Evaluation of Adverse Events Associated with the Sulfamethoxazole/Trimethoprim Combination Drug
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Extraction
2.2. Analysis of the ROR
2.3. Analysis of Onset Time of Adverse Events
3. Results
3.1. RORs and Number of Cases of Each Adverse Event Associated with ST
3.2. Time-to-Onset Analysis by the Weibull Distribution of Adverse Events
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| IDSA | Infectious Diseases Society of America |
| IQR | Interquartile range |
| JADER | Japanese Adverse Drug Event Report |
| MedDRA | Medical Dictionary for Regulatory Activities |
| PMDA | Pharmaceuticals and Medical Devices Agency |
| PT | Preferred term |
| ROR | Reported odds ratio |
| SIADH | Syndrome of inappropriate antidiuretic hormone secretion |
| SJS/TEN | Stevens–Johnson syndrome/toxic epidermal necrolysis |
| ST | Sulfamethoxazole/trimethoprim |
Appendix A
| Preferred Term Code | Case (n) |
|---|---|
| 10019670 Hepatic function abnormal | 237 |
| 10035528 Platelet count decreased | 237 |
| 10020646 Hyperkalaemia | 211 |
| 10033661 Pancytopenia | 210 |
| 10037844 Rash | 199 |
| 10001507 Agranulocytosis | 198 |
| 10042033 Stevens–Johnson syndrome | 198 |
| 10073508 Drug reaction with eosinophilia and systemic symptoms | 187 |
| 10013687 Drug eruption | 187 |
| 10047942 White blood cell count decreased | 174 |
| 10062237 Renal impairment | 168 |
| 10022611 Interstitial lung disease | 165 |
| 10044223 Toxic epidermal necrolysis | 141 |
| 10024670 Liver disorder | 131 |
| 10015218 Erythema multiforme | 125 |
| 10069339 Acute kidney injury | 120 |
| 10029354 Neutropenia | 112 |
| 10021036 Hyponatraemia | 110 |
| 10029366 Neutrophil count decreased | 108 |
| 10043554 Thrombocytopenia | 100 |
| 10016288 Febrile neutropenia | 89 |
| 10020993 Hypoglycaemia | 84 |
| 10072268 Drug-induced liver injury | 77 |
| 10002034 Anaemia | 71 |
| 10038428 Renal disorder | 62 |
| 10015150 Erythema | 61 |
| 10028584 Myelosuppression | 52 |
| 10024384 Leukopenia | 47 |
| 10013442 Disseminated intravascular coagulation | 47 |
| 10012735 Diarrhoea | 45 |
| 10025256 Lymphocyte count decreased | 43 |
| 10028813 Nausea | 40 |
| 10003481 Aspartate aminotransferase increased | 35 |
| 10001551 Alanine aminotransferase increased | 32 |
| 10066274 Cytopenia | 30 |
| 10047700 Vomiting | 28 |
| 10048302 Tubulointerstitial nephritis | 27 |
| 10039020 Rhabdomyolysis | 26 |
| 10071583 Haemophagocytic lymphohistiocytosis | 26 |
| 10060795 Hepatic enzyme increased | 26 |
| Reporting Year | Total Case (n) | Suspected ST, n (%) | Concomitant + Interaction ST, n (%) |
|---|---|---|---|
| 2004 | 24,403 | 118 (0.48) | 379 (1.55) |
| 2005 | 24,168 | 103 (0.43) | 454 (1.88) |
| 2006 | 24,281 | 110 (0.45) | 568 (2.34) |
| 2007 | 25,610 | 157 (0.61) | 543 (2.12) |
| 2008 | 28,593 | 149 (0.52) | 571 (2.00) |
| 2009 | 29,297 | 165 (0.56) | 843 (2.88) |
| 2010 | 32,972 | 169 (0.51) | 1167 (3.54) |
| 2011 | 36,173 | 175 (0.48) | 1364 (3.77) |
| 2012 | 40,778 | 194 (0.48) | 999 (2.45) |
| 2013 | 37,927 | 221 (0.58) | 1026 (2.71) |
| 2014 | 48,451 | 231 (0.48) | 904 (1.87) |
| 2015 | 50,191 | 241 (0.48) | 1282 (2.55) |
| 2016 | 54,929 | 272 (0.50) | 1174 (2.14) |
| 2017 | 60,263 | 365 (0.61) | 1303 (2.16) |
| 2018 | 61,472 | 320 (0.52) | 1219 (1.98) |
| 2019 | 60,604 | 298 (0.49) | 1108 (1.83) |
| 2020 | 51,843 | 253 (0.49) | 1100 (2.12) |
| 2021 | 82,353 | 318 (0.39) | 1374 (1.67) |
| 2022 | 71,877 | 283 (0.39) | 1530 (2.13) |
| 2023 (first quarter) | 16,767 | 61 (0.36) | 275 (1.64) |
| Total (2004–2023) | 862,952 | 4203 (0.49) | 19,183 (2.22) |
References
- Minato, Y.; Dawadi, S.; Kordus, S.L.; Sivanandam, A.; Aldrich, C.C.; Baughn, A.D. Mutual potentiation drives synergy between trimethoprim and sulfamethoxazole. Nat. Commun. 2018, 9, 1003. [Google Scholar] [CrossRef] [PubMed]
