Abstract
Oncoplastic breast-conserving surgery (OBCS), or oncoplastic surgery, has revolutionized the surgical management of breast cancer by integrating oncologic principles with reconstructive techniques to optimize both cancer control and aesthetic outcomes following breast-conserving surgery (BCS). Since its inception in the 1980s, the field has evolved significantly, incorporating a range of volume displacement and volume replacement strategies to restore breast contour after partial mastectomy. This review explores the current practices and key surgical considerations of OBCS. It highlights the role of preoperative multidisciplinary planning, patient selection, anatomical and vascular knowledge, and intraoperative technique in optimizing results. Barriers to access—including disparities in training, insurance, and geographic availability—are addressed, alongside efforts by professional societies like the American Society of Breast Surgeons (ASBS) to standardize definitions and practices. The review also outlines strategies for minimizing complications and enhancing oncologic, reconstructive, and patient-reported outcomes. By offering a comprehensive framework for clinical decision-making, this paper aims to support broader adoption and refinement of OBCS as a standard component of breast cancer care.
1. Introduction
Oncoplastic breast-conserving surgery (OBCS), or oncoplastic surgery, integrates oncologic and plastic surgery principles to optimize both cancer control and aesthetic outcomes following breast-conserving surgery (BCS). It emerged when breast-conserving therapy (BCT)—lumpectomy or partial mastectomy followed by radiotherapy—proved equally effective as mastectomy for early-stage breast cancer [1,2]. However, BCT was often associated with unsatisfactory aesthetic outcomes, such as contour deformities, fibrosis, and scarring, especially when more than 10% of breast tissue was excised [3,4]. Oncoplastic techniques were introduced to address these challenges and have since shown lower re-excision and positive margin rates [5,6], similar if not fewer complications [7,8,9,10], and enhanced patient-reported outcomes compared to BCS alone [11]. Today, OBCS is accepted as the standard of care for patients undergoing partial mastectomy worldwide [12,13]. Since its introduction in the 1980s by Dr. Werner Audretsch [14], the field has evolved continuously, emphasizing optimization of oncologic, functional, aesthetic, and patient-reported outcomes. This review summarizes the evolution, techniques, and classifications of OBCS, and outlines strategies for optimizing outcomes.
2. Evolution of OBCS and Barriers
Surgical management of breast cancer has evolved significantly, transitioning from the radical Halstedian mastectomy in the late 19th century to the widespread acceptance of BCT by the 1990s, driven by advances in surgical techniques, pathologic evaluation, chemotherapy, and radiotherapy [1,2,15,16,17,18]. In 1994, Dr. Werner Audretsch introduced the concept of “oncoplastic surgery”, integrating principles of oncologic and reconstructive surgeries [14]. Early oncoplastic techniques adapted traditional breast reduction and mastopexy methods—termed “volume displacement” approaches—to repair partial mastectomy defects [19,20]. By the early 2000s, volume replacement techniques such as locoregional flaps expanded the ability to restore breast shape after larger resections [21,22,23,24].
Despite the evolution of techniques, access to OBCS remains limited [25]. A major barrier is the lack of standardized training among breast surgeons, resulting in variability in expertise across practice settings [26,27,28]. Models of delivery of OBCS also differ internationally. In the United Kingdom, a single-surgeon model predominates, with breast surgeons who received training in oncoplastic techniques performing both the oncologic resection and reconstructive procedures [29]. In contrast, a two-surgeon model is more common in the United States and Australia, where a breast surgeon performs the oncologic resection and a plastic surgeon performs the immediate reconstruction [30]. Complex volume replacement procedures, in particular, require coordination with experienced plastic surgeons often found in high-volume academic centers. Additional barriers include insurance limitations, geographic disparities, referral biases, and racial inequities [31,32,33]. Historically, the absence of a consistent definition of OBCS created confusion not only among providers and trainees but also among patients seeking breast cancer treatment. Furthermore, the lack of specific billing codes for OBCS has further impeded broader adoption [28,34].
To address these challenges, the American Society of Breast Surgeons (ASBS) developed a consensus definition and classification system to standardize practice and expand access [35]. The ASBS defines OBCS as “breast conservation surgery incorporating an oncologic partial mastectomy with ipsilateral defect repair using volume displacement or volume replacement techniques, with contralateral symmetry surgery as appropriate”. Building upon previous frameworks such as Clough’s excision volume and surgical complexity-based model focusing primarily on volume displacement [36], the ASBS definition classifies oncoplastic procedures into volume displacement (Level 1: <20% excision; Level 2: 20–50% excision) and volume replacement (>50% excision) categories, explicitly including tissue recruitment techniques. The 20% and 50% thresholds serve as guidelines rather than rigid cut-offs, allowing for individualized decision-making based on patient anatomy and goals. A suggested guide to common oncoplastic techniques based on the ASBS classification system can be found in Figure 1. Notably, while the ASBS definition was designed primarily for clinical clarity rather than billing purposes, it remains compatible with Current Procedural Terminology (CPT) coding systems, thus supporting broader integration of OBCS into routine breast cancer care [35].
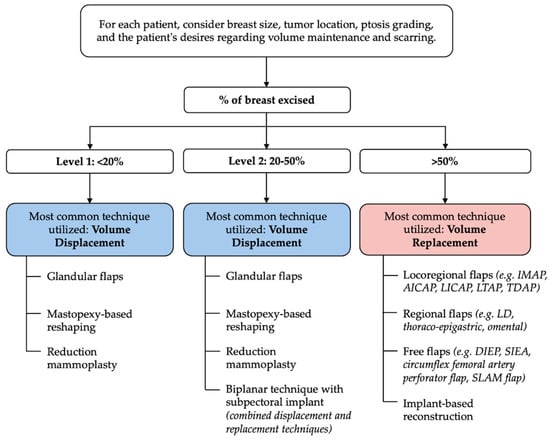
Figure 1.
Suggested guide to common oncoplastic techniques based on the American Society of Breast Surgeons (ASBS) classification system. This schematic categorizes oncoplastic approaches according to the percentage of breast volume excised: Level 1 (<20%), Level 2 (20–50%), and Volume Replacement (>50%). These thresholds serve as general guidelines rather than strict cut-offs. Surgical decision-making should be individualized, taking into account factors such as breast size, tumor location, ptosis, availability of remote tissue, and the patient’s preferences regarding volume preservation and scarring.
Efforts to expand access to OBCS have also focused on strengthening training and credentialing pathways. In the United States, the ASBS offers formal oncoplastic surgery certification program to recognize surgeons meeting benchmarks in clinical experience, procedural training, and quality assurance in OBCS [37]. In the United Kingdom, growing patient demand for immediate reconstruction following BCS led to the development of a national breast surgery curriculum mandating oncoplastic training, hands-on simulation courses, and the emergence of “stem breast surgeons”—a surgeon trained in either breast or plastic surgery who receives cross-specialty education in both oncologic resection and reconstruction, enabled by a joint initiative between the Association of Breast Surgery (ABS) and the British Association of Plastic, Reconstructive and Aesthetic Surgeons (BAPRAS) [38]. More recently, new initiatives such as an online Master of Surgery degree in Oncoplastic Breast Surgery based at the University of East Anglia in the UK [39], and changes to the surgical curricula that may enable breast surgeons to specialize in more advanced reconstructive skills such as autologous flap reconstruction without general surgery on-call duties, have further expanded training pathways. Regardless of the model, it is essential that practices include providers—either individually trained or through collaborative teams—capable of delivering OBCS as a standard offering. This necessitates not only theoretical training and practical skill development but also formal oversight, documentation, and credentialing to ensure surgical quality. Greater transparency in surgeon qualifications also empowers patients to identify and advocate for OBCS as a treatment option.
