Quality of Life Outcomes Following Total Temporomandibular Joint Replacement: A Systematic Review of Long-Term Efficacy, Functional Improvements, and Complication Rates Across Prosthesis Types
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Eligibility Criteria
- Population: Patients of any age undergoing total temporomandibular joint replacement (TMJR) for end-stage TMJ pathology, including osteoarthritis, rheumatoid arthritis, juvenile idiopathic arthritis, trauma, ankylosis, condylar resorption, or neoplasms.
- Intervention: Alloplastic TMJR using stock or custom prostheses. Studies including adjunctive procedures (e.g., fat grafting, orthognathic surgery) were included if TMJR outcomes were clearly delineated.
- Comparator: Studies with or without comparator groups were eligible. Comparisons could include pre- vs. postoperative outcomes, stock vs. custom devices, or TMJR vs. other surgical options.
- Outcomes:
- ○
- Primary outcomes: Pain reduction (e.g., VAS scores), functional recovery (e.g., maximum interincisal opening [MIO]), and quality of life (e.g., SF-36, TMJ-specific PROMs, narrative assessments).
- ○
- Secondary outcomes: Complication rates (e.g., heterotopic ossification, infection, prosthesis failure, persistent pain), long-term prosthesis survival, and the integration of advanced technologies (e.g., CAD/CAM, 3D printing, AR).
- Study Design: Randomized controlled trials (RCTs), prospective and retrospective cohort studies, and case series including ≥5 patients were included.
- Case reports or series with <5 patients;
- In vitro or biomechanical-only studies;
- Editorials, reviews, commentaries, or abstracts without peer-reviewed data;
- Non-English language studies without available translations.
2.3. Search Strategy
- PubMed (MEDLINE);
- Embase;
- Cochrane Library.
2.4. Study Selection
2.5. Data Extraction
- Study characteristics (authors, year, country, design);
- Sample size and demographics;
- Indications for TMJR;
- Prosthesis type (stock vs. custom; manufacturer if available);
- Surgical adjuncts (e.g., fat grafting, VSP):
- ○
- Follow-up duration;
- ○
- Outcome measures;
- ○
- Pain scores (pre- and post-op);
- ○
- MIO (pre- and post-op);
- ○
- QoL measures (validated instruments or narrative satisfaction).
- Complications (type and rate);
- Use of technology (e.g., CAD/CAM, 3D printing, AR, tissue engineering);
- Discrepancies in data extraction were resolved through consensus.
2.6. Risk of Bias Assessment
- Randomized controlled trials were assessed using the Cochrane Risk of Bias 2 (RoB 2) tool (11-13 Cavendish Square, London, W1G 0AN, United Kingdom)
- Observational studies were evaluated using the Newcastle–Ottawa Scale (NOS).
2.7. Data Synthesis and Analysis
- Due to heterogeneity in study design, outcome measures, and follow-up durations, meta-analysis was not feasible. A qualitative synthesis was performed.
- Findings were organized under the following domains:
- Pain reduction;
- Functional recovery (MIO, diet);
- Quality of life outcomes;
- Complications and management;
- Prosthesis type comparison (custom vs. stock);
- Pediatric TMJR outcomes;
- Technological innovations (CAD/CAM, AR, 3D printing, tissue engineering).