- Gleckman, R.; Blagg, N.; Joubert, D.W. Trimethoprim: Mechanisms of action, antimicrobial activity, bacterial resistance, pharmacokinetics, adverse reactions, and therapeutic indications. Pharmacotherapy 1981, 1, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Elajez, R.; Nisar, S.; Adeli, M. Does trimethoprim-sulfamethoxazole prophylaxis induce myelosuppression in primary immune deficiency disease patients; A retrospective, 3 groups comparative study. Asian Pac. J. Allergy Immunol. 2023, 41, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, T.; Seo, K.; Yoshino, Y.; Asako, K.; Kikuchi, H.; Kono, H.; Ota, Y. Efficacies of atovaquone, pentamidine, and trimethoprim/sulfamethoxazole for the prevention of Pneumocystis jirovecii pneumonia in patients with connective tissue diseases. J. Infect. Chemother. 2019, 25, 351–354. [Google Scholar] [CrossRef]
- Haseeb, A.; Abourehab, M.A.S.; Almalki, W.A.; Almontashri, A.M.; Bajawi, S.A.; Aljoaid, A.M.; Alsahabi, B.M.; Algethamy, M.; AlQarni, A.; Iqbal, M.S.; et al. Trimethoprim-sulfamethoxazole (bactrim) dose optimization in Pneumocystis jirovecii Pneumonia (PCP) management: A systematic review. Int. J. Environ. Res. Public Health 2022, 19, 2833. [Google Scholar] [CrossRef]
- Mikasa, K.; Aoki, N.; Aoki, Y.; Abe, S.; Iwata, S.; Ouchi, K.; Kasahara, K.; Kadota, J.; Kishida, N.; Kobayashi, O.; et al. The JAID/JSC Guideline to Clinical Management of Infectious Diseases (Respiratory Infections). Kansenshogaku Zasshi 2014, 88, 1–109. [Google Scholar] [CrossRef]
- Cockerill, F.R.; Edson, R.S. Trimethoprim-sulfamethoxazole. Mayo Clin. Proc. 1991, 66, 1260–1269. [Google Scholar] [CrossRef]
- Stevens, D.L.; Bisno, A.L.; Chambers, H.F.; Dellinger, E.P.; Goldstein, E.J.C.; Gorbach, S.L.; Hirschmann, J.V.; Kaplan, S.L.; Montoya, J.G.; Wade, J.C. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2014, 59, e10–e52. [Google Scholar] [CrossRef]
- Panel on Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV; U.S. Department of Health and Human Services: Rockville, MD, USA, 2023. Available online: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection (accessed on 21 June 2025).
- Maschmeyer, G.; Carratalà, J.; Buchheidt, D.; Hamprecht, A.; Heussel, C.P.; Kahl, C.; Karthaus, M.; Kern, W.; Kolbe, K.; Krause, R.; et al. Diagnosis and antimicrobial therapy of lung infiltrates in febrile neutropenic patients—Guidelines of the Infectious Diseases Working Party of the German Society of Hematology and Oncology. Eur. J. Cancer 2009, 45, 2462–2472. [Google Scholar] [CrossRef]
- Dworkin, M.S.; Hanson, D.L.; Kaplan, J.E.; Jones, J.L.; Ward, J.W. Prophylaxis with trimethoprim-sulfamethoxazole for human immunodeficiency virus–infected patients: Impact on risk for infectious diseases. Clin. Infect. Dis. 2001, 33, 393–398. [Google Scholar] [CrossRef]
- Sato, T.; Ito, R.; Kawamura, M.; Fujimura, S. The Risk of Emerging Resistance to Trime-thoprim/Sulfamethoxazole in Staphylococcus aureus. Infect. Drug Resist. 2022, 15, 4779–4784. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kawamura, M.; Furukawa, E.; Fujimura, S. Screening method for trimethoprim/sulfamethoxazole-resistant small colony variants of Staphylococcus aureus. J. Glob. Antimicrob. Resist. 2018, 15, 1–5. [Google Scholar] [CrossRef]
- Shimizu, Y.; Hirai, T.; Ogawa, Y.; Yamada, C.; Kobayashi, E. Characteristics of risk factors for acute kidney injury among inpatients administered sulfamethoxazole/trimethoprim: A retrospective observational study. J. Pharm. Health Care Sci. 2022, 8, 20. [Google Scholar] [CrossRef]
- Marak, C.; Nunley, M.; Guddati, A.K.; Kaushik, P.; Bannon, M.; Ashraf, A. Severe hyponatremia due to trimethoprim-sulfamethoxazole-induced SIADH. SAGE Open Med. Case Rep. 2022, 10, 2050313X221132654. [Google Scholar] [CrossRef]
- Fukasawa, T.; Urushihara, H.; Takahashi, H.; Okura, T.; Kawakami, K. Risk of Stevens-Johnson syndrome and toxic epidermal necrolysis associated with antibiotic use: A case-crossover study. J. Allergy Clin. Immunol. Pract. 2023, 11, 3463–3472. [Google Scholar] [CrossRef]
- Wang, C.; Fang, W.; Li, Z.; Sun, L. Clinical features, risk factors, diagnosis, and treatment of trimethoprim-sulfamethoxazole-induced hypoglycemia. Front. Endocrinol. 2023, 14, 1059522. [Google Scholar] [CrossRef]
- BAKTAR® Combination Tablets/BAKTAR® Mini Combination Tablets/BAKTAR® Combination Granules. Published July 2023. Available online: https://pins.japic.or.jp/pdf/newPINS/00070053.pdf (accessed on 19 June 2024).