3. Pre-Operative Evaluation
Patients with breast cancer undergoing lumpectomy or partial mastectomy should be referred for evaluation and education regarding OBCS. A multidisciplinary approach involving surgical oncology, medical oncology, radiation oncology, and plastic surgery has been shown to improve outcomes [40]. Preoperative assessment must systematically evaluate factors that influence both oncologic and reconstructive planning, including tumor size, focality, estimated resection size relative to breast volume, tumor location, degree of ptosis, skin quality, history of prior radiation, and the anticipated need for adjuvant therapies.
The most critical oncologic principle remains complete tumor excision with negative margins. OBCS often allows larger resections without compromising aesthetic outcomes, reducing positive margin rates compared to standard partial mastectomy [41]. For tumors initially too large for breast conservation, neoadjuvant therapy can be employed to downstage the disease [42,43,44]. In these cases, pre- and post-treatment breast MRI is recommended to assess response and reevaluate surgical candidacy [45,46]. Patients’ goals and preferences are equally vital; factors such as desired breast size, tolerance for incisions, and acceptance of surgical complexity must be discussed thoroughly. Setting realistic expectations regarding symmetry, breast contour, potential radiation effects, and possible complications is essential for patient satisfaction and informed consent.
Detailed anatomical understanding is crucial for surgical planning. Successful OBCS relies on maintaining perfusion to glandular flaps and preserving nerve supply to optimize nipple-areolar complex (NAC) viability and minimize fat necrosis. Pedicle design must account for vascular anatomy. Tumor location, body habitus, and breast size guide technique selection: patients with larger, ptotic breasts are better suited for volume displacement techniques, whereas those with smaller breasts may require volume replacement strategies using flaps. Aesthetically placed incisions (e.g., periareolar, inframammary, or axillary) and meticulous glandular rearrangement are essential to optimize outcomes and minimize visible scarring. Preoperative planning also considers the need for contralateral symmetry procedures, which may be performed synchronously or delayed based on patient factors. Notably, breast reduction itself may confer a reduced breast cancer risk in older women, adding an additional benefit to symmetry-achieving surgery [47,48].
4. Classifications of Oncoplastic Techniques
4.1. Volume Displacement Techniques
Volume displacement techniques are the most commonly employed form of oncoplastic techniques, particularly in patients with moderate-sized breasts, small-to-medium tumors, and mild ptosis (Grade I) [49]. These approaches involve rearrangement of the residual breast tissue after tumor excision to restore breast contour, typically resulting in a modest reduction in breast volume. Displacement strategies are most appropriate when up to 20–50% of breast volume is excised [35], and patients must be counseled about the expected decrease in breast size. Proper patient selection is key; women with adequate glandular tissue, some skin laxity, and moderate breast size achieve the best aesthetic outcomes, as these factors support tissue mobility.
A variety of glandular rearrangement techniques are employed depending on tumor location and the volume of resection [36]. These include glandular flap advancement [36], rotational glandular flaps [50,51], mastopexy-based reshaping [52,53,54], and reduction mammoplasty techniques [36,55]. Familiar skin incision designs, such as vertical or Wise-pattern incisions, are adapted to accommodate oncologic resection patterns [52,56]. The choice of pedicle and skin excision pattern is influenced by the tumor’s quadrant, breast size, and degree of ptosis. Novel strategies, such as the biplanar oncoplastic approach—which combines glandular reshaping with small-volume subpectoral implants—are being explored in selected patients to help restore volume symmetry (Figure 2) [57]. This is an example of emerging techniques that integrate volume replacement elements into displacement frameworks to address limitations of volume displacement approaches.
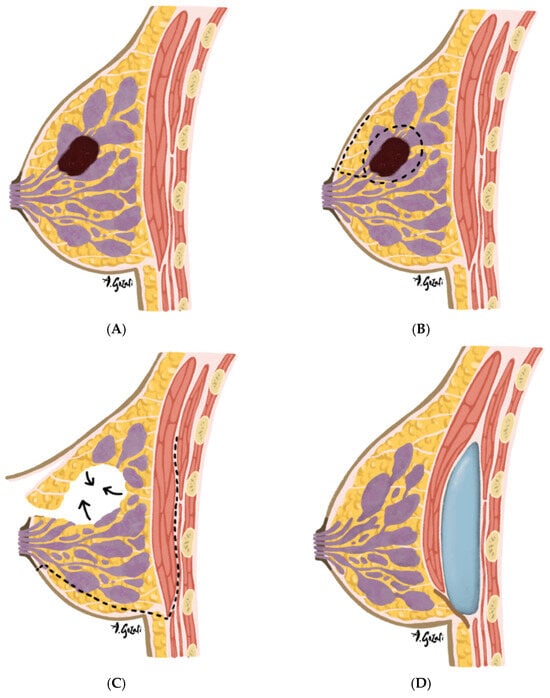
Figure 2.
(A–D). Biplanar oncoplastic surgery is a hybrid technique combining volume displacement and volume replacement strategies in patients with small, non-ptotic breasts undergoing breast-conserving surgery. This method is indicated when tumor resection would result in significant contour deformity and volume reduction that cannot be corrected with glandular rearrangement alone. A prosthetic device is placed in a subpectoral pocket to restore volume while minimizing additional donor site morbidity. The technique is especially suitable for peripheral tumors in the upper or outer quadrants. (A) Preoperative tumor localization: Tumor located in the upper outer quadrant of the breast—a favorable site for biplanar oncoplastic surgery due to ease of access and preservation of vascularity during rearrangement; (B) Tumor Excision: Incision design is based on tumor location, with common approaches including circumvertical and periareolar patterns. Skin flaps are elevated within the plane of the investing fascia, and the tumor is excised with a limited margin of surrounding healthy breast tissue. The specimen is oriented, weighed, and submitted for pathological analysis; (C) Parenchymal rearrangement following negative margin confirmation: Tissue rearrangement is performed through parenchymal and skin flap mobilization to fill the resection cavity and maintain breast contour. Attention is given to preserving vascular supply to minimize the risk of necrosis; (D) Subpectoral implant placement: A subpectoral pocket is developed by elevating the pectoralis major muscle from its inferior-lateral border via a transparenchymal tunnel or secondary inframammary fold incision. A small prosthetic device (implant or tissue expander) is inserted to replace lost volume and restore symmetry.
An intimate knowledge of breast anatomy—including vascular, lymphatic, and neural structures—is fundamental to the success of volume displacement procedures [58]. The breast is supplied by an anastomotic arterial network derived from the internal mammary, axillary, and intercostal arteries, which also forms the vascular basis for most glandular flaps used in OBCS. This is complemented by a robust subdermal venous plexus through which venous drainage occurs. Equally important is the preservation of lymphatic drainage and sensory innervation, particularly to the NAC, which receives input primarily from the fourth intercostal nerve and branches of the medial intercostal and supraclavicular nerves. Respecting these anatomical territories during dissection helps reduce complications such as flap necrosis, venous congestion, and postoperative sensory deficits such as postoperative numbness and neuropathic pain [59].
Displacement techniques are not without limitations. Extensive resections and undermining of tissue may compromise flap mobility or blood supply, increasing the risk of complications such as fat necrosis, wound dehiscence, delayed healing, or even partial or complete NAC loss [60].
4.2. Volume Replacement Techniques
Volume replacement techniques utilize autologous tissue to restore breast shape and volume after partial mastectomy, particularly in patients with small-to-moderate-sized breasts, high tumor-to-breast size ratios, or insufficient residual breast tissue following resection [61]. These approaches are most beneficial when more than 50% of the breast volume is removed, or in cases of 20–50% volume loss when the patient desires preservation of breast size [35]. Flap choice is determined by tumor location, the volume required for reconstruction to achieve the desired breast size, and patient-specific factors such as tissue availability and vascular anatomy.