2.8. Ethical Considerations
3. Results
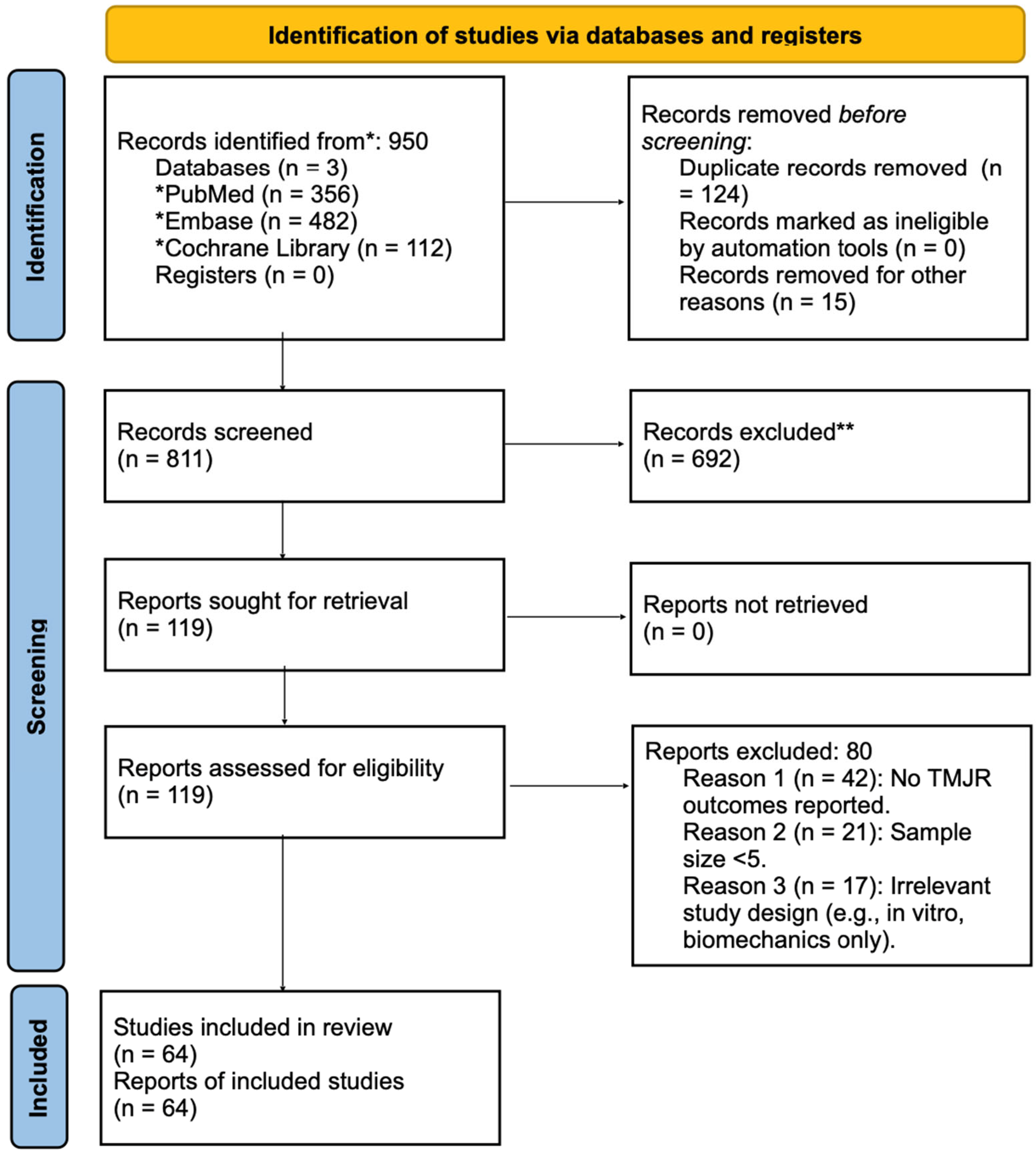
3.1. Study Selection
- The database search identified a total of 950 records: PubMed (n = 356), Embase (n = 482), and Cochrane Library (n = 112). After removing 124 duplicates and 15 non-relevant records (e.g., non-human studies, editorials), 811 records were screened by title and abstract. Of these, 692 were excluded based on the predefined eligibility criteria.
- Full-text review was conducted on 119 studies, with 80 excluded for the following reasons:
- No reported TMJR outcomes (n = 42);
- Sample size fewer than 5 patients (n = 21);
- In vitro or non-clinical/technical studies (n = 17);
- A total of 64 studies were included in the final qualitative synthesis: 39 studies from database screening and an additional 25 identified through manual reference screening and citation tracking.
3.2. Study Characteristics
- Age range: 13–72 years;
- Gender: Slight female predominance;
- Prosthesis types: 38 custom-fabricated, 19 stock, 8 hybrid or semi-custom;
- Follow-up periods: 6 months to over 20 years;
- Adjunctive procedures reported: fat grafting, orthognathic surgery, virtual surgical planning (VSP), CAD/CAM-guided surgery, and augmented reality-assisted navigation.
3.3. Quality of Life Outcomes
- The SF-36 Health Survey;
- TMJ-specific patient-reported outcome measures (PROMs);
- Narrative satisfaction reports or structured interviews.