- DailyMed—Sulfamethoxazole and Trimethoprim Double Strength—Sulfamethoxazole and Trimethoprim Tablet. NPublished 2020. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=804f8363-8ea1-4a57-bbd1-7fd9b6b4d29b (accessed on 19 June 2024).
- Co-Trimoxazole 80 mg/400 mg Per 5 mL Adult Suspension—Summary of Product Characteristics (SmPC)-(emc). Published 2021. Available online: https://www.medicines.org.uk/emc/product/6996/smpc (accessed on 19 June 2024).
- Ishida, T.; Kawada, K.; Morisawa, S.; Jobu, K.; Morita, Y.; Miyamura, M. Risk factors for pseudoaldosteronism with yokukansan use: Analysis using the Japanese adverse drug report (JADER) database. Biol. Pharm. Bull. 2020, 43, 1570–1576. [Google Scholar] [CrossRef]
- Ishida, T.; Kawada, K.; Jobu, K.; Kawazoe, T.; Tamura, N.; Miyamura, M. Analysis of drug-induced liver injury from Bofutsushosan administration using Japanese Adverse Drug Event Report (JADER) database. Biol. Pharm. Bull. 2022, 45, 460–466. [Google Scholar] [CrossRef]
- Sauzet, O.; Carvajal, A.; Escudero, A.; Molokhia, M.; Cornelius, V.R. Illustration of the Weibull shape parameter signal detection tool using electronic healthcare record data. Drug Saf. 2013, 36, 995–1006. [Google Scholar] [CrossRef]
- Noguchi, Y.; Katsuno, H.; Ueno, A.; Otsubo, M.; Yoshida, A.; Kanematsu, Y.; Sugita, I.; Esaki, H.; Tachi, T.; Tsuchiya, T.; et al. Signals of gastroesophageal reflux disease caused by incretin-based drugs: A disproportionality analysis using the Japanese adverse drug event report database. J. Pharm. Health Care Sci. 2018, 4, 15. [Google Scholar] [CrossRef]
- Ohyama, K.; Iida, M.; Akiyama, S.; Yamazaki, H.; Hori, Y. Time-to-onset analysis of rhabdomyolysis due to different proton pump inhibitors using a pharmacovigilance database. In Vivo 2024, 38, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, S.; Ishida, T.; Kawada, K.; Jobu, K.; Morisawa, S.; Tamura, N.; Takuma, D.; Yoshioka, S.; Miyamura, M. Central nervous system ischemia associated with bevacizumab: An analysis of the Japanese Adverse Drug Event Report database. Biol. Pharm. Bull. 