Techniques for Success in Nipple-Sparing Mastectomy and Immediate Reconstruction
Abstract
1. Introduction
2. Materials and Methods
2.1. Preoperative Evaluation and Patient Selection
2.2. Surgical Techniques
2.2.1. Incision Placement
2.2.2. Mastectomy Flap Quality
2.2.3. Direct to Implant Reconstruction
2.2.4. Subpectoral Versus Prepectoral Reconstruction
2.2.5. Use of Soft Tissue Support
2.2.6. Neurotization
2.2.7. Staged Reconstruction with Tissue Expanders
2.3. Clinical Outcomes
2.3.1. Oncologic
2.3.2. Aesthetic
2.3.3. Patient Satisfaction
2.3.4. Limitations
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Valero, M.G.; Muhsen, S.; Moo, T.-A.; Zabor, E.C.; Stempel, M.; Pusic, A.; Gemignani, M.L.; Morrow, M.; Sacchini, V.S. Increase in utilization of nipple-sparing mastectomy for breast cancer: Indications, complications, and oncologic outcomes. Ann. Surg. Oncol. 2020, 27, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Huang, J.; Chagpar, A.B. Is nipple sparing mastectomy associated with increased complications, readmission and length of stay compared to skin sparing mastectomy? Am. J. Surg. 2020, 219, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.J.; Salibian, A.A.; Bekisz, J.M.; Axelrod, D.M.; Guth, A.A.; Shapiro, R.L.; Schnabel, F.R.; Karp, N.S.; Choi, M. Long-term cancer recurrence rates following nipple-sparing mastectomy: A 10-year follow-up study. Plast. Reconstr. Surg. 2022, 150, 13S–19S. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.J.; Ramesh, S.; Bekisz, J.M.; Guth, A.A.; Axelrod, D.M.; Shapiro, R.L.; Hiotis, K.; Schnabel, F.R.; Karp, N.S.; Choi, M. Low cancer occurrence rate following prophylactic nipple-sparing mastectomy. Plast. Reconstr. Surg. 2024, 153, 37e–43e. [Google Scholar] [CrossRef]
- Romanoff, A.; Zabor, E.C.; Stempel, M.; Sacchini, V.; Pusic, A.; Morrow, M. A comparison of patient-reported outcomes after nipple-sparing mastectomy and conventional mastectomy with reconstruction. Ann. Surg. Oncol. 2018, 25, 2909–2916. [Google Scholar] [CrossRef]
- Wei, C.H.; Scott, A.M.; Price, A.N.; Miller, H.C.; Klassen, A.F.; Jhanwar, S.M.; Mehrara, B.J.; Disa, J.J.; McCarthy, C.; Matros, E. Psychosocial and sexual well-being following nipple-sparing mastectomy and reconstruction. Breast J. 2016, 22, 10–17. [Google Scholar] [CrossRef]
- Kelly, B.N.; Faulkner, H.R.; Smith, B.L.; Korotkin, J.E.; Lanahan, C.R.; Brown, C.; Gadd, M.A.; Specht, M.C.; Hughes, K.S.; Oseni, T.S. Nipple-sparing mastectomy versus skin-sparing mastectomy: Does saving the nipple impact short- and long-term patient satisfaction? Ann. Surg. Oncol. 2022, 29, 1033–1040. [Google Scholar] [CrossRef]
- Amro, C.; Sorenson, T.J.; Boyd, C.J.; Hemal, K.; Vernice, N.A.; Park, J.J.; Cohen, O.D.; Choi, M.; Karp, N.S. The Evolution of Implant-Based Breast Reconstruction: Innovations, Trends, and Future Directions. J. Clin. Med. 2024, 13, 7407. [Google Scholar] [CrossRef]
- Frey, J.D.; Alperovich, M.; Levine, J.P.; Choi, M.; Karp, N.S. Does Smoking History Confer a Higher Risk for Reconstructive Complications in Nipple-Sparing Mastectomy? Breast J. 2017, 23, 415–420. [Google Scholar] [CrossRef]
- Zaborowski, A.M.; Roe, S.; Rothwell, J.; Evoy, D.; Geraghty, J.; McCartan, D.; Prichard, R.S. A systematic review of oncological outcomes after nipple-sparing mastectomy for breast cancer. J. Surg. Oncol. 2023, 127, 361–368. [Google Scholar] [CrossRef]