Locoregional flaps based on chest wall perforators offer reliable options for local tissue recruitment with minimal donor site morbidity and no need for microvascular anastomosis. These include the internal mammary artery perforator (IMAP) flap [62] (Figure 3), anterior intercostal artery perforator (AICAP) flap [63] (Figure 4), lateral intercostal artery perforator (LICAP) flap [64,65], lateral thoracic artery perforator (LTAP) flap [65], and thoracodorsal artery perforator (TDAP) flap [66,67,68,69] (Figure 5). Ordered anatomically from medial to lateral, these flaps differ in arc of rotation and tissue availability (Figure 6). Although comparative volumetric data are limited, reports suggest the TDAP flap can address defects involving more than 20% of breast volume [70], while the LICAP and IMAP flaps can replace up to 100 g and 125 g of tissue, respectively [62,71]. However, these locoregional flaps may not always provide sufficient volume for larger defects, and they may result in additional chest wall incisions.
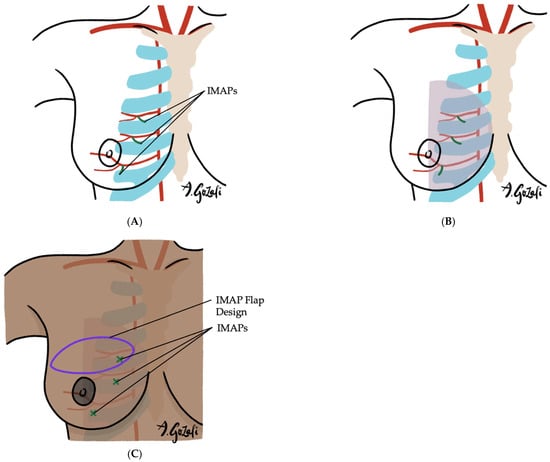
Figure 3.
(A–C). Internal Mammary Artery Perforator (IMAP) Flaps. (A) Vascular supply of IMAP flaps is based on the perforating branches of the internal mammary artery; (B) IMAP flaps are especially suitable for defects in the medial half of the breast; (C) IMAP flap designs are versatile and can be tailored according to the location of the defect.
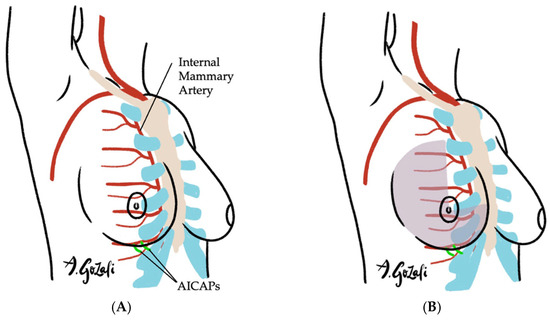
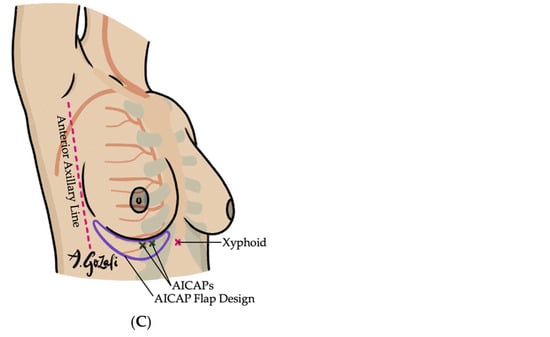
Figure 4.
(A–C). Anterior Intercostal Artery Perforator (AICAP) Flaps. (A) Vascular supply of AICAP flaps is based on the perforating branches of the anterior intercostal artery perforator, which branches off the internal mammary artery; (B) AICAP flaps are especially suitable for defects located in lateral breast or inferior medial quadrant; (C) When designing AICAP flaps, the upper boundary is typically the IMF, with the flap width spanning from the anterior axillary line to the xyphoid, subdivided into thirds and the appropriate third is chosen based on location of the defect and the perforators.
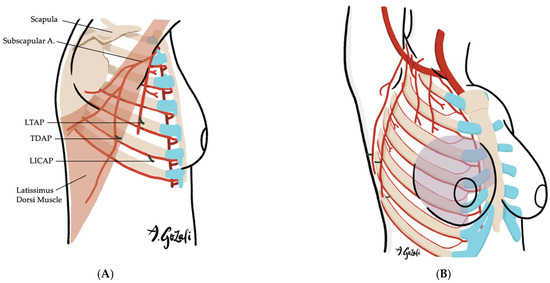
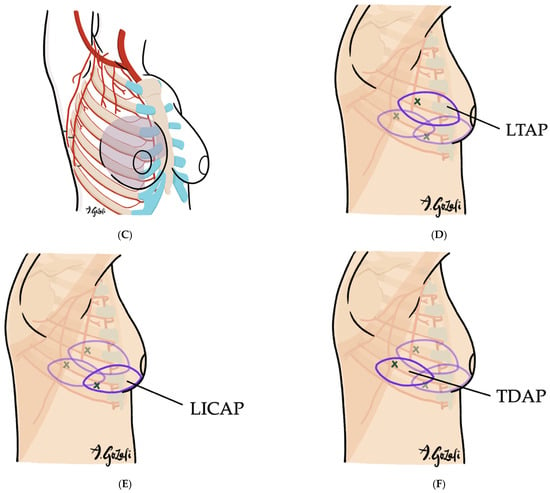
Figure 5.
(A–F). Lateral Thoracic Artery Perforator (LTAP) Flaps, Thoracodorsal Artery Perforator (TDAP) Flaps, and Lateral Intercostal Artery Perforator (LICAP) Flaps. (A) Vascular supply of LTAP flaps is based on perforators of the lateral thoracic artery (a branch of the axillary artery); LICAP flaps are supplied by perforators of the lateral intercostal artery (a branch of the posterior intercostal artery); TDAP flaps are supplied by the perforators of the thoracodorsal artery (a branch of the subscapular artery); (B) LTAP and LICAP flaps are suitable for lateral breast defects; (C) TDAP flaps are suitable for large surface area defects located in lateral breast or superior medial quadrant; (D–F) The flaps are designed on the lateral chest wall based on the location of the perforators.
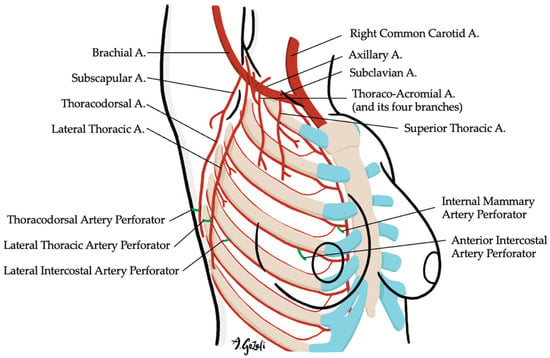
Figure 6.
Chest wall perforators available for locoregional flap as volume replacement techniques.
Regional flaps such as the traditional latissimus dorsi (LD) flap, thoracoepigastric flap, and omental flap offer more substantial tissue volume without the complexity of microsurgical anastomosis. The LD flap remains a workhorse for volume replacement, particularly when chest wall perforators are insufficient [72,73]. The thoracoepigastric flap, supplied by perforators from the superior epigastric artery, is well-suited for inferior defects, both lateral and medial [61,74,75]. It is best used in patients with adequate subcutaneous tissue and skin in the upper abdomen, with no prior scarring in the ipsilateral upper abdomen, which may compromise the pedicle. The omental flap—harvested laparoscopically or via an open approach—offers pliability and robust vascularity, especially for medial or lower-pole reconstructions [76,77] (Figure 7).