2022, 45, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Meidahl Petersen, K.; Eplov, K.; Kjær Nielsen, T.; Jimenez-Solem, E.; Petersen, M.; Broedbaek, K.; Daugaard Popik, S.; Kallehave Hansen, L.; Enghusen Poulsen, H.; Trærup Andersen, J. The effect of trimethoprim on serum folate levels in humans: A randomized, double-blind, placebo-controlled trial. Am. J. Ther. 2016, 23, e382–e387. [Google Scholar] [CrossRef] [PubMed]
- Delanaye, P.; Mariat, C.; Cavalier, E.; Maillard, N.; Krzesinski, J.M.; White, C.A. Trimethoprim, creatinine and creatinine-based equations. Nephron Clin. Pract. 2011, 119, c187–c193; discussion c193–c194. [Google Scholar] [CrossRef]
- Nomura, K.; Takahashi, K.; Hinomura, Y.; Kawaguchi, G.; Matsushita, Y.; Marui, H.; Anzai, T.; Hashiguchi, M.; Mochizuki, M. Effect of database profile variation on drug safety assessment: An analysis of spontaneous adverse event reports of Japanese cases. Drug Des. Devel. Ther. 2015, 9, 3031–3041. [Google Scholar] [CrossRef]
- Smith, E.J.; Light, J.A.; Filo, R.S.; Yum, M.N. Interstitial nephritis caused by trimethoprim-sulfamethoxazole in renal transplant recipients. JAMA 1980, 244, 360–361. [Google Scholar] [CrossRef]
- Linton, A.L.; Clark, W.F.; Driedger, A.A.; Turnbull, D.I.; Lindsay, R.M. Acute interstitial nephritis due to drugs: Review of the literature with a report of nine cases. Ann. Intern. Med. 1980, 93, 735–741. [Google Scholar] [CrossRef]
- Ju, M.; Zheng, M.; Yuan, J.; Lin, D.; Qian, Y. Prevalence and risk factors of trimethoprim/sulfamethoxazole-related acute kidney injury in pediatric patients: An observational study from a public database. Transl. Pediatr. 2022, 11, 1285–1291. [Google Scholar] [CrossRef]
- Velázquez, H.; Perazella, M.A.; Wright, F.S.; Ellison, D.H. Renal mechanism of trimethoprim-induced hyperkalemia. Ann. Intern. Med. 1993, 119, 296–301. [Google Scholar] [CrossRef]
- Chalasani, N.; Bonkovsky, H.L.; Fontana, R.; Lee, W.; Stolz, A.; Talwalkar, J.; Reddy, K.R.; Watkins, P.B.; Navarro, V.; Barnhart, H.; et al. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN prospective study. Gastroenterology 2015, 148, 1340–1352.e7. [Google Scholar] [CrossRef]