- Spear, S.L.; Willey, S.C.; Feldman, E.D.; Cocilovo, C.; Sidawy, M.; Al-Attar, A.; Hannan, C.; Seiboth, L.; Nahabedian, M.Y. Nipple-sparing mastectomy for prophylactic and therapeutic indications. Plast. Reconstr. Surg. 2011, 128, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Weber, W.P.; Haug, M.; Kurzeder, C.; Bjelic-Radisic, V.; Koller, R.; Reitsamer, R.; Fitzal, F.; Biazus, J.; Brenelli, F.; Urban, C. Oncoplastic Breast Consortium consensus conference on nipple-sparing mastectomy. Breast Cancer Res. Treat. 2018, 172, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Doremus, N.V.; Vega, K.; Tecce, M.G.; Kanchwala, S. Expanding the Use of Nipple Sparing Mastectomy: A Review of the Indications and Techniques. Surg. Oncol. Insight 2024, 1, 100062. [Google Scholar] [CrossRef]
- Jadeja, P.; Ha, R.; Rohde, C.; Ascherman, J.; Grant, R.; Chin, C.; Connolly, E.; Kalinsky, K.; Feldman, S.; Taback, B. Expanding the criteria for nipple-sparing mastectomy in patients with poor prognostic features. Clin. Breast Cancer 2018, 18, 229–233. [Google Scholar] [CrossRef]
- Burdge, E.C.; Yuen, J.; Hardee, M.; Gadgil, P.V.; Das, C.; Henry-Tillman, R.; Ochoa, D.; Korourian, S.; Suzanne Klimberg, V. Nipple skin-sparing mastectomy is feasible for advanced disease. Ann. Surg. Oncol. 2013, 20, 3294–3302. [Google Scholar] [CrossRef]
- Youn, S.; Lee, E.; Peiris, L.; Olson, D.; Lesniak, D.; Rajaee, N. Spare the nipple: A systematic review of tumor nipple-distance and oncologic outcomes in nipple-sparing mastectomy. Ann. Surg. Oncol. 2023, 30, 8381–8388. [Google Scholar] [CrossRef]
- Kracoff-Sella, S.L.; Allweis, T.M.; Bokov, I.; Kadar-Sfarad, H.; Shifer, Y.; Golzman, E.; Egozi, D. Tumor-to-nipple distance in selecting patients for nipple-sparing mastectomy. Plast. Reconstr. Surg.–Glob. Open 2020, 8, e2963. [Google Scholar] [CrossRef]
- Frey, J.D.; Salibian, A.A.; Lee, J.; Harris, K.; Axelrod, D.M.; Guth, A.A.; Shapiro, R.L.; Schnabel, F.R.; Karp, N.S.; Choi, M. Oncologic trends, outcomes, and risk factors for locoregional recurrence: An analysis of tumor-to-nipple distance and critical factors in therapeutic nipple-sparing mastectomy. Plast. Reconstr. Surg. 2019, 143, 1575–1585. [Google Scholar] [CrossRef]
- Dent, B.L.; Miller, J.A.; Eden, D.J.; Swistel, A.; Talmor, M. Tumor-to-nipple distance as a predictor of nipple involvement: Expanding the inclusion criteria for nipple-sparing mastectomy. Plast. Reconstr. Surg. 2017, 140, 1e–8e. [Google Scholar] [CrossRef]
- Park, S.; Yoon, C.; Bae, S.J.; Cha, C.; Kim, D.; Lee, J.; Ahn, S.G.; Roh, T.S.; Kim, Y.S.; Jeong, J. Comparison of complications according to incision types in nipple-sparing mastectomy and immediate reconstruction. Breast 2020, 53, 85–91. [Google Scholar] [CrossRef]
- Tang, R.; Coopey, S.B.; Merrill, A.L.; Rai, U.; Specht, M.C.; Gadd, M.A.; Colwell, A.S.; Austen Jr, W.G.; Brachtel, E.F.; Smith, B.L. Positive nipple margins in nipple-sparing mastectomies: Rates, management, and oncologic safety. J. Am. Coll. Surg. 2016, 222, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Haslinger, M.L.; Sosin, M.; Bartholomew, A.J.; Crocker, A.; Gulla, A.; Willey, S.C.; Pittman, T.A.; Tousimis, E.A. Positive nipple margin after nipple-sparing mastectomy: An alternative and oncologically safe approach to preserving the nipple–areolar complex. Ann. Surg. Oncol. 2018, 25, 2303–2307. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.L.; Foster, R.; Mukhtar, R.A.; Esserman, L.J.; Ewing, C.; Alvarado, M.; Wong, J.; Piper, M. Expanding Candidacy for Nipple-sparing Mastectomy in Women with Large or Ptotic Breasts: Staged Reconstruction Outcomes. Plast. Reconstr. Surg.–Glob. Open 2023, 11, e4767. [Google Scholar] [CrossRef] [PubMed]
