1. Introduction
Klippel–Trenaunay syndrome (KTS) is a congenital vascular malformation syndrome involving low-flow vascular anomalies such as capillary malformations, venous malformations (VMs), and lymphatic malformations. These abnormalities can result in limb asymmetry and disfigurement due to the overgrowth of soft tissue and/or bone [
1]. KTS arises from vascular developmental defects during fetal growth and is classified within the PIK3CA-related overgrowth spectrum [
2]. Limb overgrowth is typically observed at birth or during infancy and progressively worsens with age. Associated complications include pain, skin ulceration, infection, and lymphorrhea [
3]. Currently, no definitive treatment exists for KTS, and patients require lifelong symptomatic management involving sclerotherapy, embolization, excision, compression therapy, and pharmacotherapy [
1]. For controlling limb overgrowth, the available options include lower extremity orthoses, corrective orthopedic surgery [
4], and pharmacological agents such as mTOR inhibitors [
2].
Compression therapy has been reported to alleviate symptoms of low-flow vascular malformations in the extremities, including pain, swelling, ulceration, and coagulopathy [
5]. As a conservative and minimally invasive treatment, it is considered an appropriate first-line option for KTS. Similarly to VMs, lower extremity varicosities can cause venous stasis, and compression therapy is effective in relieving associated symptoms such as pain and edema [
6]. Despite its clinical use, the quantitative and qualitative benefits of compression therapy for limb vascular malformations in KTS remain poorly characterized. Additionally, standard ready-made elastic garments often do not fit well in patients with KTS due to limb overgrowth and anatomical deformities.
This study aimed to objectively evaluate the efficacy and safety of compression therapy using custom-made elastic garments in patients with KTS and VM. Since KTS is a syndrome of combined vascular malformation, its clinical character is mixed and patients can show various clinical features depending on which low-flow vascular malformation mainly develops. Our prior pilot study suggested that compression therapy seemed to be more effective in patients with KTS showing the apparent clinical character of VM than other KTS patients, and we emphasized this by adding the term VM in this report. The primary outcome was the change in lower limb circumference. Secondary outcomes included pain, modified Rankin Scale (mRS) score for disability, body water content, vital signs, and adverse events. Changes in garment elasticity were also assessed to evaluate material durability.
2. Materials and Methods
2.1. Study Design and Participants
This prospective, single-arm, multi-center interventional study was conducted between August 2021 and January 2022 at four institutions in Japan: Shinshu University School of Medicine, Fukuoka Children’s Hospital, Kobe University School of Medicine, and Aichi Children’s Health and Medical Center. The study was registered in the Japan Registry of Clinical Trials (jRCT1030210271), with recruitment commencing on 24 August 2021.
Eligible participants were diagnosed with KTS involving unilateral venous malformation (VM) of a lower limb according to diagnostic criteria from the Japan Intractable Diseases Information Center (
Table 1). All diagnoses were confirmed by magnetic resonance imaging prior to enrollment. The inclusion criteria were as follows: age ≥ 2 years, modified Rankin Scale (mRS) grade 1–4, and the ability to walk independently or with assistance. The exclusion criteria included bilateral KTS, recent treatment within 12 weeks (e.g., sclerotherapy or surgery), planned interventions during the study period, known allergy to garment materials, regular use of diuretics, recent heart failure within the past year, or any condition incompatible with study participation.
From August 2021 to January 2022, we enrolled participants for this multi-center, single-arm, prospective interventional study at four centers across Japan: Shinshu University School of Medicine, Fukuoka Children’s Hospital, Kobe University School of Medicine, and Aichi Children’s Health and Medical Center. This investigation was registered in the Japan Registry of Clinical Trials listed the International Clinical Trials Registry Platform (JRPN-jRCT1030210271), with recruitment starting on the same day (24th August 2021). The patient population was defined as those with KTS with the VM of one lower leg meeting the diagnostic criteria of the Japan Intractable Diseases Information Center (
Table 1). All VM cases were evaluated by magnetic resonance imaging at diagnosis prior to the study. The inclusion criteria were age ≥ 2 years, mRS scale grade 1–4, and being able to walk even with an assistance device. The exclusion criteria included having a KTS lesion in both lower limbs; being <12 weeks after treatment including sclerotherapy, excision, or another interventional trial; having plans for other treatments within the study period; allergy to the stocking material; regular use of diuretic medicine; clinical history of heart failure in the prior year; and other health or social conditions incompatible with this trial.
2.2. Sample Size
A prior pilot study of 13 KTS patients using custom-made compression garments for 4 weeks demonstrated a 3.0% mean difference (SD: 5.0) in limb circumference ratio. A one-sided paired t-test with alpha = 0.05 and 80% power determined that 15 subjects would be required. To accommodate potential dropouts, a sample size of 20 patients was targeted.