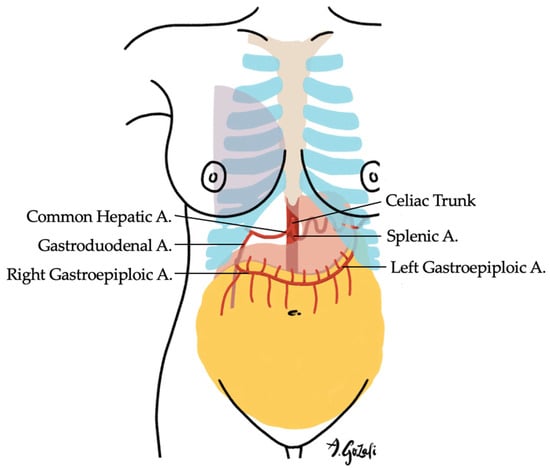
Figure 7.
The Omental Flap is supplied by the left or right gastroepiploic arteries (one is usually more dominant than the other). It is suitable for medial breast defects, especially when >20% of breast tissue is excised.
Free tissue transfers are reserved for extensive volume deficits or when previous surgeries, radiation, or chest wall scarring preclude the use of locoregional or regional options. Abdominally-based flaps such as the deep inferior epigastric perforator (DIEP) and superficial inferior epigastric artery (SIEA) flaps are most commonly used [78,79]. Smaller-volume free flaps, such as those based on the medial circumflex femoral artery perforator, may be useful particularly in central breast defects [80], and the superficially-based low-abdominal mini (SLAM) flap has recently been described as a means of providing modest volume replacement in thin patients while preserving future abdominal donor sites [81] (Figure 8). Free flap reconstruction remains technically demanding, with challenges including recipient vessel access and flap inset, especially in previously irradiated or surgically altered fields.
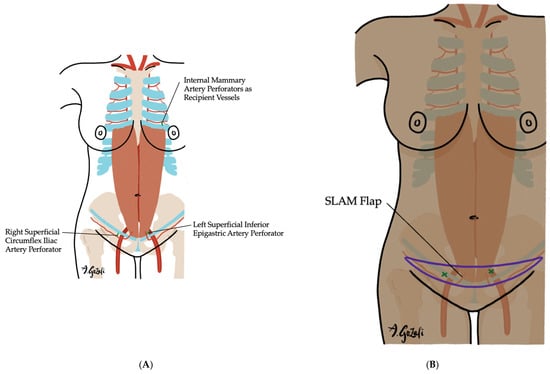
Figure 8.
The Superficially based low-abdominal mini (SLAM) flap. (A) Vascular supply of the SLAM flap is determined by the dominant vessel perfusing the designed flap, typically either the superficial inferior epigastric artery or the superficial circumflex iliac artery. This flap is ideal for small-breasted, thin patients undergoing 20–50% volume excision who wish to preserve breast volume; (B) The flap is named for its flap design, which is a narrow strip of lower abdominal tissue that allows for preservation of abdominal donor site for future reconstruction need.
Complications in volume replacement include flap loss, donor site morbidity, and delayed wound healing. Success relies heavily on careful patient selection, preoperative imaging of perforator anatomy if indicated, and experienced surgeons. Despite the growing use of these techniques, there remains a lack of standardized reporting on key variables—such as preoperative breast size, tumor location, and positive margin definitions (ranging from 1–2 mm to <10 mm across institutions)—making comparison of outcomes difficult [7].
Table 1 summarizes the volume displacement and replacement techniques mentioned in this review as well as their indications (Table 1).

Table 1.
Summary of Oncoplastic Breast-Conserving Surgery Techniques and Their Indications.
5. Outcome Optimization
Optimizing outcomes in OBCS requires a coordinated approach that integrates patient-reported, aesthetic, reconstructive, and oncologic goals. Among these, patient-reported outcomes have become increasingly central, as they reflect the patient’s own assessment of satisfaction, body image, and quality of life. Compared to standard BCS, OBCS consistently yields higher patient-reported outcome measures [11,82]. However, achieving high satisfaction depends on proper patient selection, technical execution, and managing expectations, particularly in the context of anticipated adjuvant therapy, which may alter the final cosmetic result.
Setting expectations before surgery is key to optimizing patient-reported outcomes. Patients should be informed not only about oncologic control but also about scar placement, potential loss of nipple or skin sensation, possible changes in breast shape due to adjuvant therapy, and the need for contralateral symmetrizing procedures. Whenever feasible, symmetry procedures should be performed during the same operation to enhance aesthetic outcomes, minimize recovery time, and reduce cost [83,84,85].
Success in OBCS also depends heavily on patient and procedural factors. Optimization of modifiable risk factors—such as body mass index (BMI), smoking cessation, and diabetes control—can reduce complication rates and improve healing. Unlike aesthetic breast surgery, OBCS involves tissue that has already been compromised by cancer excision, leading to reduced vascularity and altered architecture. Therefore, strategies that prioritize vascular preservation, minimization of tissue trauma, and avoidance of excessive tension during closure are essential for successful reconstruction.
From a reconstructive standpoint, the most common complications are early wound healing issues. Approximately 80% of complications after OBCS occur prior to initiation of radiation therapy, and about 8% of patients may experience delays in adjuvant treatment due to surgical complications [86]. Prevention of wound healing complications is therefore critical, as treatment delays can negatively impact oncologic outcomes. As tissue subjected to partial mastectomy already suffers disruption of vascular networks and structural integrity, key strategies include selecting robust pedicles based on vascular anatomy, minimizing closure tension, and handling tissue gently during glandular advancement to preserve vascular perfusion and promote wound healing. Strategies to minimize tension include selecting appropriate pedicles based on tumor location—such as using two shorter pedicles for lateral defects rather than one elongated pedicle [87]—to preserve perfusion and reduce complication rates. When advancing glandular tissue, sutures should be placed within sturdy glandular parenchyma rather than predominantly fatty tissue to avoid tearing, bleeding, and fat necrosis. Designing the resection cavity to collapse predictably, such as in the Benelli mastopexy where tissue is oriented to close radially around the NAC, helps maintain contour and NAC position [88]. Additionally, preserving tissue mobility by limiting dissection to either the subcutaneous plane or the chest wall fascia—but not both—reduces vascular disruption. Strategic incision planning (e.g., periareolar, inframammary, or crescent incisions) and careful tunneling techniques further support favorable cosmetic outcomes.
From an oncologic standpoint, the primary goal remains achieving negative margins while preserving the breast. OBCS facilitates wider excisions compared to standard partial mastectomy, reducing positive margin and re-excision rates [89]. However, the glandular rearrangement that defines OBCS can complicate intraoperative margin assessment. Techniques such as intraoperative margin assessment tool [90], ultrasound guidance, and frozen section analysis are invaluable tools for ensuring complete tumor removal. Still, definitions of an adequate margin vary across institutions as mentioned before, making multidisciplinary coordination and intraoperative judgement critical.
Proper undermining is essential for both oncologic access and effective glandular redistribution, but must be carefully balanced to avoid compromising vascularity. Skin and parenchymal undermining should be sufficient to allow for tension-free tissue advancement, especially for peripheral tumors. However, excessive undermining—particularly of the superficial fat layer—can increase the risk of fat necrosis and wound healing complications. Subareolar undermining should be minimized to preserve NAC vascularity and positioning. These technical details are critical to achieving both aesthetic and functional outcomes, particularly in patients undergoing adjuvant radiation.
Ultimately, successful OBCS hinges on integration: combining oncologic safety with aesthetic and functional goals, while maintaining alignment with patient expectations. A multidisciplinary, patient-centered approach—starting with counseling and extending through reconstruction and adjuvant therapy—is essential to maximize outcomes across all domains.