- Ostapenko, E.; Nixdorf, L.; Devyatko, Y.; Exner, R.; Math, P.; Wimmer, K.; Haeusler, T.; Fitzal, F. Ptotic versus nonptotic breasts in nipple-sparing mastectomy and immediate prepectoral breast reconstruction. Plast. Reconstr. Surg.–Glob. Open 2023, 11, e5032. [Google Scholar] [CrossRef]
- Momeni, A.; Kanchwala, S.; Sbitany, H. Oncoplastic procedures in preparation for nipple-sparing mastectomy and autologous breast reconstruction: Controlling the breast envelope. Plast. Reconstr. Surg. 2020, 145, 914–920. [Google Scholar] [CrossRef]
- Spear, S.L.; Rottman, S.J.; Seiboth, L.A.; Hannan, C.M. Breast reconstruction using a staged nipple-sparing mastectomy following mastopexy or reduction. Plast. Reconstr. Surg. 2012, 129, 572–581. [Google Scholar] [CrossRef]
- Economides, J.M.; Graziano, F.; Tousimis, E.; Willey, S.; Pittman, T.A. Expanded algorithm and updated experience with breast reconstruction using a staged nipple-sparing mastectomy following mastopexy or reduction mammaplasty in the large or ptotic breast. Plast. Reconstr. Surg. 2019, 143, 688e–697e. [Google Scholar] [CrossRef]
- Yazar, S.; Bengur, F.B.; Altinkaya, A.; Kara, H.; Uras, C. Nipple-sparing mastectomy and immediate implant-based reconstruction with or without skin reduction in patients with large ptotic breasts: A case-matched analysis. Aesthetic Plast. Surg. 2021, 45, 956–967. [Google Scholar] [CrossRef]
- Aliotta, R.E.; Scomacao, I.; Duraes, E.F.; Kwiecien, G.J.; Durand, P.D.; Fanning, A.; Moreira, A. Pushing the envelope: Skin-only mastopexy in single-stage nipple-sparing mastectomy with direct-to-implant breast reconstruction. Plast. Reconstr. Surg. 2021, 147, 38–45. [Google Scholar] [CrossRef]
- Mosharrafa, A.M.; Mosharrafa, T.M.; Zannis, V.J. Direct-to-implant breast reconstruction with simultaneous nipple-sparing mastopexy utilizing an inferiorly based adipodermal flap: Our experience with prepectoral and subpectoral techniques. Plast. Reconstr. Surg. 2020, 145, 1125–1133. [Google Scholar] [CrossRef]
- Pontell, M.; Saad, N.; Brown, A.; Rose, M.; Ashinoff, R.; Saad, A. Single Stage Nipple-Sparing Mastectomy and Reduction Mastopexy in the Ptotic Breast. Plast. Surg. Int. 2018, 2018, 9205805. [Google Scholar] [CrossRef]
- Ghidei, L.; Bansil, H.A.; Stuckey, A.; Pandya, S.; Edmonson, D.; Michaud, P.; Gass, J. Nipple-sparing mastectomy and ptosis: Using a free nipple graft with tissue expander reconstruction. Plast. Reconstr. Surg.–Glob. Open 2020, 8, e2623. [Google Scholar] [CrossRef]
- Deptula, P.; Yesantharao, P.; Wapnir, I.; Nguyen, D. Staged approach to autologous reconstruction in the ptotic breast: A comparative study. Ann. Plast. Surg. 2021, 86, S395–S402. [Google Scholar] [CrossRef] [PubMed]
- Loreti, A.; Fanelli, B.; Abate, O.; Spallone, D.; Arelli, F.; Bruno, E.; Marcasciano, M.; La Pinta, M.; Meli, E.Z.; Fortunato, L. Surgical delay of nipple areola complex: A powerful technique to extend the indication of nipple-sparing mastectomy. Clin. Breast Cancer 2023, 23, 255–264. [Google Scholar] [CrossRef]