- Reported QoL benefits included:
- Reduced pain and disability;
- Improved chewing, speech, and diet;
- Enhanced social confidence, sleep quality, and emotional well-being.
- Notable studies:
- Gupta et al. reported significant SF-36 improvements across pain, vitality, and social domains [7];
- Alba et al. observed airway, speech, and psychosocial gains in pediatric patients [11];
- Balel and Mercuri documented reduced somatization and anxiety [12].
3.4. Functional Outcomes and Pain Reduction
- Preoperative range: 8–25 mm;
- Postoperative range: 30–42 mm;
- Mean gain: 26–36 mm.
- Consistent across unilateral and bilateral TMJR;
3.5. Complications and Management (Table 1)
- While TMJR is generally safe, reported complications included the following:
| Complication | Approximate Rate | Notes |
|---|---|---|
| Heterotopic ossification (HO) | ~20% (without fat graft); <5% (with fat graft) | Prevented using autologous fat interposition [13,14] |
| Prosthetic infection | 3–4.9% | Managed with antibiotics, debridement, or staged revision [15,16] |
| Mechanical failure/ loosening | ~4–5% | Often seen in long-term follow-up; usually revised to custom [16,22,24,45] |
| Chronic pain | 20–30% | Frequently due to central sensitization; treated with multimodal pain therapy [17,34] |
| Facial nerve injury | ~10% transient; ~1–2% permanent | Minimized with careful dissection and nerve monitoring [46] |
3.6. Stock vs. Custom Prostheses
3.7. Pediatric TMJR
- No clear evidence of mandibular growth inhibition during 2–8 years of follow-up;
- Ongoing monitoring is needed as some patients may require revision during growth;
3.8. Technological Innovations
- Emerging technologies improving TMJR include the following:
4. Discussion
4.1. Interpretation of Findings
4.2. Quality of Life Improvements
- Functional gains supported emotional and psychological recovery, reinforcing TMJR’s role in restoring holistic well-being.
4.3. Prosthesis Type Comparison: Custom vs. Stock
4.4. Pediatric TMJR Applications
- No definitive evidence of growth inhibition has been reported in studies with follow-up of 2–8 years.
- However, long-term surveillance is essential. Pediatric TMJR patients may require prosthesis revision as they mature.
4.5. Complication Management (Table 2)
| Complication | Prevention/Management |
|---|---|
| Heterotopic ossification (HO) | Autologous fat grafting (reduces incidence to <5%) [13,14] |
| Infection | Perioperative antibiotics, debridement, staged revision if needed [15,16] |
| Prosthesis failure | Revision surgery, often converting to custom device [16,32,34] |
| Chronic pain | Multimodal therapy targeting central sensitization and myofascial dysfunction [17,33] |
| Facial nerve injury | Surgical planning, nerve monitoring, meticulous dissection [46] |
4.6. Technological Innovations
- Smart implants with embedded sensors are being explored to monitor joint loading and detect early mechanical wear [62].
4.7. Future Directions
- Long-term outcome registries (e.g., UK TMJR database) to track prosthesis performance, complication rates, and QoL at 10–20 years [30].
- Standardized outcome reporting using validated metrics (e.g., SF-36, TMJ-specific PROMs, VAS, MIO) across all centers.
- Multidisciplinary care models involving surgeons, engineers, pain specialists, and rehabilitation professionals.