2.3. Protocol
After obtaining informed consent, baseline evaluations were performed and limb measurements were taken to fabricate custom-made elastic garments. Patients who had previously used compression devices were instructed to discontinue use at least three days prior to baseline assessment. No edema-reducing measures (e.g., limb elevation or compression) were used during this period.
The custom-made garments were produced by THUASNE (France) to deliver consistent 30 mmHg compression across the lower limb. Garment length varied based on lesion extent, with some extending to the buttocks. All garments reached at least the knee. Patients were instructed to wear the garment during waking hours, excluding bathing and sleep. Assessments were conducted before and after 26 weeks of treatment.
2.4. Outcomes
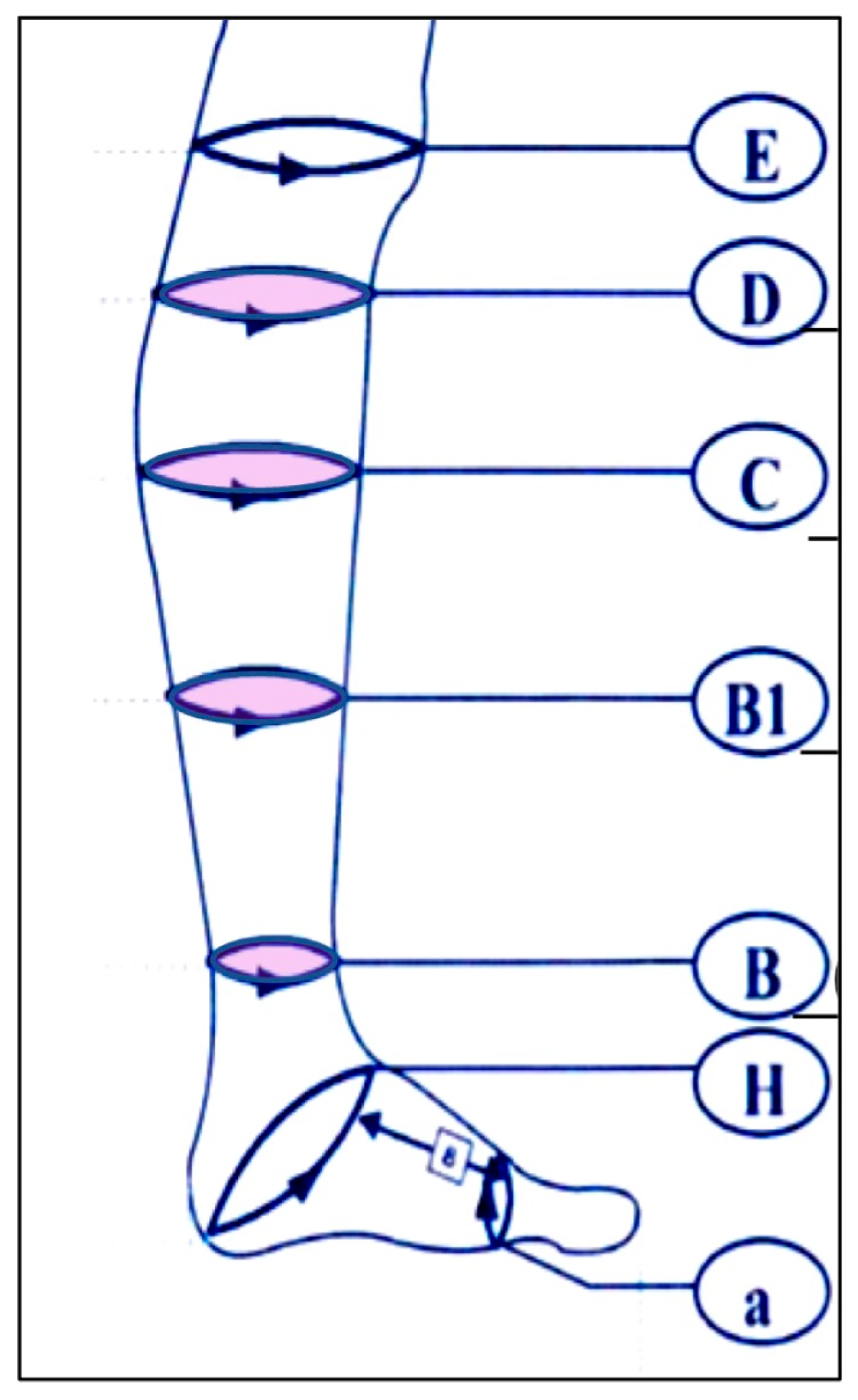
The primary endpoint was the change in circumference ratio (affected to unaffected limb) at four anatomical locations: the thinnest part of the ankle (B), junction of the calcaneal tendon and gastrocnemius muscle (B1), thickest part of the lower leg (C), and superior end of the tibial tuberosity (D). The ratios at each point were compared between baseline and week 26 (
Figure 1).