- Lee, P.L.; Ma, I.T.; Mark Asher Schusterman, I.; Beiriger, J.; Ahrendt, G.; De La Cruz, C.; Diego, E.J.; Steiman, J.G.; McAuliffe, P.F.; Gimbel, M.L. Surgical Nipple Delay and its Expanded Indications for Nipple-sparing Mastectomy. Plast. Reconstr. Surg.–Glob. Open 2023, 11, e4783. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Coopey, S.B.; Colwell, A.S.; Specht, M.C.; Gadd, M.A.; Kansal, K.; McEvoy, M.P.; Merrill, A.L.; Rai, U.; Taghian, A. Nipple-sparing mastectomy in irradiated breasts: Selecting patients to minimize complications. Ann. Surg. Oncol. 2015, 22, 3331–3337. [Google Scholar] [CrossRef]
- Choi, M.; Frey, J.D. Optimizing aesthetic outcomes in breast reconstruction after nipple-sparing mastectomy. Aesthetic Surg. J. 2020, 40, S13–S21. [Google Scholar] [CrossRef]
- Lin, A.M.; Lorenzi, R.; Van Der Hulst, J.E.; Liao, E.C.; Austen Jr, W.G.; Webster, A.; Smith, B.L.; Colwell, A.S. A decade of nipple-sparing mastectomy: Lessons learned in 3035 immediate implant-based breast reconstructions. Plast. Reconstr. Surg. 2024, 153, 277–287. [Google Scholar] [CrossRef]
- Colwell, A.S.; Tessler, O.; Lin, A.M.; Liao, E.; Winograd, J.; Cetrulo, C.L.; Tang, R.; Smith, B.L.; Austen Jr, W.G. Breast reconstruction following nipple-sparing mastectomy: Predictors of complications, reconstruction outcomes, and 5-year trends. Plast. Reconstr. Surg. 2014, 133, 496–506. [Google Scholar] [CrossRef]
- Salibian, A.A.; Bekisz, J.M.; Frey, J.D.; Thanik, V.D.; Levine, J.P.; Karp, N.S.; Choi, M. Comparing incision choices in immediate microvascular breast reconstruction after nipple-sparing mastectomy: Unique considerations to optimize outcomes. Plast. Reconstr. Surg. 2021, 148, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- Visconti, G.; Franceschini, G.; Bianchi, A.; Barone-Adesi, L.; Garganese, G.; Masetti, R.; Salgarello, M. Transaxillary nipple-sparing mastectomy and direct-to-implant breast reconstruction using a simplified endoscopic approach: Indications, cosmetic outcomes and technical refinements. Aesthetic Plast. Surg. 2020, 44, 1466–1475. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, Y.; Liang, F.; Wang, Y.; Lv, Q.; Du, Z. Endoscopic-assisted nipple-sparing mastectomy with direct-to-implant subpectoral breast reconstruction in the management of breast cancer. Plast. Reconstr. Surg.–Glob. Open 2021, 9, e3978. [Google Scholar] [CrossRef]
- Gau, R.-Y.; Chou, H.-H.; Tsai, H.-P.; Shen, S.-C.; Kuo, W.-L.; Chu, C.-H.; Ho, H.-y.; Huang, J.-J.; Lin, Y.-C.; Huang, Y.-T. Long-term follow-up of surgical outcomes and oncological results of nipple-sparing mastectomy with immediate reconstruction through a single axillary incision with different approach methods. Ann. Surg. Oncol. 2025, 32, 2092–2102. [Google Scholar] [CrossRef]
- Farr, D.E.; Haddock, N.T.; Tellez, J.; Radi, I.; Alterio, R.; Sayers, B.; Zeh, H. Safety and feasibility of single-port robotic-assisted nipple-sparing mastectomy. JAMA Surg. 2024, 159, 269–276. [Google Scholar] [CrossRef]
- Lee, J.; Park, H.S.; Lee, H.; Lee, D.W.; Song, S.Y.; Lew, D.H.; Kim, J.Y.; Park, S.; Kim, S.I. Post-operative complications and nipple necrosis rates between conventional and robotic nipple-sparing mastectomy. Front. Oncol. 2021, 10, 594388. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, J.; Lee, D.W.; Song, S.Y.; Lew, D.H.; Kim, S.I.; Cho, Y.U. Robot-assisted nipple-sparing mastectomy with immediate breast reconstruction: An initial experience. Sci. Rep. 2019, 9, 15669. [Google Scholar]
