. Dial. Transpl. 2022, 37, 1731–1741. [Google Scholar] [CrossRef]
- Combs, S.A.; Teixeira, J.P.; Germain, M.J. Pruritus in Kidney Disease. Semin. Nephrol. 2015, 35, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yosipovitch, G. New insights into the pathophysiology and treatment of chronic itch in patients with end-stage renal disease, chronic liver disease, and lymphoma. Int. J. Dermatol. 2010, 49, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J.; Cao, G.; Tang, W.X.; Lv, X.Y.; Huang, S.M.; Qin, W.; Ping, F.; Ye, T. A randomized controlled trial of high-permeability haemodialysis against conventional haemodialysis in the treatment of uraemic pruritus. Clin. Exp. Dermatol. 2009, 34, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, S.; Kim, J.H. Real-world Evidence versus Randomized Controlled Trial: Clinical Research Based on Electronic Medical Records. J. Korean Med. Sci. 2018, 33, e213. [Google Scholar] [CrossRef]
- Skarbinski, J.; Fischer, H.; Hong, V.; Liu, L.; Yau, V.M.; Incerti, D.; Qian, L.; Ackerson, B.K.; Amsden, L.B.; Shaw, S.F.; et al. Real-World Evidence to Supplement Randomized Clinical Trials: Tocilizumab for Severe COVID-19 Pneumonia vs. a Cohort Receiving Standard of Care. Clin. Pharmacol. Ther. 2023, 114, 1073–1081. [Google Scholar] [CrossRef]
- Sheldrick, R.C. Randomized Trials vs. Real-world Evidence: How Can Both Inform Decision-making? JAMA 2023, 329, 1352–1353. [Google Scholar] [CrossRef]
- Franklin, J.M.; Glynn, R.J.; Suissa, S.; Schneeweiss, S. Emulation Differences vs. Biases When Calibrating Real-World Evidence Findings Against Randomized Controlled Trials. Clin. Pharmacol. Ther. 2020, 107, 735–737. [Google Scholar] [CrossRef]
- Deleuran, M.; Vestergaard, C. Real-world evidence vs. randomized control trials. Br. J. Dermatol. 2020, 182, 275–276. [Google Scholar] [CrossRef]
- Craig, J.C.; Irwig, L.M.; Stockler, M.R. Evidence-based medicine: Useful tools for decision making. Med. J. Aust. 2001, 174, 248–253. [Google Scholar] [CrossRef]
- Strippoli, G.F.; Craig, J.C.; Schena, F.P. The number, quality, and coverage of randomized controlled trials in nephrology. J. Am. Soc. Nephrol. 2004, 15, 411–419. [Google Scholar] [CrossRef]
- Grooteman, M.P.; van den Dorpel, M.A.; Bots, M.L.; Penne, E.L.; van der Weerd, N.C.; Mazairac, A.H.; den Hoedt, C.H.; van der Tweel, I.; Lévesque, R.; Nubé, M.J.; et al. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J. Am. Soc. Nephrol. 