- Li, Y.J.; Phillips, E.J.; Dellinger, A.; Nicoletti, P.; Schutte, R.; Li, D.; Ostrov, D.A.; Fontana, R.J.; Watkins, P.B.; Stolz, A.; et al. Human leukocyte antigen B14:01 and B35:01 are associated with trimethoprim-sulfamethoxazole induced liver injury. Hepatology 2021, 73, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.A.; Knowles, S.R.; Shear, N.H. Sulfonamide hypersensitivity: Fact and fiction. J. Allergy Clin. Immunol. Pract. 2019, 7, 2116–2123. [Google Scholar] [CrossRef]
- Illing, P.T.; Mifsud, N.A.; Purcell, A.W. Allotype specific interactions of drugs and HLA molecules in hypersensitivity reactions. Curr. Opin. Immunol. 2016, 42, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Naisbitt, D.J.; Hough, S.J.; Gill, H.J.; Pirmohamed, M.; Kitteringham, N.R.; Park, B.K. Cellular disposition of sulphamethoxazole and its metabolites: Implications for hypersensitivity. Br. J. Pharmacol. 1999, 126, 1393–1407. [Google Scholar] [CrossRef]
- Rossio, R.; Arcudi, S.; Peyvandi, F.; Piconi, S. Persistent and severe hypoglycemia associated with trimethoprim-sulfamethoxazole in a frail diabetic man on polypharmacy: A case report and literature review. Int. J. Clin. Pharmacol. Ther. 2018, 56, 86–89. [Google Scholar] [CrossRef]
- Ogata, H.; Kitamura, S.; Fujiwara, M.; Shimizu, M.; Tan, C.; Zhao, S.; Maejima, Y.; Shimomura, K. Dose dependent effect of sulfamethoxazole on inhibiting KATP channel of mouse pancreatic β cell. Dose Response 2023, 21, 15593258231203611. [Google Scholar] [CrossRef]
- Klein-Schwartz, W.; Stassinos, G.L.; Isbister, G.K. Treatment of sulfonylurea and insulin overdose. Br. J. Clin. Pharmacol. 2016, 81, 496–504. [Google Scholar] [CrossRef]
- Asmar, B.I.; Maqbool, S.; Dajani, A.S. Hematologic abnormalities after oral trimethoprim-sulfamethoxazole therapy in children. Am. J. Dis. Child. 1981, 135, 1100–1103. [Google Scholar] [CrossRef]