6. Conclusions
OBCS has transformed the landscape of surgical management of breast cancer by uniting oncologic safety with long-term aesthetic and functional restoration. The field has advanced from simple volume displacement procedures to a diverse spectrum of techniques—including complex volume replacement and free tissue transfer techniques—that now allow for tailored, patient-specific approaches. This evolution is accompanied by clear evidence of improved patient-reported outcomes and reduced rates of positive margins and re-excisions.
While technical progress has expanded the reconstructive toolbox, practical and systemic barriers—such as lack of access to trained providers and inconsistent reporting standards—continue to limit equitable implementation and comparative research. Recent efforts to address these challenges include the ASBS consensus definition and classification system, which provides a standardized framework for OBCS. Beyond improving clarity and accessibility, this system has shown potential as a predictor of clinical outcomes: a recent study demonstrated that patients undergoing Level II excision had significantly higher odds of developing delayed wound healing [91]. Importantly, growing evidence suggests that BCS with radiation therapy offers survival benefits compared to mastectomy in treating early-stage breast cancer, reinforcing the role of OBCS not just as a sound option but as the preferred treatment in eligible patients [92,93]. These findings underscore the need to further establish uniform outcome metrics, support prospective data collection, and ensure broader dissemination of OBCS expertise across diverse healthcare settings in order to expand patient access to oncoplastic techniques that improve both aesthetic and survival outcomes.
This review underscores the importance of optimizing outcomes across oncologic, reconstructive, aesthetic, and patient-reported domains. Whether through refined surgical strategies—such as minimizing closure tension, preserving pedicle perfusion, or tailoring flap choice to anatomy—or through preoperative planning and expectation setting, maximizing outcomes in OBCS requires an intentional, multidisciplinary, and patient-centered approach. Continued innovation, research, and policy support will be essential to ensure that the benefits of OBCS are equitably and consistently delivered to all patients undergoing BCS for breast cancer.
Author Contributions
Conceptualization, A.G. and M.P.; writing—original draft preparation, A.G.; writing—review and editing, A.G. and M.P.; illustration, A.G.; supervision, M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| OBCS | Oncoplastic Breast-Conserving Surgery |
| BCS | Breast-Conserving Surgery |
| BCT | Breast-Conserving Therapy |
| ASBS | American Society of Breast Surgeons |
| CPT | Current Procedural Terminology |
| NAC | Nipple-Areolar Complex |
| IMAP | Internal Mammary Artery Perforator |
| AICAP | Anterior Intercostal Artery Perforator |
| LICAP | Lateral Intercostal Artery Perforator |
| LTAP | Lateral Thoracic Artery Perforator |
| TDAP | Thoracodorsal Artery Perforator |
| LD | Latissimus Dorsi |
| DIEP | Deep Inferior Epigastric Perforator |
| SIEA | Superficial Inferior Epigastric Artery |
| SLAM | Superficially-based Low-Abdominal Mini |
| BMI | Body Mass Index |
References
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.-H.; Wolmark, N. Twenty-Year Follow-up of a Randomized Trial Comparing Total Mastectomy, Lumpectomy, and Lumpectomy plus Irradiation for the Treatment of Invasive Breast Cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-Year Follow-up of a Randomized Study Comparing Breast-Conserving Surgery with Radical Mastectomy for Early Breast Cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, R.A.; Valasiadou, P.; Wilson, A.R.M.; Al-Ghazal, S.K.; Macmillan, R.D. Cosmesis and satisfaction after breast-conserving surgery correlates with the percentage of breast volume excised. Br. J. Surg. 2003, 90, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
- Clough, K.B.; Cuminet, J.; Fitoussi, A.; Nos, C.; Mosseri, V. Cosmetic sequelae after conservative treatment for breast cancer: Classification and results of surgical correction. Ann. Plast. Surg. 1998, 41, 471–481. [Google Scholar] [CrossRef]
- Switalla, K.M.; Falade, I.O.; Quirarte, A.; Baxter, M.; Kaur, M.; Sakr, R.A.; Corso, G.; Mukhtar, R.A. Positive Margin Rates After Breast-Conserving Surgery by Histologic Subtype: A Systematic Review and Meta-analysis Evaluating the Impact of Oncoplastic Surgery. Ann. Surg. Oncol. 2025, 32, 4899–4909. [Google Scholar] [CrossRef]
- Losken, A.; Pinell-White, X.; Hart, A.M.; Freitas, A.M.; Carlson, G.W.; Styblo, T.M. The Oncoplastic Reduction Approach to Breast Conservation Therapy: Benefits for Margin Control. Aesthet. Surg. J. 2014, 34, 1185–1191. [Google Scholar] [CrossRef]
- Rutherford, C.; Barker, S.; Romics, L. A systematic review of oncoplastic volume replacement breast surgery: Oncological safety and cosmetic outcome. Ann. R. Coll. Surg. Engl. 2022, 104, 5–17. [Google Scholar] [CrossRef]
- Crown, A.; Scovel, L.G.; Rocha, F.G.; Scott, E.J.; Wechter, D.G.; Grumley, J.W. Oncoplastic breast conserving surgery is associated with a lower rate of surgical site complications compared to standard breast conserving surgery. Am. J. Surg. 2019, 217, 138–141. [Google Scholar] [CrossRef]
- Carter, S.A.; Lyons, G.R.; Kuerer, H.M.; Bassett, R.L.; Oates, S.; Thompson, A.; Caudle, A.S.; Mittendorf, E.A.; Bedrosian, I.; Lucci, A.; et al. Operative and Oncologic Outcomes in 9861 Patients with Operable Breast Cancer: Single-Institution Analysis of Breast Conservation with Oncoplastic Reconstruction. Ann. Surg. Oncol. 2016, 23, 3190–3198. [Google Scholar] [CrossRef]
- De La Cruz, L.; Blankenship, S.A.; Chatterjee, A.; Geha, R.; Nocera, N.; Czerniecki, B.J.; Tchou, J.; Fisher, C.S. Outcomes After Oncoplastic Breast-Conserving Surgery in Breast Cancer Patients: A Systematic Literature Review. Ann. Surg. Oncol. 2016, 23, 3247–3258. [Google Scholar] [CrossRef]