- Ryu, J.M.; Kim, J.Y.; Choi, H.J.; Ko, B.; Kim, J.; Cho, J.; Lee, M.H.; Choi, J.E.; Kim, J.H.; Lee, J. Robot-assisted nipple-sparing mastectomy with immediate breast reconstruction: An initial experience of the Korea Robot-Endoscopy Minimal Access Breast Surgery Study Group (KoREa-BSG). Ann. Surg. 2022, 275, 985–991. [Google Scholar] [CrossRef]
- Vicini, E.; De Lorenzi, F.; Invento, A.; Corso, G.; Radice, D.; Bozzo, S.; Fontana, S.K.R.; Caldarella, P.; Veronesi, P.; Galimberti, V. Is Nipple-Sparing Mastectomy Indicated after Previous Breast Surgery? A Series of 387 Institutional Cases. Plast. Reconstr. Surg. 2021, 148, 21–30. [Google Scholar] [CrossRef]
- Lv, W.; Fu, P.; Wu, P. Updated findings of skin flap thickness and residual breast tissue after mastectomy for breast cancer: A systematic review of the literature. Updates Surg. 2024, 76, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Pagliara, D.; Montella, R.A.; Garganese, G.; Bove, S.; Costantini, M.; Rinaldi, P.M.; Pino, V.; Grieco, F.; Rubino, C.; Salgarello, M. Improving decision-making in prepectoral direct-to-implant reconstruction after nipple sparing mastectomy: The key role of flap thickness ratio. Clin. Breast Cancer 2023, 23, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Han, M.W.; Hong, K.Y. Prospective clinical trial for predicting mastectomy skin flap necrosis with Indocyanine green angiography in implant-based Prepectoral breast reconstruction. Aesthetic Plast. Surg. 2024, 48, 4937–4944. [Google Scholar] [CrossRef]
- Lauritzen, E.; Damsgaard, T.E. Use of Indocyanine Green Angiography decreases the risk of complications in autologous-and implant-based breast reconstruction: A systematic review and meta-analysis. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 1703–1717. [Google Scholar] [CrossRef]
- Kim, M.J.; Mok, J.H.; Lee, I.J.; Lim, H. Mastectomy skin flap stability prediction using indocyanine green angiography: A randomized prospective trial. Aesthetic Surg. J. 2023, 43, NP1052–NP1060. [Google Scholar] [CrossRef]
- Choi, M.; Frey, J.D.; Alperovich, M.; Levine, J.P.; Karp, N.S. “Breast in a day”: Examining single-stage immediate, permanent implant reconstruction in nipple-sparing mastectomy. Plast. Reconstr. Surg. 2016, 138, 184e–191e. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.M.; Christensen, J.M.; Liao, E.C.; Cetrulo Jr, C.L.; Smith, B.L.; Austen Jr, W.G.; Winograd, J.; Colwell, A.S. Postmastectomy radiation therapy on permanent implants or tissue expanders: Which is better? Ann. Surg. 2021, 274, e974–e979. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Yang, Y.-J.; Lee, D.-W.; Song, S.-Y.; Lew, D.-H.; Yang, E.-J. Prevention of postoperative complications by prepectoral versus subpectoral breast reconstruction: A systematic review and meta-analysis. Plast. Reconstr. Surg. 2024, 153, 10e–24e. [Google Scholar] [CrossRef]
- Holland, M.; Su, P.; Piper, M.; Withers, J.; Harbell, M.W.; Bokoch, M.P.; Sbitany, H. Prepectoral breast reconstruction reduces opioid consumption and pain after mastectomy: A head-to-head comparison with submuscular reconstruction. Ann. Plast. Surg. 2022, 89, 492–499. [Google Scholar] [CrossRef]
- Fang, H.A.; Soto, E.; Pigg, R.; Smith, M.; Boyd, C.J.; Ananthasekar, S.; Fix, R.J.; Kilic, A.; Denney, B.; Patcha, P. The safety of fat grafting: An institutional retrospective review. Ann. Plast. Surg. 2022, 88, S473–S477. [Google Scholar] [CrossRef]
- Bekisz, J.M.; Salibian, A.A.; Frey, J.D.; Choi, M.; Karp, N.S. Picking the right plane: A comparison of total submuscular, dual-plane, and prepectoral implant–based breast reconstruction. Plast. Reconstr. Surg. 2022, 150, 737e–746e. [Google Scholar] [CrossRef] [PubMed]