2012, 23, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Caskey, F.J.; Procter, S.; MacNeill, S.J.; Wade, J.; Taylor, J.; Rooshenas, L.; Liu, Y.; Annaw, A.; Alloway, K.; Davenport, A.; et al. The high-volume haemodiafiltration vs. high-flux haemodialysis registry trial (H4RT): A multi-centre, unblinded, randomised, parallel-group, superiority study to compare the effectiveness and cost-effectiveness of high-volume haemodiafiltration and high-flux haemodialysis in people with kidney failure on maintenance dialysis using linkage to routine healthcare databases for outcomes. Trials 2022, 23, 532. [Google Scholar] [CrossRef] [PubMed]
- Susantitaphong, P.; Siribamrungwong, M.; Jaber, B.L. Convective therapies versus low-flux hemodialysis for chronic kidney failure: A meta-analysis of randomized controlled trials. Nephrol. Dial. Transpl. 2013, 28, 2859–2874. [Google Scholar] [CrossRef]
- Neri, L.; Gurevich, K.; Zarya, Y.; Plavinskii, S.; Bellocchio, F.; Stuard, S.; Barbieri, C.; Canaud, B. Practice Patterns and Outcomes of Online Hemodiafiltration: A Real-World Evidence Study in a Russian Dialysis Network. Blood Purif. 2021, 50, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Imamovic, G.; Hrvacevic, R.; Kapun, S.; Marcelli, D.; Bayh, I.; Grassmann, A.; Scatizzi, L.; Maslovaric, J.; Canaud, B. Survival of incident patients on high-volume online hemodiafiltration compared to low-volume online hemodiafiltration and high-flux hemodialysis. Int. Urol. Nephrol. 2014, 46, 1191–1200. [Google Scholar] [CrossRef]
- Canaud, B.; Barbieri, C.; Marcelli, D.; Bellocchio, F.; Bowry, S.; Mari, F.; Amato, C.; Gatti, E. Optimal convection volume for improving patient outcomes in an international incident dialysis cohort treated with online hemodiafiltration. Kidney Int. 2015, 88, 1108–1116. [Google Scholar] [CrossRef]
- Canaud, B.; Bayh, I.; Marcelli, D.; Ponce, P.; Merello, J.I.; Gurevich, K.; Ladanyi, E.; Ok, E.; Imamovic, G.; Grassmann, A.; et al. Improved survival of incident patients with high-volume haemodiafiltration: A propensity-matched cohort study with inverse probability of censoring weighting. Nephron 2015, 129, 179–188. [Google Scholar] [CrossRef]
- Maduell, F.; Varas, J.; Ramos, R.; Martin-Malo, A.; Perez-Garcia, R.; Berdud, I.; Moreso, F.; Canaud, B.; Stuard, S.; Gauly, A.; et al. Hemodiafiltration Reduces All-Cause and Cardiovascular Mortality in Incident Hemodialysis Patients: A Propensity-Matched Cohort Study. Am. J. Nephrol. 2017, 46, 288–297. [Google Scholar] [CrossRef]
- Mercadal, L.; Franck, J.E.; Metzger, M.; Urena Torres, P.; de Cornelissen, F.; Edet, S.; Béchade, C.; Vigneau, C.; Drüeke, T.; Jacquelinet, C.; et al. Hemodiafiltration Versus Hemodialysis and Survival in Patients With ESRD: The French Renal Epidemiology and Information Network (REIN) Registry. Am. J. Kidney Dis. 2016, 68, 247–255. [Google Scholar] [CrossRef]