- Doodnauth, A.V.; Sivakumar, S.; Mulatu, Y.; Alicea, E.; McFarlane, S.I. Acute severe throm-bocytopenia event associated with trimethoprim-sulfamethoxazole use. Am. J. Med. Case Rep. 2021, 9, 155–157. [Google Scholar] [CrossRef]
- Justice, J.; Mukherjee, E.; Martin-Poz, M.; Phillips, E. Updates in the pathogenesis of SJS/TEN. Allergol. Int. 2025, 74, 361–371. [Google Scholar] [CrossRef]
- Roujeau, J.C.; Kelly, J.P.; Naldi, L.; Rzany, B.; Stern, R.S.; Anderson, T.; Begaud, B.; Shapiro, S.; Kaufman, D.W.; Brup-pacher, R. Medication use and the risk of Stevens–Johnson syndrome or toxic epidermal necrolysis. N. Engl. J. Med. 1995, 333, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Knox, C.; Phillips, E.J. Worldwide prevalence of antibiotic-associated Stevens-Johnson syndrome and toxic epidermal necrolysis: A systematic review and meta-analysis. JAMA Dermatol. 2023, 159, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Wang, C.W.; Lu, C.W.; Chen, C.B.; Lee, H.E.; Hung, S.I.; Choon, S.E.; Yang, C.H.; Liu, M.T.; Chen, T.J.; et al. The function of HLA-B*13:01 involved in the pathomechanism of dap-sone-induced severe cutaneous adverse reactions. JAMA Dermatol. 2018, 138, 1546–1554. [Google Scholar] [CrossRef]
- Matsuda, S.; Aoki, K.; Kawamata, T.; Kimotsuki, T.; Kobayashi, T.; Kuriki, H.; Nakayama, T.; Okugawa, S.; Sugimura, Y.; Tomita, M.; et al. Bias in spontaneous reporting of adverse drug reactions in Japan. PLoS ONE 2015, 10, e0126413. [Google Scholar] [CrossRef]
- Sakai, T. A checklist of important points in research using JADER. Jpn. J. Pharmacoepidemiol. 2020, 25, 64–73. [Google Scholar] [CrossRef]
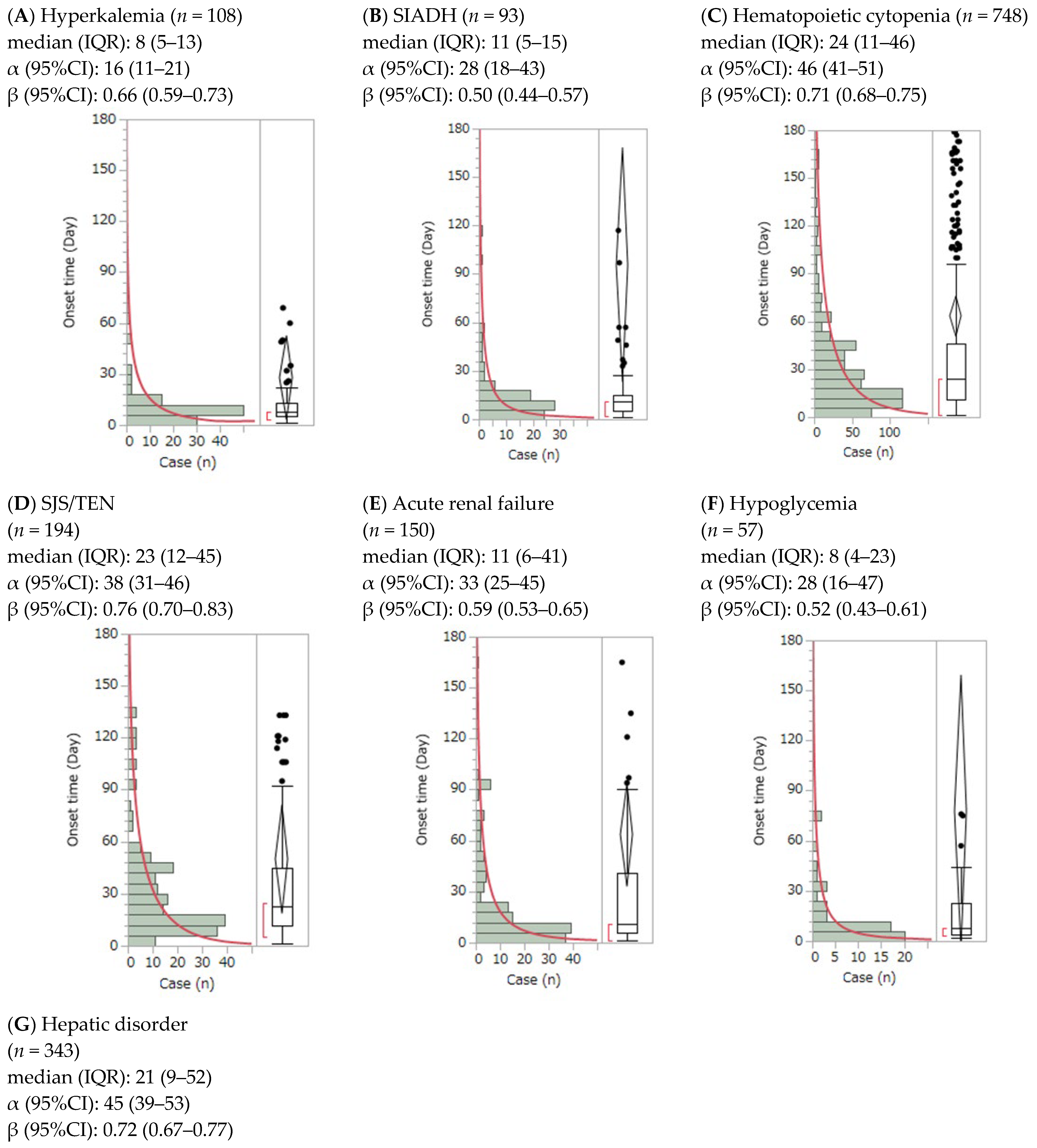

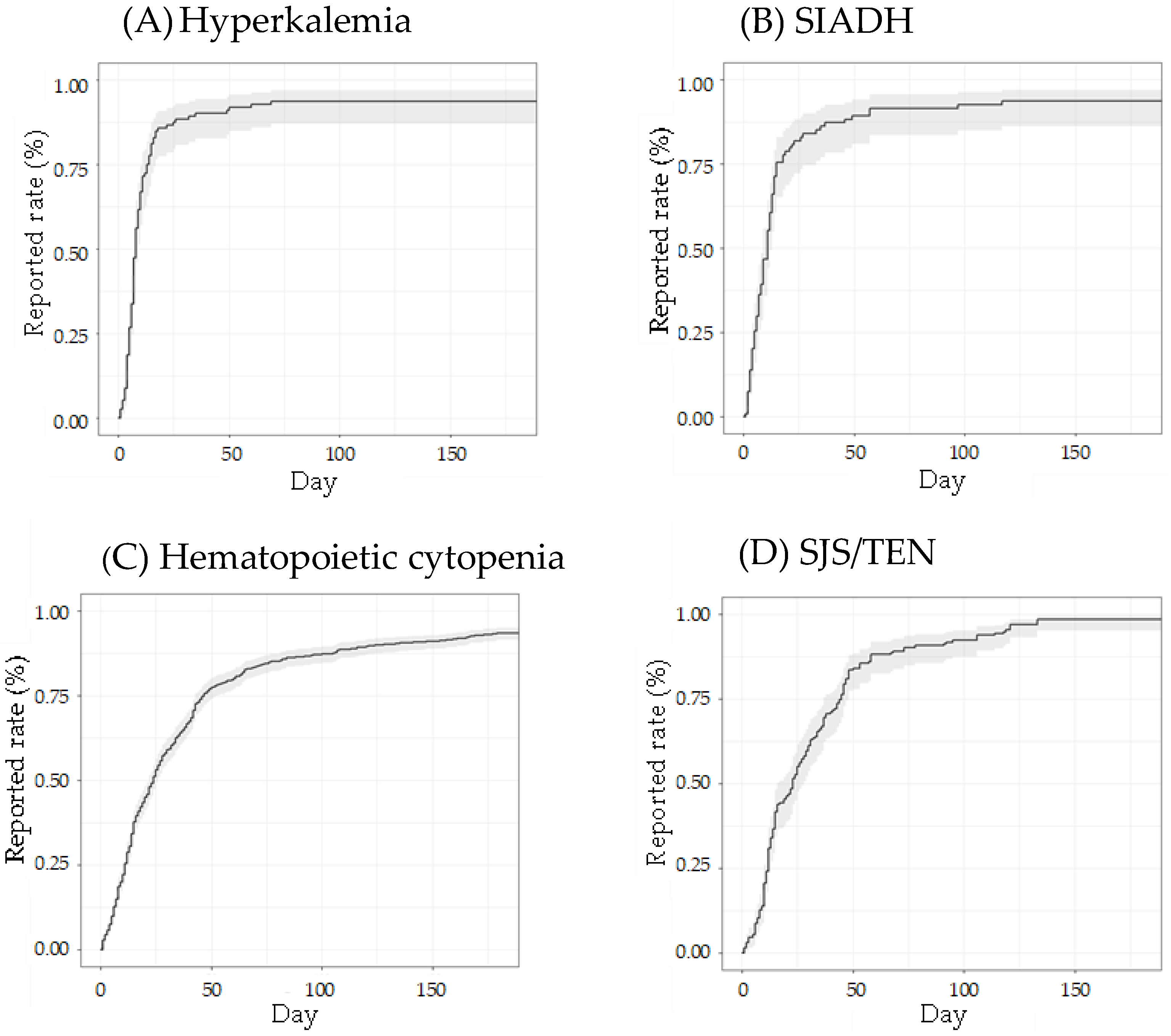
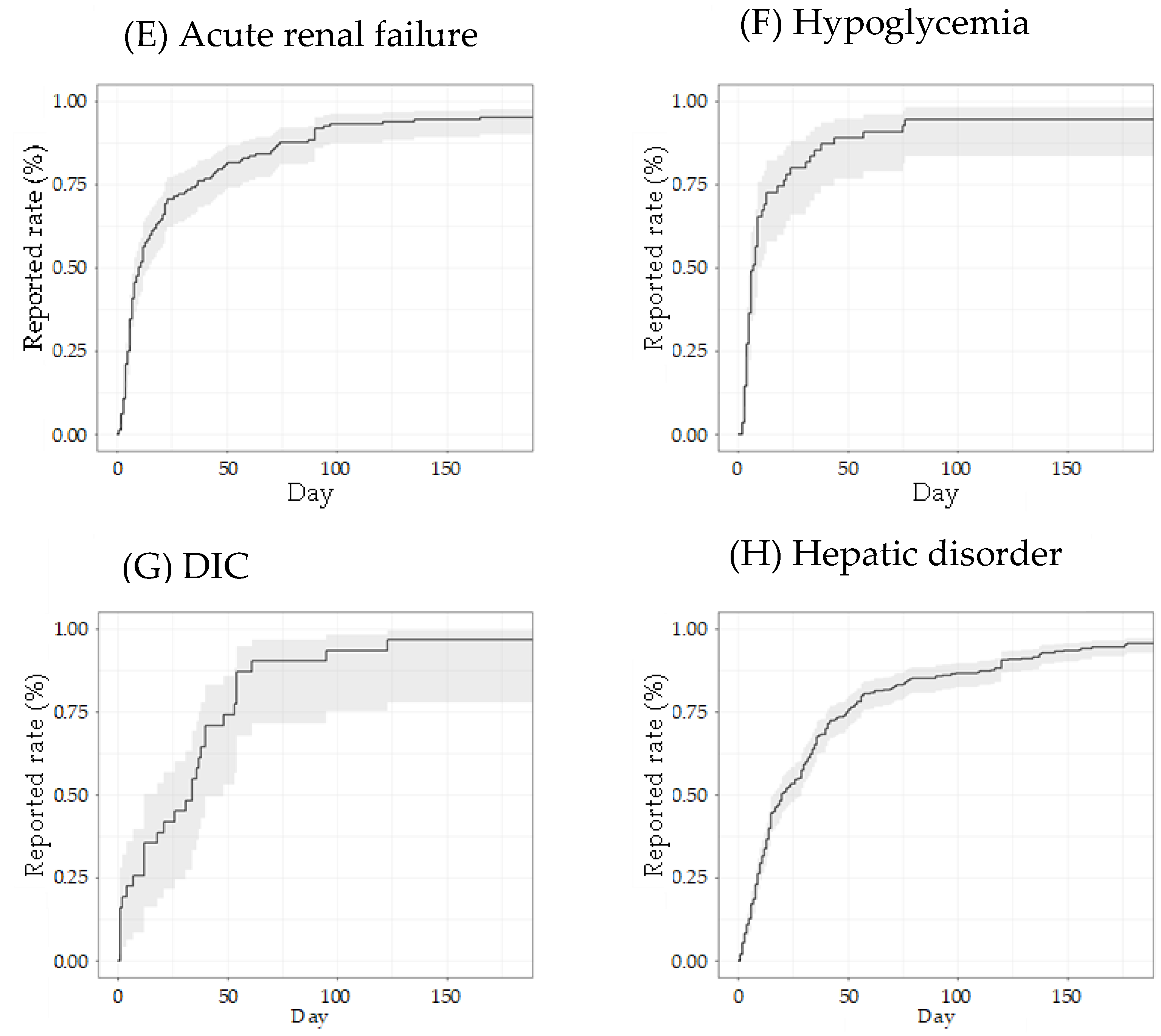
| Adverse Event | Suspected | Concomitant + Interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | No Case | Ratio (%) | ROR (95%CI) | p Value | Case | No Case | Ratio (%) | ROR
(95% CI) | p Value | |
| Hyperkalemia | 230 | 3973 | 5.5 | 11.3 (9.85–13.0) | <0.001 | 110 | 19,073 | 0.6 | 1.08 (0.88–1.31) | 0.42 |
| SIADH | 136 | 4067 | 3.2 | 4.67 (3.90–5.55) | <0.001 | 188 | 18,995 | 1.0 | 1.37 (1.18–1.58) | <0.001 |