Secondary outcomes included changes in pain, mRS score, body water content, vital signs (blood pressure, pulse), garment elasticity, and adverse events. Pain was assessed using patient/guardian questionnaires and clinician ratings at the beginning and the end of the study on a 1–5 scale (1 = no pain; 5 = severe pain). The questionnaire items included garment discomfort, pain severity, difficulty donning/removing the garment, activity restrictions (e.g., bathing, toileting), and symptoms such as wounds, bleeding, lymphorrhea, or infection.
Partial body water content was evaluated using bioelectrical impedance analysis (InBody S10, InBody Japan, Tokyo, Japan) at baseline and after 26 weeks. Measurements were taken 10–30 min after garment removal. Vital signs were measured on Day 1 (pre- and 10–30 min post-application) and the final day (pre- and post-removal).
Garment elasticity was measured by stretching two fixed garment points to a standard distance using a digital scale before use and at 26 weeks.
Adverse events were documented throughout the study period.
2.5. Statistical Analysis
The primary outcome data were analyzed using one-sided paired t-tests (significance: p < 0.05). Two-sided t-tests were used for water content, vital signs, and garment elasticity. Ordinal outcomes (pain and mRS) were analyzed with Wilcoxon signed-rank tests (p < 0.05). Analyses were conducted using IBM SPSS Statistics version 23.0 (IBM Corp., Armonk, NY, USA).
3. Results
This study included 7 male and 13 female patients aged 2 to 54 years (mean: 10.9 years). All participants successfully wore their custom-made elastic garment for the full 6-month study period. The mean height gain among the participants was 3.26 cm, and the mean weight gain was 1.65 kg.
Table 2 summarizes the mean changes in limb circumference for both the affected and unaffected sides.
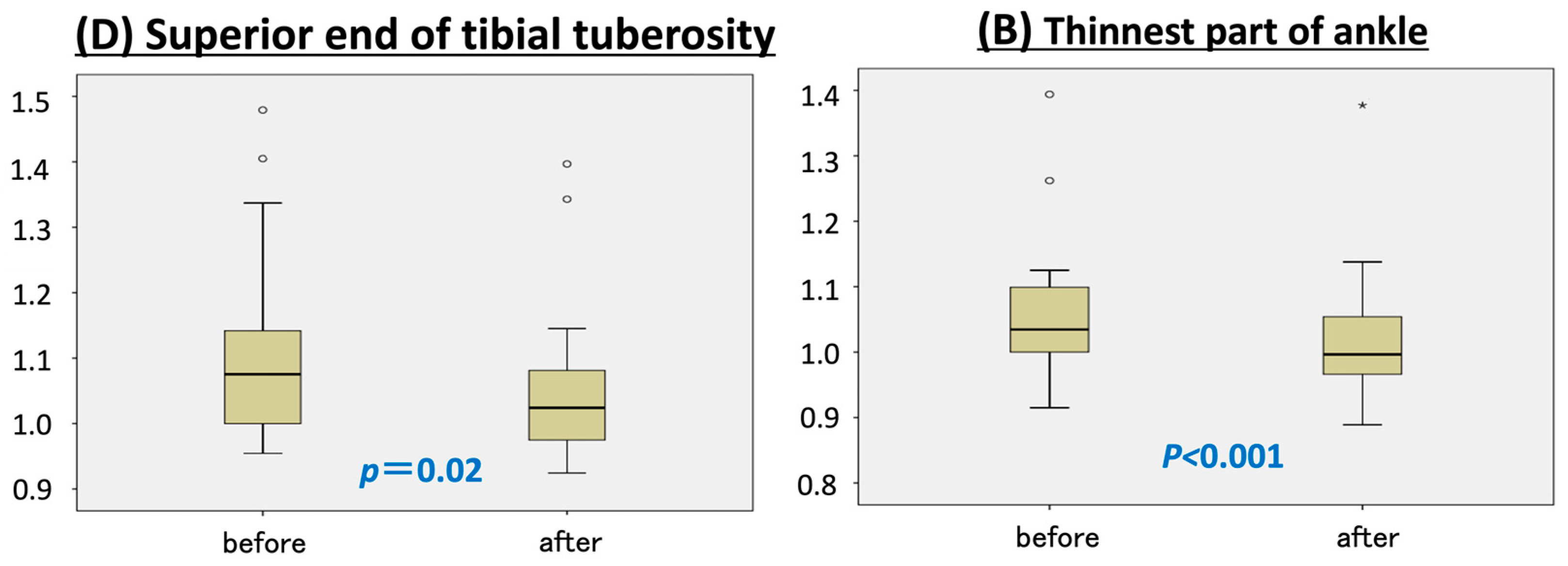
At the superior end of the tibial tuberosity (point D), the affected limb decreased by 4 mm in circumference, while the unaffected limb increased by 7 mm compared to baseline, resulting in a statistically significant decrease in the circumference ratio (
p = 0.02) (
Figure 2).