- See, E.J.; Hedley, J.; Agar, J.W.M.; Hawley, C.M.; Johnson, D.W.; Kelly, P.J.; Lee, V.W.; Mac, K.; Polkinghorne, K.R.; Rabindranath, K.S.; et al. Patient survival on haemodiafiltration and haemodialysis: A cohort study using the Australia and New Zealand Dialysis and Transplant Registry. Nephrol. Dial. Transpl. 2019, 34, 326–338. [Google Scholar] [CrossRef]
- Jirka, T.; Cesare, S.; Di Benedetto, A.; Perera Chang, M.; Ponce, P.; Richards, N.; Tetta, C.; Vaslaky, L. Mortality risk for patients receiving hemodiafiltration versus hemodialysis. Kidney Int. 2006, 69, 2087–2093, author reply in Kidney Int. 2006, 70, 1524–1525. [Google Scholar] [CrossRef]
- Valderrama, L.A.; Barrera, L.; Cantor, E.J.; Muñoz, J.; Arango, J.; Tobon, C.; Canaud, B. Mortality in High-Flux Hemodialysis vs. High-Volume Hemodiafiltration in Colombian Clinical Practice: A Propensity Score Matching Study. Kidney Dial. 2022, 2, 209–220. [Google Scholar][Green Version]
- da Rocha, E.P.; Kojima, C.A.; Modelli de Andrade, L.G.; Costa, D.M.; Magalhaes, A.O.; Rocha, W.F.; de Vasconcelos Junior, L.N.; Rosa, M.G.; Wagner Martins, C.S. Comparing Survival Outcomes between Hemodialysis and Hemodiafiltration Using Real-World Data from Brazil. J. Clin. Med. 2024, 13, 594. [Google Scholar] [CrossRef]
- Zhang, Y.; Winter, A.; Ferreras, B.A.; Carioni, P.; Arkossy, O.; Anger, M.; Kossmann, R.; Usvyat, L.A.; Stuard, S.; Maddux, F.W. Real-world effectiveness of hemodialysis modalities: A retrospective cohort study. BMC Nephrol. 2025, 26, 9. [Google Scholar] [CrossRef]
- Strogoff-de-Matos, J.P.; Canziani, M.E.F.; Barra, A.B.L. Mortality on Hemodiafiltration Compared to High-Flux Hemodialysis: A Brazilian Cohort Study. Am. J. Kidney Dis. 2025. [CrossRef]
- Marcelli, D.; Kirchgessner, J.; Amato, C.; Steil, H.; Mitteregger, A.; Moscardo, V.; Carioni, C.; Orlandini, G.; Gatti, E. EuCliD (European Clinical Database): A database comparing different realities. J. Nephrol. 2001, 14 (Suppl. S4), S94–S100. [Google Scholar][Green Version]
- Barbieri, C.; Neri, L.; Stuard, S.; Mari, F.; Martin-Guerrero, J.D. From electronic health records to clinical management systems: How the digital transformation can support healthcare services. Clin. Kidney J. 2023, 16, 1878–1884. [Google Scholar] [CrossRef]
- Schouten, A.E.M.; Fischer, F.; Blankestijn, P.J.; Vernooij, R.W.M.; Hockham, C.; Strippoli, G.F.M.; Canaud, B.; Hegbrant, J.; Barth, C.; Cromm, K.; et al. A health economic evaluation of the multinational, randomized controlled CONVINCE trial—Cost-utility of high-dose online hemodiafiltration compared to high-flux hemodialysis. Kidney Int. 2025, 107, 728–739. [Google Scholar] [CrossRef]