- As TMJR evolves, its success will depend not only on structural reconstruction but also on biologic integration, patient-centered design, and long-term functional outcomes.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dimitroulis, G. Overview of TMJ disorders. Int. J. Oral Maxillofac. Surg. 2014, 43, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, L.G. Alloplastic TMJ replacement: Rationale for custom devices. Int. J. Oral Maxillofac. Surg. 2012, 41, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Wolford, L.M. Early challenges with Proplast-Teflon implants. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1997, 83, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.; Abdulqader, D.; Audi, K.; Ng, M.; Audi, S.; Vaderhobli, R.M. Temporomandibular Total Joint Replacement Implant Devices: A Systematic Review of Their Outcomes. J. Long Term Eff. Med. Implants 2021, 31, 91–98. [Google Scholar] [CrossRef]
- Mercuri, L.G. Past, present, and future of TMJ replacement. Br. J. Oral Maxillofac. Surg. 2024, 62, 91–96. [Google Scholar] [CrossRef]
- Rajkumar, A.; Sidebottom, A.J. Ten-year outcomes following TMJ Concepts total joint replacement. Int. J. Oral Maxillofac. Surg. 2022, 51, 665–668. [Google Scholar] [CrossRef]
- Gupta, B.; Ahmed, N.; Sidebottom, A.J. Quality of life outcomes after TMJR using modified SF-36 questionnaire. Br. J. Oral Maxillofac. Surg. 2020, 58, 304–308. [Google Scholar] [CrossRef]
- Burgess, M.; Bowler, M.; Jones, R.; Hase, M.; Murdoch, B. Improved outcomes after alloplastic TMJ replacement: Analysis of a multicenter study from Australia and New Zealand. J. Oral Maxillofac. Surg. 2014, 72, 1251–1257. [Google Scholar] [CrossRef]
- Westermark, A. TMJR outcomes with Biomet prostheses: Up to 8 years follow-up. Int. J. Oral Maxillofac. Surg. 2010, 39, 951–955. [Google Scholar] [CrossRef]
- Alakailly, X.; Schwartz, D.; Alwanni, N. Patient-centered QoL measures after TMJR. Int. J. Oral Maxillofac. Surg. 2017, 46, 204–207. [Google Scholar] [CrossRef]
- Alba, B.; Harmon, K.A.; La-Anyane, O.; Jones, K.; Figueroa, A.; Tragos, C. Bilateral Temporomandibular Joint Reconstruction with Custom Alloplastic Implants for Pediatric Ankylosis. J. Craniofac. Surg. 2024, 35, 1502–1506. [Google Scholar] [CrossRef] [PubMed]
- Balel, Y.; Mercuri, L.G. Emotional well-being improvements after TMJR. J. Oral Maxillofac. Surg. 2023, 81, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Lu, C.; Zhao, J.; He, D. Heterotopic ossification after alloplastic temporomandibular joint replacement: A case cohort study. BMC Musculoskelet. Disord. 2022, 23, 638. [Google Scholar]
- Wolford, L.M.; Wilkinson, T.M.; Tzou, C.H.; Ortiz, J.A. Fat grafting prevents heterotopic ossification in TMJR. J. Oral Maxillofac. Surg. 2016, 74, 1215–1227. [Google Scholar] [CrossRef]
- Gruber, E.A.; McNeill, C.; Shafer, D.M. Medium-term outcomes of TMJ Concepts prostheses. Br. J. Oral Maxillofac. Surg. 2015, 53, 412–415. [Google Scholar] [CrossRef]
- Amarista, F.J.; Mercuri, L.G.; Perez, D. TMJR prosthesis revision and replacement: A multicenter study. J. Oral Maxillofac. Surg. 2020, 78, 1692–1703. [Google Scholar] [CrossRef]
- Linsen, S.S.; Teschke, M.; Heim, N.; Mercuri, L.G. Chronic pain risk post-TMJR independent of indication. Br. J. Oral Maxillofac. Surg. 2023, 61, 337–343. [Google Scholar] [CrossRef]
- De Meurechy, N.K.G.; Politis, C. Custom vs stock TMJ prosthesis: A comparative analysis. Craniomaxillofac. Trauma Reconstr. 2020, 13, 179–187. [Google Scholar]
- Ackland, D.C.; Robinson, D.; Redhead, M.; Lee, P.V.S.; Dimitroulis, G. 3D-printed personalized TMJR design and outcomes. J. Mech. Behav. Biomed. Mater. 2017, 69, 404–411. [Google Scholar] [CrossRef]