| Hematopoietic cytopenia | 1232 | 2971 | 29.3 | 3.53 (3.31–3.79) | <0.001 | 5744 | 13,439 | 29.9 | 3.79 (3.67–3.91) | <0.001 |
| Cytopenia affecting more | 311 | 3892 | 7.4 | 4.22 (3.75–4.75) | <0.001 | 909 | 18,274 | 4.7 | 2.68 (2.50–2.88) | <0.001 |
| Erythropenia | 43 | 4160 | 1.0 | 4.06 (2.93–5.50) | <0.001 | 102 | 19,081 | 0.5 | 2.12 (1.72–2.59) | <0.001 |
| Leukopenia | 701 | 3502 | 16.7 | 2.80 (2.58–3.04) | <0.001 | 4147 | 15,036 | 21.6 | 4.05 (3.91–4.20) | <0.001 |
| Thrombocytopenia | 334 | 3869 | 7.9 | 2.56 (2.28–2.86) | <0.001 | 2267 | 16,916 | 11.8 | 4.20 (4.01–4.39) | <0.001 |
| Hypersensitivity | 1167 | 3036 | 27.8 | 2.78 (2.59–2.97) | <0.001 | 1651 | 17,532 | 8.6 | 0.67 (0.64–0.71) | <0.001 |
| SJS/TEN | 343 | 3860 | 8.2 | 7.19 (6.41–8.04) | <0.001 | 149 | 19,080 | 0.8 | 0.61 (0.51–0.72) | <0.001 |
| Anaphylactic reaction | 46 | 4157 | 1.1 | 0.23 (0.17–0.31) | <0.001 | 522 | 18,661 | 2.7 | 0.58 (0.53–0.64) | <0.001 |
| Acute renal failure | 317 | 3886 | 7.5 | 2.39 (2.12–2.68) | <0.001 | 713 | 18,470 | 3.7 | 1.13 (1.04–1.21) | 0.002 |
| Hypoglycemia | 100 | 4103 | 2.4 | 2.33 (1.89–2.84) | <0.001 | 84 | 18,089 | 0.5 | 0.41 (0.33–0.51) | <0.001 |
| DIC | 47 | 4156 | 1.1 | 2.25 (1.64–2.99) | <0.001 | 188 | 18,995 | 1.0 | 1.99 (1.71–2.31) | <0.001 |
| Hepatic disorder | 589 | 3614 | 14.0 | 2.07 (1.90–2.26) | <0.001 | 1487 | 17,696 | 7.8 | 1.07 (1.01–1.12) | 0.021 |
| Interstitial lung disease | 165 | 4038 | 3.9 | 0.88 (0.75–1.03) | 0.12 | 855 | 18,328 | 4.5 | 1.01 (0.94–1.08) | 0.79 |
| Nausea and vomiting symptoms | 55 | 4148 | 1.3 | 0.81 (0.61–1.06) | 0.14 | 327 | 18,856 | 1.7 | 1.06 (0.95–1.19) | 0.28 |
| Noninfectious diarrhea | 45 | 4158 | 1.1 | 0.78 (0.57–1.05) | 0.11 | 380 | 18,803 | 2.0 | 1.47 (1.33–1.63) | <0.001 |
| Rhabdomyolysis | 26 | 4177 | 0.6 | 0.69 (0.45–1.01) | 0.059 | 58 | 19,125 | 0.3 | 0.33 (0.25–0.43) | <0.001 |
| Adverse Events | Adjusted ROR (95% CI) | p-Value |
|---|---|---|
| Hyperkalemia | 9.45 (8.10—1.10) | <0.001 |
| SIADH | 4.75 (3.98—5.65) | <0.001 |