- Rose, M.; Svensson, H.; Handler, J.; Hoyer, U.; Ringberg, A.; Manjer, J. Patient-reported outcome after oncoplastic breast surgery compared with conventional breast-conserving surgery in breast cancer. Breast Cancer Res. Treat. 2020, 180, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, A.; Cutress, R.; Gandhi, A.; Harcourt, D.; Little, K.; Mansell, J.; Murphy, J.; Pennery, E.; Tillett, R.; Vidya, R.; et al. Oncoplastic breast surgery: A guide to good practice. Eur. J. Surg. Oncol. 2021, 47, 2272–2285. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.P.; Soysal, S.D.; Zeindler, J.; Kappos, E.A.; Babst, D.; Schwab, F.; Kurzeder, C.; Haug, M. Current standards in oncoplastic breast conserving surgery. The Breast 2017, 34, S78–S81. [Google Scholar] [CrossRef] [PubMed]
- Audretsch, W.P.; Rezai, M.; Kolotas, C.; Zamboglou, N.; Schnabel, T.; Bojar, H. Tumor-Specific Immediate Reconstruction in Breast Cancer Patients. Perspect. Plast. Surg. 2008, 11, 71–100. [Google Scholar] [CrossRef]
- Blichert-Toft, M.; Nielsen, M.; Düring, M.; Møller, S.; Rank, F.; Overgaard, M.; Mouridsen, H.T. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol. 2008, 47, 672–681. [Google Scholar] [CrossRef]
- Poggi, M.M.; Danforth, D.N.; Sciuto, L.C.; Smith, S.L.; Steinberg, S.M.; Liewehr, D.J.; Menard, C.; Lippman, M.E.; Lichter, A.S.; Altemus, R.M. Eighteen-year results in the treatment of early breast carcinoma with mastectomy versus breast conservation therapy. Cancer 2003, 98, 697–702. [Google Scholar] [CrossRef]
- Van Dongen, J.A.; Voogd, A.C.; Fentiman, I.S.; Legrand, C.; Sylvester, R.J.; Tong, D.; van der Schueren, E.; Helle, P.A.; van Zijl, K.; Bartelink, H. Long-Term Results of a Randomized Trial Comparing Breast-Conserving Therapy with Mastectomy: European Organization for Research and Treatment of Cancer 10801 Trial. JNCI J. Natl. Cancer Inst. 2000, 92, 1143–1150. [Google Scholar] [CrossRef]
- Arriagada, R.; Lê, M.G.; Rochard, F.; Contesso, G. Conservative treatment versus mastectomy in early breast cancer: Patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J. Clin. Oncol. 1996, 14, 1558–1564. [Google Scholar] [CrossRef]
- Clough, K.B.; Nos, C.; Salmon, R.J.; Soussaline, M.; Durand, J.C. Conservative treatment of breast cancers by mammaplasty and irradiation: A new approach to lower quadrant tumors. Plast. Reconstr. Surg. 1995, 96, 363–370. [Google Scholar] [CrossRef]
- Galimberti, V.; Zurrida, S.; Luini, A.; Greco, M.; Zanini, V.; Callegari, M.; Grisotti, A.; Veronesi, P.; Catania, S. Central small size breast cancer: How to overcome the problem of nipple and areola involvement. Eur. J. Cancer 1993, 29, 1093–1096. [Google Scholar] [CrossRef]
- Raufdeen, F.; Murphy, J.; Ahluwalia, M.; Coroneos, C.J.; Thoma, A. Outcomes in volume replacement and volume displacement techniques in oncoplastic breast conserving surgery: A systematic review. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 2846–2855. [Google Scholar] [CrossRef] [PubMed]
- Yiannakopoulou, E.C.; Mathelin, C. Oncoplastic breast conserving surgery and oncological outcome: Systematic review. Eur. J. Surg. Oncol. EJSO 2016, 42, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, M.; Van Landuyt, K.; Monstrey, S.; Blondeel, P. Pedicled perforator flaps in breast reconstruction: A new concept. Br. J. Plast. Surg. 2004, 57, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.A.; Straker, V.F.; Rainsbury, R.M. Extending the role of breast-conserving surgery by immediate volume replacement. Br. J. Surg. 1997, 84, 101–105. [Google Scholar]
- Panchal, H.; Shukla, D.; Razdan, S.N.; El-Tamer, M.; Matros, E.; Henderson, P.W. American trends in oncoplastic breast surgery for 2006–2015: A retrospective analysis of NSQIP database. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 644–710. [Google Scholar] [CrossRef]
- Willcox, L.M.; Losken, A.; Garcia Nores Gdel, P. Oncoplastic surgery in the USA: A review of where we started, where we are today and where we are headed. Gland Surg. 2024, 13, 749–759. [Google Scholar] [CrossRef]
- Maliko, N.; Schok, T.; Bijker, N.; Wouters, M.W.; Strobbe, L.J.; Hoornweg, M.J.; Peeters, M.-J.T.V. Oncoplastic Breast Conserving Surgery: Is There a Need for Standardization? Results of a Nationwide Survey. Breast Care 2023, 18, 90–96. [Google Scholar] [CrossRef]
- Maxwell, J.; Roberts, A.; Cil, T.; Somogyi, R.; Osman, F. Current Practices and Barriers to the Integration of Oncoplastic Breast Surgery: A Canadian Perspective. Ann. Surg. Oncol. 2016, 23, 3259–3265. [Google Scholar] [CrossRef]
- Challoner, T.; Skillman, J.; Wallis, K.; Vourvachis, M.; Whisker, L.; Hardwicke, J. Oncoplastic techniques: Attitudes and changing practice amongst breast and plastic surgeons in Great Britain. Breast 2017, 34, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.W.; Chatterjee, A.; Losken, A. Standards in oncoplastic breast-conserving surgery. Ann. Breast Surg. 2022, 6, 37, Published online 2021. Available online: https://abs.amegroups.org/article/view/7042 (accessed on 2 January 2021). [CrossRef]
- Johnstone, T.; Thawanyarat, K.; Rowley, M.; Francis, S.; Camacho, J.M.; Singh, D.; Navarro, Y.; Shah, J.K.; Nazerali, R.S. Racial Disparities in Postoperative Breast Reconstruction Outcomes: A National Analysis. J. Racial. Ethn. Health Disparities 2024, 11, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Keegan, G.; Rizzo, J.R.; Joseph, K.A. Disparities in breast cancer among patients with disabilities: Care gaps, accessibility, and best practices. JNCI J. Natl. Cancer Inst. 2023, 115, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, A.R.; Willcox, L.M.; Abolghasemi, D.M.; Jiang, R.; Wei, Z.Z.; Arciero, C.A.; Subhedar, P.D. Did Medicaid Expansion Mitigate Disparities in Post-mastectomy Reconstruction Rates? Am. Surg. 2022, 88, 846–851. [Google Scholar] [CrossRef]
- Molina, B.J.; Shelby, R.D.; Janis, J.E. Key Areas for Development in Oncoplastic Breast Reconstruction. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3273. [Google Scholar] [CrossRef]
- Chatterjee, A.; Gass, J.; Patel, K.; Holmes, D.; Kopkash, K.; Peiris, L.; Peled, A.; Ryan, J.; El-Tamer, M.; Reiland, J. A Consensus Definition and Classification System of Oncoplastic Surgery Developed by the American Society of Breast Surgeons. Ann. Surg. Oncol. 2019, 26, 3436–3444. [Google Scholar] [CrossRef]
- Clough, K.B.; Kaufman, G.J.; Nos, C.; Buccimazza, I.; Sarfati, I.M. Improving Breast Cancer Surgery: A Classification and Quadrant per Quadrant Atlas for Oncoplastic Surgery. Ann. Surg. Oncol. 2010, 17, 1375–1391. [Google Scholar] [CrossRef]
- ASBrS. Oncoplastic Surgery Certification. Available online: https://www.breastsurgeons.org/certification/oncoplastic (accessed on 10 May 2025).