- Zou, L.; He, D.; Ellis, E. A Comparison of Clinical Follow-Up of Different Total Temporomandibular Joint Replacement Prostheses: A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2018, 76, 294–303. [Google Scholar] [CrossRef]
- Gonzalez-Perez, L.M.; Gonzalez-Perez-Somarriba, B.; Centeno, G. Five-year outcomes after TMJ replacement with stock prostheses. Br. J. Oral Maxillofac. Surg. 2020, 58, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Sidebottom, A.J.; Salha, R. Management of the failing temporomandibular joint replacement. Br. J. Oral Maxillofac. Surg. 2013, 51, 594–598. [Google Scholar] [CrossRef]
- Vorrasi, J.; Harris, H.; Karras, M.; Basir Barmak, A.; Kolokythas, A. Prosthetic temporomandibular joint replacement (TJR): Stock or custom? A single institution pilot comparison. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2023, 135, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Linsen, S.S.; Schön, A.; Mercuri, L.G.; Teschke, M. Bilateral TMJR biomechanical outcomes. J. Oral Maxillofac. Surg. 2021, 79, 2433–2443. [Google Scholar] [CrossRef] [PubMed]
- Sembronio, S.; Tel, A.; Robiony, M. The use of cutting/positioning devices for custom-fitted temporomandibular joint alloplastic reconstruction: Current knowledge and development of a new system. Int. J. Oral Maxillofac. Surg. 2021, 50, 530–537. [Google Scholar] [CrossRef]
- Xu, X.; Luo, D.; Guo, C.; Rong, Q. A custom-made temporomandibular joint prosthesis for fabrication by selective laser melting: Finite element analysis. Med. Eng. Phys. 2017, 46, 1–11. [Google Scholar] [CrossRef]
- Niloy, I.; Liu, R.H.; Pham, N.M.; Yim, C.M.R. Augmented reality-assisted TMJ total joint replacement. J. Oral Maxillofac. Surg. 2024, 82, 632–640. [Google Scholar] [CrossRef]
- Singh, A.; Roychoudhury, A.; Bhutia, O.; Yadav, R.; Bhatia, R.; Yadav, P. Longitudinal changes in electromyographic activity of masseter and anterior temporalis muscles before and after alloplastic total joint replacement in patients with temporomandibular ankylosis: A prospective study. Br. J. Oral Maxillofac. Surg. 2022, 60, 896–903. [Google Scholar] [CrossRef]
- Nedrelow, D.S.; Rassi, A.; Ajeeb, B.; Jones, C.P.; Huebner, P.; Ritto, F.G.; Williams, W.R.; Fung, K.M.; Gildon, B.W.; Townsend, J.M.; et al. Regenerative Engineering of a Biphasic Patient-Fitted Temporomandibular Joint Condylar Prosthesis. Tissue Eng. Part. C Methods. 2023, 29, 307–320. [Google Scholar] [CrossRef]
- Sidebottom, A.J. UK national TMJR database experience. Br. J. Oral Maxillofac. Surg. 2017, 55, 446–451. [Google Scholar]
- Sinn, D.P.; Tandon, R.; Tiwana, P.S. Alloplastic TMJR in growing patients: Preliminary outcomes. J. Oral Maxillofac. Surg. 2021, 79, 2267.e1–2267.e16. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Mao, Y.; Zhou, Z.; Zheng, J.; Zhen, J.; Qiu, Y.; Zhang, S.; Qin, H.; Yang, C. Biomechanical evaluation of Chinese customized three-dimensionally printed total temporomandibular joint prostheses: A finite element analysis. J. Craniomaxillofac. Surg. 2018, 46, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Resnick, C.M. TMJR in growing patients: Challenges and future strategies. Oral Maxillofac. Surg. Clin. N. Am. 2018, 30, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Bach, E.; Sigaux, N.; Fauvernier, M.; Cousin, A.S. Reasons for failure of total temporomandibular joint replacement: A systematic review and meta-analysis. Int. J. Oral. Maxillofac. Surg. 2022, 51, 1059–1068. [Google Scholar] [CrossRef]
- Mercuri, L.G. The role of VSP and 3D printing in TMJR evolution. Br. J. Oral Maxillofac. Surg. 2023, 61, 401–409. [Google Scholar]