| Hematopoietic cytopenia | 3.56 (3.32—3.81) | <0.001 |
| Cytopenia affecting more | 4.35 (3.87—4.90) | <0.001 |
| Erythropenia | 3.65 (2.65—5.02) | <0.001 |
| Leukopenia | 2.79 (2.57—3.03) | <0.001 |
| Thrombocytopenia | 2.45 (2.19—2.76) | <0.001 |
| Hypersensitivity | 2.66 (2.48—2.86) | <0.001 |
| SJS/TEN | 6.74 (6.01—7.56) | <0.001 |
| Anaphylactic reaction | 0.20 (0.15—0.28) | <0.001 |
| Acute renal failure | 2.21 (1.95—2.50) | <0.001 |
| Hypoglycemia | 2.41 (1.96—2.96) | <0.001 |
| Hepatic disorder | 1.98 (1.81—2.17) | <0.001 |
| Interstitial lung disease | 0.88 (0.75—1.03) | 0.11 |
| Nausea and vomiting symptoms | 0.79 (0.60—1.04) | 0.092 |
| Noninfectious diarrhea | 0.78 (0.58—1.05) | 0.099 |
| Rhabdomyolysis | 0.58 (0.39—0.88) | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagawa, T.; Ishida, T.; Jobu, K.; Morisawa, S.; Akagaki, K.; Kato, T.; Maruyama, T.; Yagi, Y.; Kihara, T.; Suzuki, S.; et al. Evaluation of Adverse Events Associated with the Sulfamethoxazole/Trimethoprim Combination Drug. J. Clin. Med. 2025, 14, 4819. https://doi.org/10.3390/jcm14144819
Sagawa T, Ishida T, Jobu K, Morisawa S, Akagaki K, Kato T, Maruyama T, Yagi Y, Kihara T, Suzuki S, et al. Evaluation of Adverse Events Associated with the Sulfamethoxazole/Trimethoprim Combination Drug. Journal of Clinical Medicine. 2025; 14(14):4819. https://doi.org/10.3390/jcm14144819
Chicago/Turabian StyleSagawa, Takaya, Tomoaki Ishida, Kohei Jobu, Shumpei Morisawa, Keita Akagaki, Takahiro Kato, Takumi Maruyama, Yusuke Yagi, Tomomi Kihara, Sanae Suzuki, and et al. 2025. "Evaluation of Adverse Events Associated with the Sulfamethoxazole/Trimethoprim Combination Drug" Journal of Clinical Medicine 14, no. 14: 4819. https://doi.org/10.3390/jcm14144819
APA StyleSagawa, T., Ishida, T., Jobu, K., Morisawa, S., Akagaki, K., Kato, T., Maruyama, T., Yagi, Y., Kihara, T., Suzuki, S., Endo, M., Matsunaga, N., & Hamada, Y. (2025). Evaluation of Adverse Events Associated with the Sulfamethoxazole/Trimethoprim Combination Drug. Journal of Clinical Medicine, 14(14), 4819. https://doi.org/10.3390/jcm14144819