Similar results were observed at the thinnest part of the ankle (point B), where the affected limb became 5 mm thinner and the healthy limb increased in size by 2.5 mm (
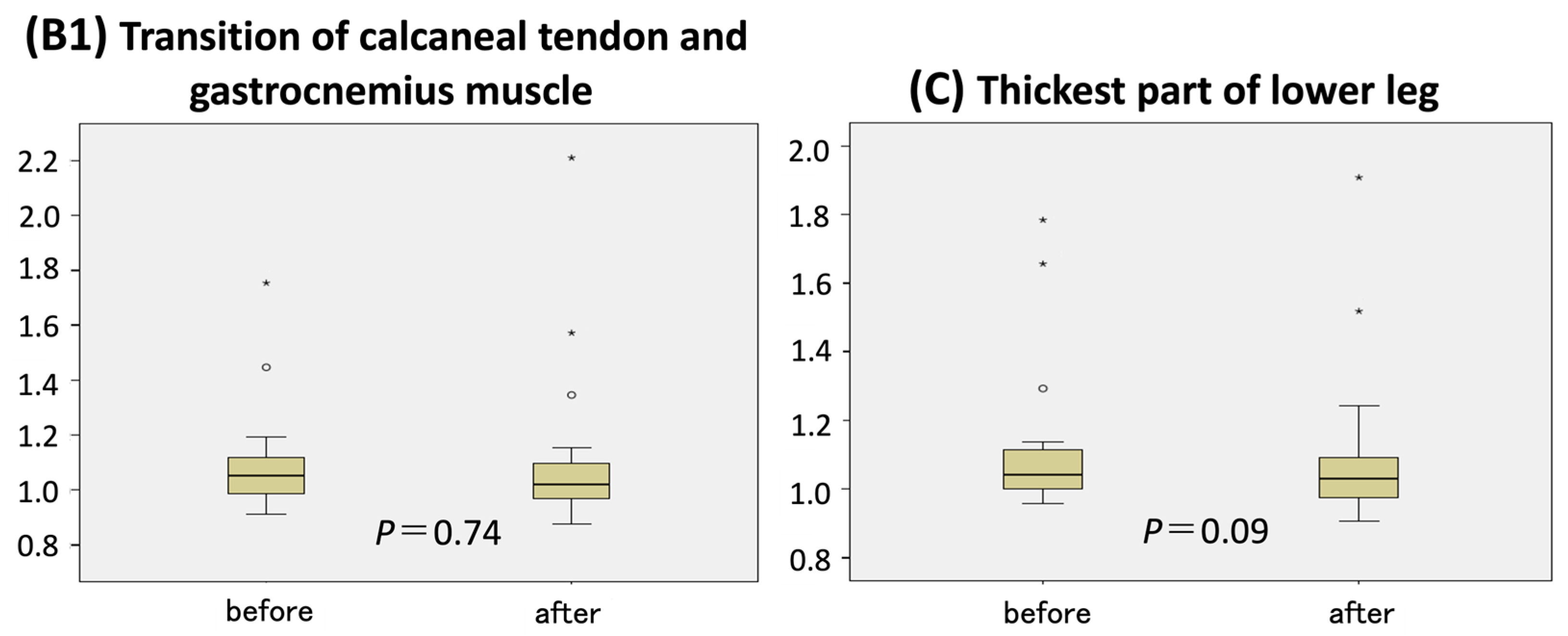
p < 0.001). No significant changes in circumference ratio were observed at the remaining measurement points—B1 (junction of the calcaneal tendon and gastrocnemius) and C (thickest part of the lower leg) (
Figure 3).
Pain and mRS scores showed a trend toward improvement after 6 months of compression therapy. Pain while wearing the garment decreased from a baseline mean of 1.7 to 1.5; pain without the garment declined from 1.5 to 1.44. The average mRS score improved from 1.6 to 1.4. Although some participants reported minor discomfort, such as a sensation of heat or itchiness, no restrictions in daily activities were noted.