- Belmouaz, M.; Goussard, G.; Joly, F.; Sibille, A.; Martin, C.; Betous, T.; Thierry, A.; Bauwens, M.; Bridoux, F. Comparison of High-Flux, Super High-Flux, Medium Cut-Off Hemodialysis and Online Hemodiafiltration on the Removal of Uremic Toxins. Blood Purif. 2023, 52, 309–318. [Google Scholar] [CrossRef]
- Blankestijn, P.J. Do Medium Cut-Off Dialyzers Offer Any Clinical Benefit or Should We Focus on Hemodiafiltration? J. Am. Soc. Nephrol. 2025. [Google Scholar] [CrossRef]
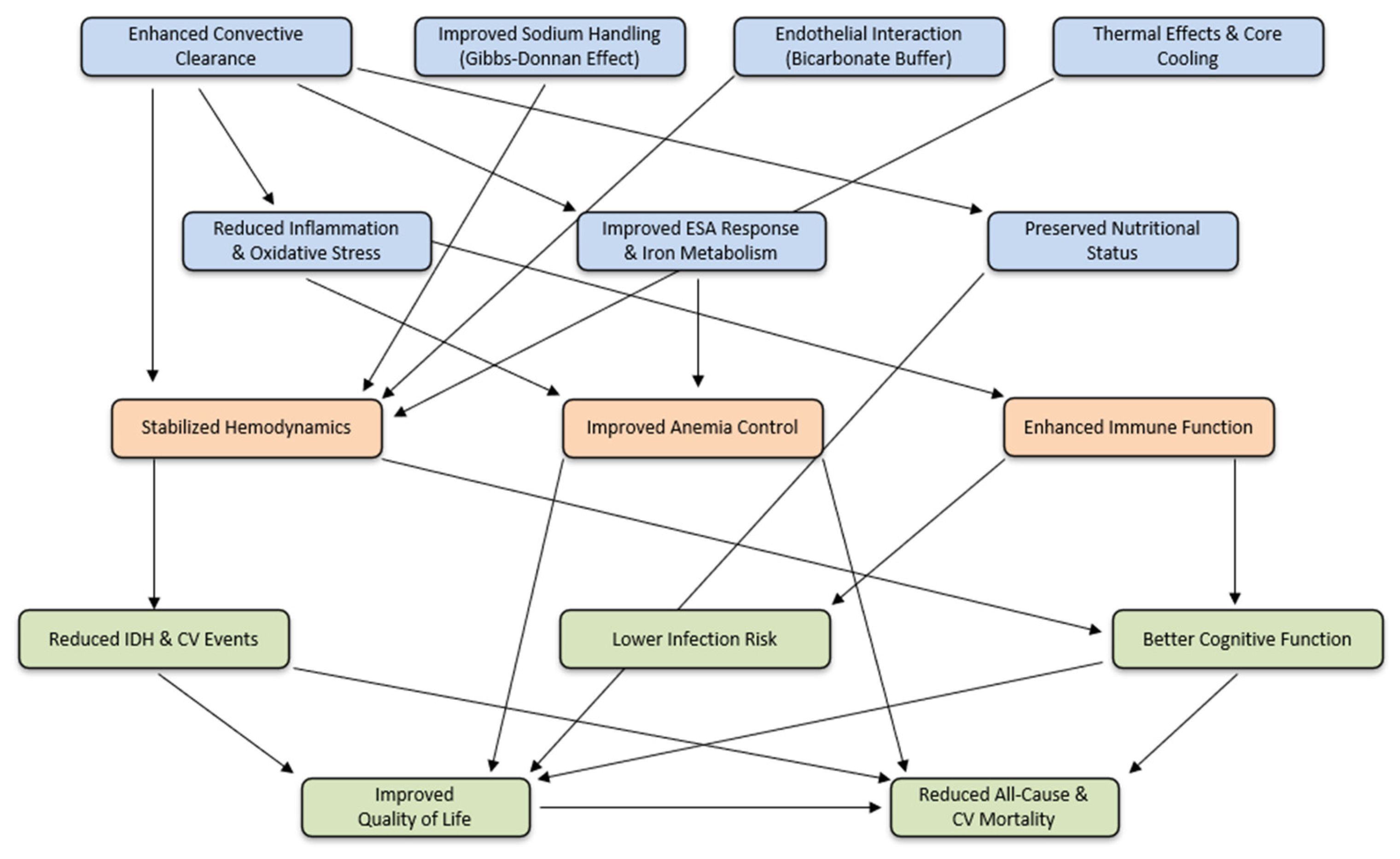
| Time Frame | Outcome Domains | HDF Key Findings |
|---|---|---|
| Short Term | ↑ toxin clearance | ↑ removal of small/MM. |
| within days | ↑ hemodynamic stability | ↓ IDH, ↑ thermal balance, ↓ inflammation,… |
| to weeks | ↓ inflammation | ↓ hsCRP, ↓ IL-6, ↓ TNF-α, ↓ β2M, ↓ pentraxin… |
| ↓ oxidative stress | ↓ AGEs, ↓ oxidized LDL, ↑ TAC, … | |
| ↑ anemia management | ↓ ESA resistance/hepcidin, ↑ RC survival | |
| ↑ kidney protection | ↑ hemodynamic stability, ↓ inflammation | |
| ↓ intradialytic cramps | ↓ IDH (?) | |
| ↓ skin hyperpigmentation | ↓ melanin | |
| Mid Term | ↓ amyloidosis, ↓ JP | ↓ β2M, ↓ inflammation |
| months to 2 | ↑ nutritional status | ↑ LT mass, ↑ physical activity, ↓ leptin |
| years | ↓ infection risk | ↓ uremic toxins, ↓ intestinal ischemia, … |
| ↑ CV benefits | Direct and indirect effects on CV system | |
| ↑ peripheral neuropathy | ↓ oxidative stress, ↓ indoxyl sulfate, ↓ β2M, … | |
| ↓ cognitive impairment | ↓ neurotoxic MM, ↓ cerebral ischemia, … | |
| ↑ HRQoL improvement | ↑ physical function, ↑ social participation, … | |
| Long Term | ↓ all-cause mortality | Convection volume ≥ 23 L/session |
| >2 years | ↓ CV mortality | Convection volume ≥ 23 L/session |
| Cost-effectiveness | ↓ cost–QALY in economic modeling |
| MMWs and PBTUs | MW (Da) | Clinical Relevance |
|---|---|---|
| Insulin [35] | 5800 | Glucose metabolism |
| PTH Fragments [32,36] | 9000 | CKD-MBD |
| AGE Products [26,37] | >10,000 | Oxidative vascular damage |
| Complement C3a/C5a [38] | 11,500 | Inflammation, immune response |
| Beta 2-Microglobulin [39,40,41,42,43,44,45,46] | 11,800 | Amyloidosis, inflammation |
| Leptin [47,48] | 16,000 | Appetite regulation |
| TNF-α [49] | 17,000 | Systemic inflammation |
| Myoglobin [50,51] | 17,000 | Rhabdomyolysis marker |
| Interleukin-1 [52] | 17,000 | Inflammation, immune signaling |
| Retinol-Binding Protein [53] | 21,000 | Insulin resistance |