- Keyser, B.R.; Banda, A.K.; Mercuri, L.G.; Warburton, G.; Sullivan, S.M. Alloplastic total temporomandibular joint replacement in skeletally immature patients: A pilot survey. Int. J. Oral. Maxillofac. Surg. 2020, 49, 1202–1209. [Google Scholar] [CrossRef]
- Khattak, Y.R.; Ghaffar, N.; Gulzar, M.A.; Rahim, S.; Rafique, F.; Jan, Z.; Iqbal, S.; Ahmad, I. Can growing patients with end-stage TMJ pathology be successfully treated with alloplastic temporomandibular joint reconstruction? - A systematic review. Oral Maxillofac. Surg. 2024, 28, 529–537. [Google Scholar] [CrossRef]
- Arif, H.; Ashraf, R.; Khan, F.; Khattak, Y.R.; Nisar, H.; Ahmad, I. Total temporomandibular joint reconstruction prosthesis in hemifacial microsomia: A systematic review. Orthod. Craniofac. Res. 2024, 27, 15–26. [Google Scholar] [CrossRef]
- Kanatsios, S.; Thomas, A.M.; Tocaciu, S. Comparative outcomes of stock vs custom TMJR. J. Craniomaxillofac. Surg. 2022, 50, 322–327. [Google Scholar] [CrossRef]
- Aagaard, E.; Thygesen, T. Patient outcomes following TMJR with custom Biomet prosthesis. Int. J. Oral Maxillofac. Surg. 2014, 43, 1229–1235. [Google Scholar] [CrossRef]
- Schwartz, H.C.; Relle, R.J. Distraction osteogenesis for TMJ reconstruction. J. Oral Maxillofac. Surg. 2008, 66, 718–723. [Google Scholar] [CrossRef]
- Mian, M.; Ackland, D.; Fink, S.; Wang, N.; Dimitroulis, G. Accuracy of custom temporomandibular joint replacement surgery using a virtual surgical planning protocol. Oral Maxillofac. Surg. 2021, 25, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Gerbino, G.; Zavattero, E.; Bosco, G.; Berrone, S.; Ramieri, G. Temporomandibular joint reconstruction with stock and custom-made devices: Indications and results of a 14-year experience. J. Craniomaxillofac. Surg. 2017, 45, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jiao, K. Engineering patient-specific TMJ scaffolds. Sci. Transl. Med. 2020, 12, eabb6683. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, L.G.; Giobbie-Hurder, A. Long-term outcomes after alloplastic TMJR following failed materials. J. Oral Maxillofac. Surg. 2004, 62, 1088–1096. [Google Scholar] [CrossRef]
- Schwartz, H.C. Facial nerve monitoring in TMJ surgery. J. Oral Maxillofac. Surg. 2015, 73, 2341–2347. [Google Scholar]
- Caniello, G.; Oliveira dos Santos, R.; Miglioli, K.; Borges Scribon, A. Major clinical approaches of prefabricated and customized prostheses for the temporomandibular joint: A systematic review. Med. NEXT J. Med. Health Sci. 2023, 4, 1–12. [Google Scholar] [CrossRef]
- Wojczyńska, A.; Gallo, L.M.; Bredell, M.; Leiggener, C.S. Alterations of mandibular movement patterns after total joint replacement: A case series of long-term outcomes in patients with total alloplastic temporomandibular joint reconstructions. Int. J. Oral Maxillofac. Surg. 2019, 48, 225–232. [Google Scholar] [CrossRef]
- Idle, M.R.; Lowe, D.; Rogers, S.N.; Sidebottom, A.J.; Speculand, B.; Worrall, S.F. UK temporomandibular joint replacement database: Report on baseline data. Br. J. Oral Maxillofac. Surg. 2014, 52, 203–207. [Google Scholar] [CrossRef]
- Rahman, F.; Femiano, F.; Louis, P.J.; Kau, C.H. Jaw tracking evaluation after TMJR. Medicina 2022, 58, 738. [Google Scholar] [CrossRef]
- Bhargava, D. Hybrid TMJ prosthesis outcomes. Oral Maxillofac. Surg. 2024, 28, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, L.M.; Fakih-Gomez, N.; Gonzalez-Perez-Somarriba, B.; Centeno, G.; Montes-Carmona, J.F. Two-year prospective study of outcomes following total temporomandibular joint replacement. Int. J. Oral Maxillofac. Surg. 2016, 45, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Linsen, S.S.; Reich, R.H.; Teschke, M. Kinematic analysis of mandibular movement post-TMJR. J. Oral Maxillofac. Surg. 2012, 70, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Keller, E.E.; Reid, K.I. Metal hemijoint prosthesis for advanced TMJ arthritis. J. Oral Maxillofac. Surg. 2004, 62, 320–328. [Google Scholar] [CrossRef]