No statistically significant differences in total or extracellular water content were observed between the baseline and post-treatment values in either the affected or healthy limbs, though all values showed a downward trend (
Table 3).
Vital signs did not vary significantly throughout the program (
Table 4).
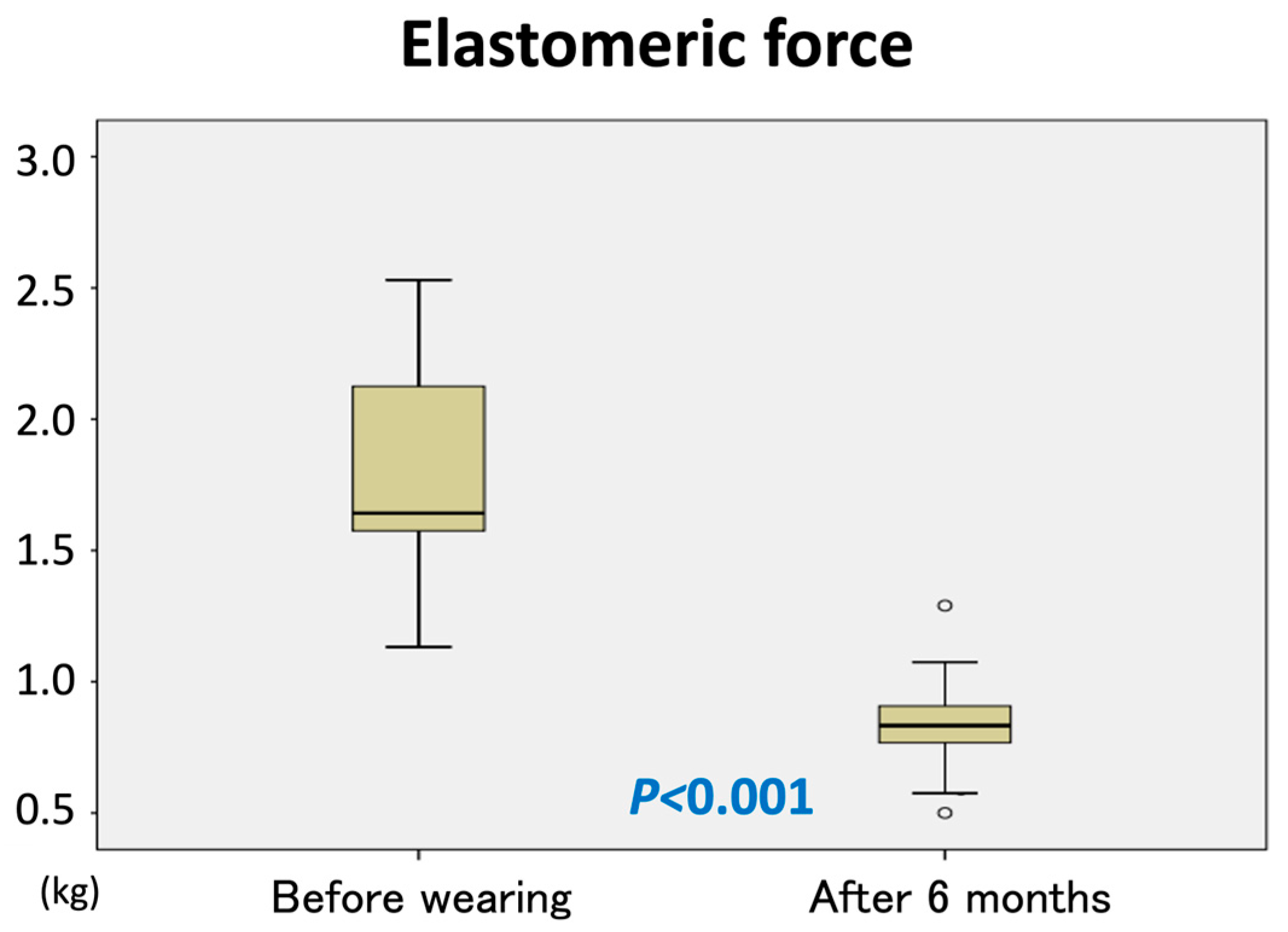
The elastic force of the garments significantly declined, measuring less than 50% of the initial force after 6 months of use (
p < 0.001) (
Figure 4).
No severe adverse events related to garment use were recorded. One patient developed a bacterial infection requiring hospitalization during the study period, but the infection site was outside the area of garment application, and a causal relationship was excluded. Another participant initially experienced difficulty donning the garment, which was resolved by fabricating a new garment. This participant successfully completed the study.