| FLC K/L [50,54,55] | 22,000 | Inflammation, dyscrasias |
| Beta-trace Protein [50] | 23,000 | GFR biomarker |
| Complement Factor D [56] | 24,000 | Complement activation |
| Hepcidin [57,58] | 25,000 | Iron regulation |
| α-1 Microglobulin [59] | 26,000 | Tubular injury, oxidative stress |
| Interleukin-6 [60] | 26,000 | Inflammation, CV risk |
| FGF 23 [61,62] | 32,000 | CKD-MBD, vascular calcification |
| α-1-Acid Glycoprotein [50] | 43,000 | Acute-phase protein |
| PB p-Cresyl Sulfate [63,64,65] | 188 | Inflammation, atherosclerosis |
| PB Indoxyl Sulfate [63,64,65] | 213 | Vascular calcification, ox. stress |
| Study | Country | Sample Size (HD/HDF) | Sub/Conv Volume (L/session) | Primary Outcome | Key Findings |
|---|---|---|---|---|---|
| ICS [70] | Italy | 70/40 | Sub: 30–40 (pre) | ISH | ↓ ISH 50.9% with HDF |
| CONTRAST [223] | NL-CA | 356/358 | Sub: 19.8 | All-cause mortality | No difference overall, but benefit with high-volume HDF |
| Turkish [33] | Turkey | 391/391 | 17.2/19.5 | All-cause mortality + CV event | No difference overall, better survival in high-efficiency HDF |
| ESHOL [34] | Spain | 450/456 | 21.8/23.9 | All-cause mortality | 30% lower all-cause mortality in HDF |
| FRENCHIE [73] | France | 191/190 | 20/21 | Intradialytic tolerance | Better tolerance; no difference in mortality |
| CONVINCE [2] | M | 677/683 | 23.0/25.5 | All-cause mortality | HVHDF ↓ all-cause morta-lity by 23% (HR 0.77) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stuard, S.; Maddux, F.W.; Canaud, B. Why High-Volume Post-Dilution Hemodiafiltration Should Be the New Standard in Dialysis Care: A Comprehensive Review of Clinical Outcomes and Mechanisms. J. Clin. Med. 2025, 14, 4860. https://doi.org/10.3390/jcm14144860
Stuard S, Maddux FW, Canaud B. Why High-Volume Post-Dilution Hemodiafiltration Should Be the New Standard in Dialysis Care: A Comprehensive Review of Clinical Outcomes and Mechanisms. Journal of Clinical Medicine. 2025; 14(14):4860. https://doi.org/10.3390/jcm14144860
Chicago/Turabian StyleStuard, Stefano, Franklin W. Maddux, and Bernard Canaud. 2025. "Why High-Volume Post-Dilution Hemodiafiltration Should Be the New Standard in Dialysis Care: A Comprehensive Review of Clinical Outcomes and Mechanisms" Journal of Clinical Medicine 14, no. 14: 4860. https://doi.org/10.3390/jcm14144860
APA StyleStuard, S., Maddux, F. W., & Canaud, B. (2025). Why High-Volume Post-Dilution Hemodiafiltration Should Be the New Standard in Dialysis Care: A Comprehensive Review of Clinical Outcomes and Mechanisms. Journal of Clinical Medicine, 14(14), 4860. https://doi.org/10.3390/jcm14144860