- Rainsbury, R.M. The development of oncoplastic breast surgery in the UK. Ann. Breast Surg. 2022, 6, 36. [Google Scholar] [CrossRef]
- Down, S.K.; Pereira, J.H.; Leinster, S.; Simpson, A. Training the oncoplastic breast surgeon-current and future perspectives. Gland Surg. 2013, 2, 126–127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roughton, M.C.; Shenaq, D.; Jaskowiak, N.; Park, J.E.; Song, D.H. Optimizing Delivery of Breast Conservation Therapy: A Multidisciplinary Approach to Oncoplastic Surgery. Ann. Plast. Surg. 2012, 69, 250. [Google Scholar] [CrossRef]
- Losken, A.; Dugal, C.S.; Styblo, T.M.; Carlson, G.W. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann. Plast. Surg. 2014, 72, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Golshan, M.; Loibl, S.; Wong, S.M.; Huober, J.B.; O’sHaughnessy, J.; Rugo, H.S.; Wolmark, N.; McKee, M.D.; Maag, D.; Sullivan, D.M.; et al. Breast Conservation After Neoadjuvant Chemotherapy for Triple-Negative Breast Cancer. JAMA Surg. 2020, 155, e195410. [Google Scholar] [CrossRef] [PubMed]
- Golshan, M.; Cirrincione, C.T.; Sikov, W.M.; Carey, L.A.; Berry, D.A.; Overmoyer, B.; Henry, N.L.; Somlo, G.; Port, E.; Burstein, H.J.; et al. Impact of neoadjuvant therapy on eligibility for and frequency of breast conservation in stage II–III HER2-positive breast cancer: Surgical results of CALGB 40601 (Alliance). Breast Cancer Res. Treat. 2016, 160, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Golshan, M.; Cirrincione, C.T.; Sikov, W.M.; Berry, D.A.; Jasinski, S.; Weisberg, T.F.; Somlo, G.; Hudis, C.; Winer, E.; Ollila, D.W. Impact of Neoadjuvant Chemotherapy in Stage II–III Triple Negative Breast Cancer on Eligibility for Breast-conserving Surgery and Breast Conservation Rates. Ann. Surg. 2015, 262, 434–439. [Google Scholar] [CrossRef]
- Loo, C.E.; Straver, M.E.; Rodenhuis, S.; Muller, S.H.; Wesseling, J.; Peeters, M.-J.T.V.; Gilhuijs, K.G. Magnetic Resonance Imaging Response Monitoring of Breast Cancer During Neoadjuvant Chemotherapy: Relevance of Breast Cancer Subtype. J. Clin. Oncol. 2011, 29, 660–666. [Google Scholar] [CrossRef]
- Chen, J.-H.; Bahri, S.; Mehta, R.S.; Kuzucan, A.; Yu, H.J.; Carpenter, P.M.; Feig, S.A.; Lin, M.; Hsiang, D.J.B.; Lane, K.T.; et al. Breast Cancer: Evaluation of Response to Neoadjuvant Chemotherapy with 3.0-T MR Imaging. Radiology 2011, 261, 735–743. [Google Scholar] [CrossRef]
- Palmieri, B.; Benuzzi, G.; Costa, A.; Grappolini, S. Breast reduction and subsequent cancer: A prophylactic perspective. Breast 2006, 15, 476–481. [Google Scholar] [CrossRef]
- Boice, J.D., Jr.; Friis, S.; McLaughlin, J.K.; Mellemkjaer, L.; Blot, W.J.; Fraumeni, J.F., Jr.; Olsen, J.H. Cancer following breast reduction surgery in Denmark. Cancer Causes Control CCC 1997, 8, 253–258. [Google Scholar] [CrossRef]
- Vindigni, V.; Marena, F.; Zanettin, C.; Bassetto, F. Breast Reconstruction: The Oncoplastic Approach. J. Clin. Med. 2024, 13, 4718. [Google Scholar] [CrossRef]
- Massey, E.J.D.; Gouveia, P.F.; Nos, C.; Poulet, B.; Sarfati, I.; Clough, K.B. A new level 1 oncoplastic technique for breast conserving surgery: Rotation glandular flap. Breast 2013, 22, 186–189. [Google Scholar] [CrossRef]
- Lee, S.; Jung, Y.; Bae, Y. Dermoglandular rotation flap with subaxillary advancement flap as an oncoplastic technique for breast cancer. Breast J. 2019, 26, 420–426. [Google Scholar] [CrossRef]
- Nardello, S.M.; Bloom, J.A.; Gaffney, K.A.; Singhal, M.; Persing, S.; Chatterjee, A. Practical oncoplastic surgery techniques needed for practice. Ann. Transl. Med. 2023, 11, 383. [Google Scholar] [CrossRef] [PubMed]
- Matkowski, R.; Szynglarewicz, B.; Kasprzak, P.; Forgacz, J.; Skalik, R.; Zietek, M.; Kornafel, D. Batwing mastopexy as oncoplastic surgical approach to periareolar tumors in upper quadrants. Tumori. J. 2012, 98, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.F.; Colen, J.S.; Ellsworth, W. Ellsworth WA 4th. Reduction and Mastopexy Techniques for Optimal Results in Oncoplastic Breast Reconstruction. Semin. Plast. Surg. 2015, 29, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Bloom, J.; Nardello, S.; Cohen, S.; Reiland, J.; Chatterjee, A. An Oncoplastic Surgery Primer: Common Indications, Techniques, and Complications in Level 1 and 2 Volume Displacement Oncoplastic Surgery. Ann. Surg. Oncol. 2019, 26, 3063–3070. [Google Scholar] [CrossRef]
- Chatterjee, A.; Dayicioglu, D.; Khakpour, N.; Czerniecki, B.J. Oncoplastic Surgery. Cancer Control J. Moffitt Cancer Cent. 2017, 24, 1073274817729043. [Google Scholar] [CrossRef]
- Nahabedian, M.Y.; Patel, K.M.; Kaminsky, A.J.; Cocilovo, C.; Miraliakbari, R. Biplanar oncoplastic surgery: A novel approach to breast conservation for small and medium sized breasts. Plast. Reconstr. Surg. 2013, 132, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Irigo, M.; Coscarelli, L.; Rancati, A. Anatomical basis of pedicles in breast reduction. Gland. Surg. 2017, 6, 154–162. [Google Scholar] [CrossRef]
- Monib, S.; Abdelaziz, M.I. Epidemiology and Predictive Factors for Persistent Breast Pain Following Breast-Conserving Surgery. Cureus 2021, 13, e14063. [Google Scholar] [CrossRef]
- Moen, M.; Holton, T.; Phung, A.; Badve, S.; Mylander, C.; Sanders, T.; Pauliukonis, M.; Jackson, R.S. Complications after Oncoplastic Breast Reduction and Impact on Time to Adjuvant Therapy. Plast. Reconstr. Surg. Glob. Open 2024, 12, e6010. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, M.C.; Park, H.Y.; Yang, J.D. Oncoplastic volume replacement techniques according to the excised volume and tumor location in small- to moderate-sized breasts. Gland Surg. 2014, 3, 14–21. [Google Scholar] [CrossRef]
- Van Huizum, M.A.; Hage, J.J.; Oldenburg, H.A.; Hoornweg, M.J. Internal Mammary Artery Perforator Flap for Immediate Volume Replacement Following Wide Local Excision of Breast Cancer. Arch. Plast. Surg. 2017, 44, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-López, C.; Ibañez, J.F.J.; Vilà, J.; Tomás, M.A.L.; López, J.N.; Miguel, I.P.; Fernandez-Llamazares-Rodriguez, J.; Higueras-Suñe, C. Anterior intercostal artery perforator flap in immediate breast reconstruction: Anatomical study and clinical application. Microsurgery 2017, 37, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Kim, D.K.; Lee, J.W.; Choi, K.Y.; Chung, H.Y.; Cho, B.C.; Park, H.Y.; Lee, J.Y.; Yang, J.D. The usefulness of pedicled perforator flap in partial breast reconstruction after breast conserving surgery in Korean women. Arch. Plast. Surg. 2018, 45, 29–36. [Google Scholar] [CrossRef] [PubMed]
- McCulley, S.J.; Schaverien, M.V.; Tan, V.K.M.; Macmillan, R.D. Lateral thoracic artery perforator (LTAP) flap in partial breast reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 686–691. [Google Scholar] [CrossRef]