Overall, the use of the custom-made compression garments was well-tolerated and did not exacerbate any subjective symptoms.
4. Discussion
4.1. Initial Prospective Interventional Trial in Vascular Malformation Patients
Although several reports have described the effectiveness of compression therapy in alleviating vascular malformation symptoms, most have been retrospective and lacked robust evidence [
7,
8,
9]. Previous studies on elastic garments primarily focused on venous disorders, such as post-thrombotic syndrome following deep vein thrombosis. One randomized controlled trial showed that wearing elastic stockings for two years significantly reduced symptoms such as edema, pain, and ulceration [
10]. Another study involving 112 patients with chronic venous disease (CEAP classification C0–C6) demonstrated improvements in pain, swelling, pigmentation, physical activity, and overall health status after 16 months of using 35–40 mmHg compression stockings [
11]. However, evidence remains limited regarding the efficacy of compression therapy in treating varicose veins without active or healed ulcers—conditions that share hemodynamic characteristics with VMs [
12,
13]. Our study is a prospective, interventional investigation that objectively demonstrates the efficacy and safety of custom-made elastic garments in patients with vascular abnormalities.
4.2. Effectiveness According to Limb Thickness
This study found that six months of wearing a custom-made 30 mmHg compression garment significantly reduced limb circumference at two measurement points: the superior end of the tibial tuberosity (point D) and the thinnest part of the ankle (point B). Of the four measured sites, the thickest part of the lower leg (point C) showed the least improvement. These findings suggest that compression therapy may be more effective in thinner regions of the limb.
According to Laplace’s law (p = T/r), where p is pressure, T is tension, and r is the radius, constant tension produces greater pressure in areas with smaller radii. Although the garments were designed to apply uniform tension, thicker regions such as point C likely received lower pressure compared to thinner regions. These results imply that custom garments with variable pressure distribution tailored to limb geometry may enhance their therapeutic effect in areas with greater girth. In this regard, the adaptation of Velcro-type garments and combinations of bandages and stockings may be effective, and testing the efficacy of these various compression therapies is a topic for future study.
4.3. Compression Therapy from Infancy and Childhood
KTS is often diagnosed at birth or in early childhood due to visible skin malformations and limb overgrowth [
14,
15]. In this study, the effectiveness of therapy was evaluated based on the circumference ratio between the affected and unaffected limbs, assuming symmetric natural growth.
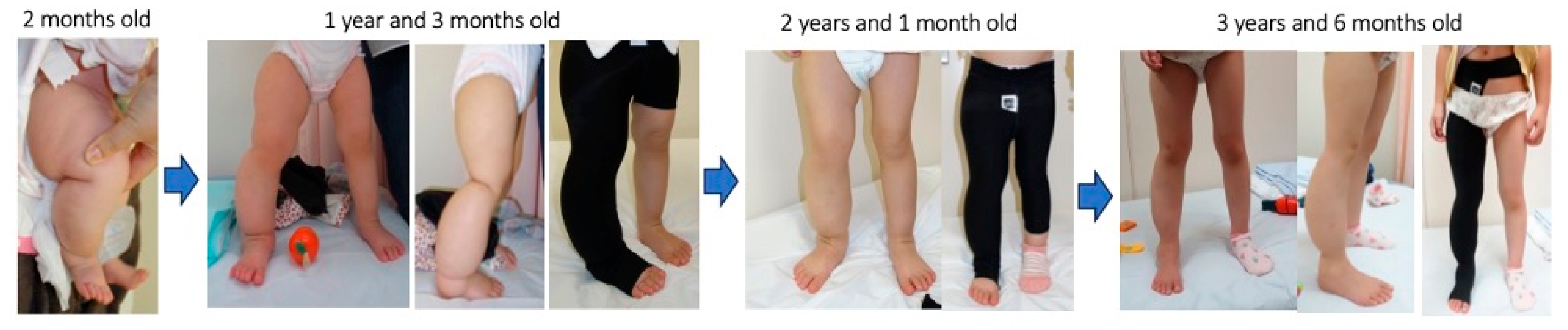
A representative case (
Figure 5) showed that the consistent use of a custom-made elastic garment from early childhood led to long-term symptom control with minimal invasiveness. To optimize outcomes and ensure adherence, it is important to minimize discomfort and daily activity restrictions. Although some participants reported sensations of heat or itching, none discontinued therapy, indicating that the garment was generally well-tolerated.