- Olate, S.; Ravelo, V.; Huentequeo, C.; Parra, M.; Unibazo, A. An Overview of Clinical Conditions and a Systematic Review of Personalized TMJ Replacement. J. Pers. Med. 2023, 13, 533. [Google Scholar] [CrossRef]
- Wojczyńska, A.; Leiggener, C.S.; Bredell, M.; Ettlin, D.A.; Erni, S.; Gallo, L.M.; Colombo, V. Alloplastic total temporomandibular joint replacements: Do they perform like natural joints? Prospective cohort study with a historical control. Int. J. Oral Maxillofac. Surg. 2016, 45, 1213–1221. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Mao, Y. Biomechanical behavior of 3D-printed TMJ prostheses. J. Craniomaxillofac. Surg. 2018, 46, 1561–1568. [Google Scholar] [CrossRef]
- Ackland, D.; Robinson, D.; Lee, P.V.S. Melbourne 3D-printed TMJ prosthesis validation. Clin. Biomech. 2018, 56, 52–60. [Google Scholar] [CrossRef]
- Del Castillo Pardo de Vera, J.L.; Cebrián Carretero, J.L.; Aragón Niño, Í.; Pampín Martínez, M.M.; Borjas Gómez, J.T.; Navarro Cuéllar, I.; López López, A.M.; Gómez Larren, E.; Navarro Vila, C.; Montes Fernández-Micheltorena, P.; et al. Virtual Surgical Planning for Temporomandibular Joint Reconstruction with Stock TMJ Prostheses: Pilot Study. Medicina 2024, 60, 339. [Google Scholar] [CrossRef]
- Cheng, K.J.; Liu, Y.F.; Wang, J.H.; Wang, R.; Xia, J.; Xu, X.; Jiang, X.F.; Dong, X.T. 3D-printed porous condylar prosthesis for temporomandibular joint replacement: Design and biomechanical analysis. Technol. Health Care 2022, 30, 1017–1030. [Google Scholar] [CrossRef]
- Cheng, K.J.; Liu, Y.F. Smart implant monitoring systems in TMJ prostheses. Technol. Health Care 2022, 30, 1025–1035. [Google Scholar]
- Mehra, P.; Nadershah, M.; Chigurupati, R. TMJR for idiopathic condylar resorption outcomes. J. Oral Maxillofac. Surg. 2016, 74, 2044–2054. [Google Scholar] [CrossRef] [PubMed]
- Terletskyi, R.; Dowgierd, K.; Chepurnyi, Y.; Kopchak, A.; Neff, A. Influence of preoperative anatomy and functional status on outcomes after total temporomandibular joint replacement with patient-specific endoprostheses: A retrospective cohort study. Dent. Med. Probl. 2025, 62, 57–64. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, L.E.; Zammuto, S.; Mercuri, L.G. Quality of Life Outcomes Following Total Temporomandibular Joint Replacement: A Systematic Review of Long-Term Efficacy, Functional Improvements, and Complication Rates Across Prosthesis Types. J. Clin. Med. 2025, 14, 4859. https://doi.org/10.3390/jcm14144859
Almeida LE, Zammuto S, Mercuri LG. Quality of Life Outcomes Following Total Temporomandibular Joint Replacement: A Systematic Review of Long-Term Efficacy, Functional Improvements, and Complication Rates Across Prosthesis Types. Journal of Clinical Medicine. 2025; 14(14):4859. https://doi.org/10.3390/jcm14144859
Chicago/Turabian StyleAlmeida, Luis Eduardo, Samuel Zammuto, and Louis G. Mercuri. 2025. "Quality of Life Outcomes Following Total Temporomandibular Joint Replacement: A Systematic Review of Long-Term Efficacy, Functional Improvements, and Complication Rates Across Prosthesis Types" Journal of Clinical Medicine 14, no. 14: 4859. https://doi.org/10.3390/jcm14144859
APA StyleAlmeida, L. E., Zammuto, S., & Mercuri, L. G. (2025). Quality of Life Outcomes Following Total Temporomandibular Joint Replacement: A Systematic Review of Long-Term Efficacy, Functional Improvements, and Complication Rates Across Prosthesis Types. Journal of Clinical Medicine, 14(14), 4859. https://doi.org/10.3390/jcm14144859