- Amin, A.A.; Rifaat, M.; Farahat, A.; Hashem, T. The role of thoracodorsal artery perforator flap in oncoplastic breast surgery. J. Egypt. Natl. Cancer Inst. 2017, 29, 83–87. [Google Scholar] [CrossRef]
- Angrigiani, C.; Rancati, A.; Artero, G.; Escudero, E.; Khouri, R.K. TDAP: Island versus propeller. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 506–511. [Google Scholar] [CrossRef]
- Angrigiani, C.; Rancati, A.; Escudero, E.; Artero, G. Extended thoracodorsal artery perforator flap for breast reconstruction. Gland Surg. 2015, 4, 519–527. [Google Scholar] [CrossRef]
- Ayhan, S.; Tuncer, S.; Demir, Y.; Kandal, S. Thoracodorsal Artery Perforator Flap: A Versatile Alternative for Various Soft Tissue Defects. J. Reconstr. Microsurg. 2008, 24, 285–293. [Google Scholar] [CrossRef]
- Mangialardi, M.L.; Baldelli, I.; Salgarello, M.; Raposio, E. Thoracodorsal Artery Perforator Flap in Partial Breast Reconstruction: A Systematic Review. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3104. [Google Scholar] [CrossRef]
- Mangialardi, M.L.; Baldelli, I.; Salgarello, M.; Raposio, E. Breast Reconstruction Using the Lateral Thoracic, Thoracodorsal, and Intercostal Arteries Perforator Flaps. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3334. [Google Scholar] [CrossRef]
- Yang, J.D.; Ryu, D.W.; Lee, J.W.; Choi, K.Y.; Chung, H.Y.; Cho, B.C.; Park, H.Y.; Byun, J.S. Usefulness of a Lateral Thoracodorsal Flap after Breast Conserving Surgery in Laterally Located Breast Cancer. Arch. Plast. Surg. 2013, 40, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.J.; Hill, H.L.; Brown, R.G. Latissimus dorsi myocutaneous flap for breast reconstruction. Br. J. Plast. Surg. 1977, 30, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Lee, J.W.; Cho, Y.K.; Kim, W.W.; Hwang, S.O.; Jung, J.H.; Park, H.Y. Surgical Techniques for Personalized Oncoplastic Surgery in Breast Cancer Patients with Small- to Moderate-Sized Breasts (Part 2): Volume Replacement. J. Breast Cancer 2012, 15, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T. Superior epigastric artery perforator flap: Preliminary report. Plast. Reconstr Surg. 2007, 120, 1e–5e. [Google Scholar] [CrossRef]
- Zaha, H. Oncoplastic volume replacement technique for the upper inner quadrant using the omental flap. Gland Surg. 2015, 4, 263–269. [Google Scholar] [CrossRef]
- Zaha, H.; Inamine, S.; Naito, T.; Nomura, H. Laparoscopically harvested omental flap for immediate breast reconstruction. Am. J. Surg. 2006, 192, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Molina, B.J.; Dayan, E.; Jablonka, E.M.; Okwali, M.; Kim, J.N.; Dayan, J.H.; Smith, M.L. Defining the Role of Free Flaps in Partial Breast Reconstruction. J. Reconstr. Microsurg. 2018, 34, 185–192. [Google Scholar] [CrossRef]
- Spiegel, A.J.; Eldor, L. Partial breast reconstruction with mini superficial inferior epigastric artery and mini deep inferior epigastric perforator flaps. Ann. Plast. Surg. 2010, 65, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.; Fujikawa, M.; Tashima, H.; Saito, T.; Sotsuka, Y.; Tomita, K.; Hosokawa, K. Immediate reconstruction using free medial circumflex femoral artery perforator flaps after breast-conserving surgery. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 1528–1533. [Google Scholar] [CrossRef]
- Salibian, A.A.; Swerdlow, M.A.; Kondra, K.; Patel, K.M. The Free Superficially Based Low-Abdominal Mini Flap for Oncoplastic Breast Reconstruction. Plast. Reconstr. Surg. 2023, 152, 959–962. [Google Scholar] [CrossRef]
- Ritter, M.; Oberhauser, I.; Montagna, G.; Zehnpfennig, L.; Schaefer, K.; Ling, B.M.; Levy, J.; Soysal, S.D.; Müller, M.; López, L.C.; et al. Comparison of patient-reported outcomes among different types of oncoplastic breast surgery procedures. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 3068–3077. [Google Scholar] [CrossRef] [PubMed]
- Grant, Y.; Thiruchelvam, P.T.R.; Kovacevic, L.; Mossialos, E.; Al-Mufti, R.; Hogben, K.; Hadjiminas, D.J.; Leff, D.R. Patient-level costs of staged unilateral versus immediate bilateral symmetrization mammoplasty in breast-conserving surgery. BJS Open 2022, 6, zrac073. [Google Scholar] [CrossRef] [PubMed]
- Gulis, K.; Rydén, L.; Bendahl, P.O.; Svensjö, T. Cosmetic Outcomes and Symmetry Comparison in Patients Undergoing Bilateral Therapeutic Mammoplasty for Breast Cancer. World J. Surg. 2021, 45, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Deigni, O.A.; Baumann, D.P.; Adamson, K.A.; Garvey, P.B.; Selber, J.C.; Caudle, A.S.; Smith, B.D.; Hanson, S.E.; Robb, G.L.; Schaverien, M.V. Immediate Contralateral Mastopexy/Breast Reduction for Symmetry Can Be Performed Safely in Oncoplastic Breast-Conserving Surgery. Plast. Reconstr. Surg. 2020, 145, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Hillberg, N.S.; Meesters-Caberg, M.A.J.; Beugels, J.; Winkens, B.; Vissers, Y.L.J.; van Mulken, T.J.M. Delay of adjuvant radiotherapy due to postoperative complications after oncoplastic breast conserving surgery. Breast 2018, 39, 110–116. [Google Scholar] [CrossRef]
- Gilani, S.N.; Cuffolo, G.; Win, M.M.; Asgeirsson, K.; Whisker, L. Volume displacement techniques in oncoplastic breast conserving surgery. Rev. Senol. Patol. Mamar.-J. Senol. Breast Dis. 2021, 34, S30–S34. [Google Scholar] [CrossRef]
- Benelli, L. A new periareolar mammaplasty: The “round block” technique. Aesthetic Plast. Surg. 1990, 14, 93–100. [Google Scholar] [CrossRef]
- Heeg, E.; Jensen, M.B.; Hölmich, L.R.; Bodilsen, A.; Tollenaar, R.A.E.M.; Lænkholm, A.V.; Offersen, B.V.; Ejlertsen, B.; Mureau, M.A.M.; Christiansen, P.M. Rates of re-excision and conversion to mastectomy after breast-conserving surgery with or without oncoplastic surgery: A nationwide population-based study. Br. J. Surg. 2020, 107, 1762–1772. [Google Scholar] [CrossRef]
- Rossou, C.; Alampritis, G.; Patel, B. Reducing re-excision rates in breast conserving surgery with Margin Probe: Systematic review. Br. J. Surg. 2023, 111, znad335. [Google Scholar] [CrossRef]
- Maggi, N.; Rais, D.; Nussbaumer, R.; Levy, J.; Schwab, F.D.; Kurzeder, C.; Heidinger, M.; Weber, W.P. The American Society of Breast Surgeons classification system for oncoplastic breast conserving surgery independently predicts the risk of delayed wound healing. Eur. J. Surg. Oncol. 2023, 49, 107032. [Google Scholar] [CrossRef]
- Christiansen, P.; Mele, M.; Bodilsen, A.; Rocco, N.; Zachariae, R. Breast-Conserving Surgery or Mastectomy?: Impact on Survival. Ann. Surg. Open 2022, 3, e205. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Tang, Y.; Jing, H.; Sun, G.; Jin, J.; Liu, Y.; Song, Y.; Wang, W.; Fang, H.; et al. Comparison of Treatment Outcomes With Breast-conserving Surgery Plus Radiotherapy Versus Mastectomy for Patients with Stage I Breast Cancer: A Propensity Score-matched Analysis. Clin. Breast Cancer 2018, 18, e975–e984. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).