4.4. Pressure Retention and Durability
Compression pressure classifications vary by country. In Japan, compression is categorized as light (18–21 mmHg), weak (23–32 mmHg), medium (34–46 mmHg), and strong (>49 mmHg). The required pressure for venous compression varies by body position: 20–25 mmHg supine, 35–40 mmHg upright, and >60 mmHg for vein occlusion [
16]. Although no universal compression standard exists for vascular anomalies, the prior literature recommends 20–30 mmHg for simple varicose veins (CEAP C2) and 30–40 mmHg for venous ulcers [
6,
17,
18]. Our study used garments applying 30 mmHg of compression. However, the garment’s elastic force decreased by more than half over six months, suggesting that replacement at least twice annually is needed to maintain therapeutic benefit. The exact timing of elasticity loss remains uncertain and requires further study.
4.5. Thrombosis in Vascular Malformations
Vascular malformations are associated with localized intravascular coagulopathy, which can lead to thrombosis in some patients. VMs may enlarge over time and cause disfigurement or pain, often due to thrombotic events resulting in phlebolith formation [
19]. Approximately one-third of pediatric VM patients exhibit elevated D-dimer levels due to localized coagulopathy, particularly in large or multifocal lesions, lesions with phleboliths, or cases of KTS with VM [
20]. Mixed-type vascular malformations, such as in KTS, are also linked to a higher incidence of deep vein thrombosis and elevated D-dimer levels due to mild coagulopathy and low-flow dynamics [
21].
Compression therapy may mitigate local coagulopathy in KTS by reducing blood stasis in addition to its mechanical slimming effects. However, the impact of compression therapy on thrombosis and coagulation parameters in VM remains unclear. Future investigations should incorporate serial coagulation testing to clarify these effects.
4.6. Limitations
This study was limited by its single-arm design and lack of a control group. Additionally, daily wearing time was not rigorously documented, which may have influenced the outcomes. The relatively small sample size also limits generalizability. It is also desirable to conduct separate studies on pediatric and adult patients. Future studies with larger cohorts and more comprehensive evaluations of coagulation markers are warranted.
5. Conclusions
Six months of wearing a custom-made 30 mmHg elastic garment significantly reduced limb circumference at two measurement points in patients with KTS and VM, without any severe adverse events. Given the observed decline in garment elasticity, replacement is recommended at least every six months to maintain therapeutic efficacy.
Author Contributions
Conceptualization, F.N., S.A., T.N., T.M. and S.Y.; methodology, F.N., S.A., T.N., T.M., M.N. and S.Y.; validation, F.N., S.A., T.N., T.M., M.N. and S.Y.; formal analysis, F.N., S.A. and S.Y.; investigation, F.N., S.A., T.N., T.M. and S.Y.; resources, F.N., S.A., T.N., T.M. and S.Y.; data curation, F.N., S.A., T.N., T.M., M.N. and S.Y.; writing—original draft preparation, M.N.; writing—review and editing, F.N., S.A., T.N., T.M., M.N. and S.Y.; visualization, M.N.; supervision, S.Y.; project administration, S.Y.; funding acquisition, S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by both the Ministry of Health, Labor and Welfare and the Ministry of Education, Culture, Sports, Science and Technology in Japan: grant numbers 20FC1031 and 24K12822.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Shinshu University School of Medicine Biological and Medical Research Ethics Committee (protocol code 5249 and 3 August 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients or their parents/guardians to publish this paper.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We are thankful to Masatomo Morita, a prosthetist and orthoticist at Matsumoto Prosthetics & Orthotics Manufacturing Co., Ltd., for his support regarding all the measurements for manufacturing the custom-made compression elastic garments. We are grateful to Trevor Ralph for editing a draft of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| KTS | Klippel–Trenaunay syndrome |
| VM | Venous malformation |
References
- Gloviczki, P.; Driscoll, D.J. Klippel-Trenaunay syndrome: Current management. Phlebology 2007, 22, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Vahidnezhad, H.; Youssefian, L.; Uitto, J. Klippel-Trenaunay syndrome belongs to the PIK3CA-related overgrowth spectrum (PROS). Exp. Dermatol. 2016, 25, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Redondo, P.; Aguado, L.; Martinez-Cuesta, A. Diagnosis and management of extensive vascular malformations of the lower limb: Part I. Clinical diagnosis. J. Am. Acad. Dermatol. 2011, 65, 893–906, quiz 907–908. [Google Scholar] [CrossRef]
- Jacob, A.G.; Driscoll, D.J.; Shaughnessy, W.J.; Stanson, A.W.; Clay, R.P.; Gloviczki, P. Klippel-Trenaunay syndrome: Spectrum and management. Mayo Clin. Proc. 1998, 73, 28–36. [Google Scholar] [CrossRef]
- Langbroek, G.B.; Horbach, S.E.; van der Vleuten, C.J.; Ubbink, D.T.; van der Horst, C.M. Compression therapy for congenital low-flow vascular malformations of the extremities: A systematic review. Phlebology 2018, 33, 5–13. [Google Scholar] [CrossRef]
- Gloviczki, P.; Comerota, A.J.; Dalsing, M.C.; Eklof, B.G.; Gillespie, D.L.; Gloviczki, M.L.; Lohr, J.M.; McLafferty, R.B.; Meissner, M.H.; Murad, M.H.; et al. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J. Vasc. Surg. 2011, 53, 2S–48S. [Google Scholar] [CrossRef]
- Enjolras, O.; Ciabrini, D.; Mazoyer, E.; Laurian, C.; Herbreteau, D. Extensive pure venous malformations in the upper or lower limb: A review of 27 cases. J. Am. Acad. Dermatol. 1997, 36, 219–225. [Google Scholar] [CrossRef]
- Dompmartin, A.; Vikkula, M.; Boon, L.M. Venous malformation: Update on aetiopathogenesis, diagnosis and management. Phlebology 2010, 25, 224–235. [Google Scholar] [CrossRef]
- Mazoyer, E.; Enjolras, O.; Laurian, C.; Houdart, E.; Drouet, L. Coagulation abnormalities associated with extensive venous malformations of the limbs: Differentiation from Kasabach-Merritt syndrome. Clin. Lab. Haematol. 2002, 24, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Brandjes, D.P.; Buller, H.R.; Heijboer, H.; Huisman, M.V.; de Rijk, M.; Jagt, H.; ten Cate, J.W. Randomised trial of effect of compression stockings in patients with symptomatic proximal-vein thrombosis. Lancet 1997, 349, 759–762. [Google Scholar] [CrossRef]
- Motykie, G.D.; Caprini, J.A.; Arcelus, J.I.; Reyna, J.J.; Overom, E.; Mokhtee, D. Evaluation of therapeutic compression stockings in the treatment of chronic venous insufficiency. Dermatol. Surg. 1999, 25, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Raetz, J.; Wilson, M.; Collins, K. Varicose Veins: Diagnosis and Treatment. Am. Fam. Physician 2019, 99, 682–688. [Google Scholar]
- Palfreyman, S.J.; Michaels, J.A. A systematic review of compression hosiery for uncomplicated varicose veins. Phlebology 2009, 24 (Suppl. S1), 13–33. [Google Scholar] [CrossRef]
- Pavone, P.; Marino, L.; Cacciaguerra, G.; Di Nora, A.; Parano, E.; Musumeci, G.; Ruggieri, M.; Polizzi, A.; Falsaperla, R. Klippel-Trenaunay Syndrome, Segmental/Focal Overgrowth Malformations: A Review. Children 2023, 10, 1421. [Google Scholar] [CrossRef]
- Hassanandani, T.; Panda, M.; Agarwal, A. Dermoscopy of Klippel-Trenaunay Syndrome in a 6-month-old Infant. Indian Dermatol. Online J. 2022, 13, 824–825. [Google Scholar] [PubMed]
- Partsch, B.; Partsch, H. Calf compression pressure required to achieve venous closure from supine to standing positions. J. Vasc. Surg. 2005, 42, 734–738. [Google Scholar] [CrossRef] [PubMed]
- Partsch, H.; Flour, M.; Smith, P.C.; International Compression, C. Indications for compression therapy in venous and lymphatic disease consensus based on experimental data and scientific evidence. Under the auspices of the IUP. Int. Angiol. 2008, 27, 193–219. [Google Scholar]
- Coleridge-Smith, P.D. Leg ulcer treatment. J. Vasc. Surg. 2009, 49, 804–808. [Google Scholar] [CrossRef]
- Nakano, T.A.; Zeinati, C. Venous Thromboembolism in Pediatric Vascular Anomalies. Front. Pediatr. 2017, 5, 158. [Google Scholar] [CrossRef]
- Hung, J.W.; Leung, M.W.; Liu, C.S.; Fung, D.H.; Poon, W.L.; Yam, F.S.; Leung, Y.C.; Chung, K.L.; Tang, P.M.; Chao, N.S.; et al. Venous Malformation and Localized Intravascular Coagulopathy in Children. Eur. J. Pediatr. Surg. 2017, 27, 181–184. [Google Scholar]
- Douma, R.A.; Oduber, C.E.; Gerdes, V.E.; van Delden, O.M.; van Eck-Smit, B.L.; Meijers, J.C.; van Beers, E.J.; Bouma, B.J.; van der Horst, C.M.; Bresser, P. Chronic pulmonary embolism in Klippel-Trenaunay syndrome. J. Am. Acad. Dermatol. 2012, 66, 71–77. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).