- Talwar, A.A.; Lanni, M.A.; Ryan, I.A.; Kodali, P.; Bernstein, E.; McAuliffe, P.B.; Broach, R.B.; Serletti, J.M.; Butler, P.D.; Fosnot, J. Prepectoral versus submuscular implant-based breast reconstruction: A matched-pair comparison of outcomes. Plast. Reconstr. Surg. 2024, 153, 281e–290e. [Google Scholar] [CrossRef] [PubMed]
- King, C.A.; Bartholomew, A.J.; Sosin, M.; Avila, A.; Famiglietti, A.L.; Dekker, P.K.; Perez-Alvarez, I.M.; Song, D.H.; Fan, K.L.; Tousimis, E.A. A critical appraisal of late complications of prepectoral versus subpectoral breast reconstruction following nipple-sparing mastectomy. Ann. Surg. Oncol. 2021, 28, 9150–9158. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.E.; Dreicer, M.; Butterworth, J.A.; Larson, K.E. Do nipple necrosis rates differ in prepectoral versus submuscular implant-based reconstruction after nipple-sparing mastectomy? Ann. Surg. Oncol. 2020, 27, 4760–4766. [Google Scholar] [CrossRef]
- Avila, A.; Bartholomew, A.J.; Sosin, M.; Deldar, R.; Griffith, K.F.; Willey, S.C.; Song, D.H.; Fan, K.L.; Tousimis, E.A. Acute postoperative complications in prepectoral versus subpectoral reconstruction following nipple-sparing mastectomy. Plast. Reconstr. Surg. 2020, 146, 715e–720e. [Google Scholar] [CrossRef] [PubMed]
- Nolan, I.T.; Farajzadeh, M.M.; Bekisz, J.M.; Boyd, C.J.; Gibson, E.G.; Salibian, A.A. Prepectoral versus Subpectoral Breast Reconstruction after Nipple-sparing Mastectomy: A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg.–Glob. Open 2024, 12, e5808. [Google Scholar] [CrossRef] [PubMed]
- Arnautovic, A.; Williams, S.; Ash, M.; Menon, A.; Shauly, O.; Losken, A. Outcomes in Implant-Based Breast Reconstruction Utilizing Biosynthetic Mesh: A Meta-Analysis. Aesthetic Surg. J. 2025, sjaf002. [Google Scholar] [CrossRef]
- Nolan, I.T.; Farajzadeh, M.M.; Boyd, C.J.; Bekisz, J.M.; Gibson, E.G.; Salibian, A.A. Do we need acellular dermal matrix in prepectoral breast reconstruction? A systematic review and meta-analysis. J. Plast. Reconstr. Aesthetic Surg. 2023, 86, 251–260. [Google Scholar] [CrossRef]
- DeLong, M.R.; Tandon, V.J.; Bertrand, A.A.; MacEachern, M.; Goldberg, M.; Salibian, A.; Pusic, A.L.; Festekjian, J.H.; Wilkins, E.G. Review of outcomes in prepectoral prosthetic breast reconstruction with and without surgical mesh assistance. Plast. Reconstr. Surg. 2021, 147, 305–315. [Google Scholar] [CrossRef]
- Boyd, C.J.; Bekisz, J.M.; Choi, M.; Karp, N.S. Catch-22: Acellular dermal matrix and US food and drug administration premarket approval—How can we construct studies? Plast. Reconstr. Surg. 2022, 150, 1363–1366. [Google Scholar] [CrossRef]
- Salibian, A.A.; Bekisz, J.M.; Kussie, H.C.; Thanik, V.D.; Levine, J.P.; Choi, M.; Karp, N.S. Do we need support in prepectoral breast reconstruction? Comparing outcomes with and without ADM. Plast. Reconstr. Surg.–Glob. Open 2021, 9, e3745. [Google Scholar] [CrossRef]
- Boyd, C.; Hemal, K.; Perez, S.; Kabir, R.; Thanik, V.; Levine, J.; Cohen, O.; Choi, M.; Karp, N. Soft Tissue Support in Prepectoral Tissue Expander Reconstruction: Do We Need It? Plast. Reconstr. Surg.–Glob. Open 2023, 11, 30–31. [Google Scholar] [CrossRef]
- Frey, J.D.; Salibian, A.A.; Karp, N.S.; Choi, M. Implant-based breast reconstruction: Hot topics, controversies, and new directions. Plast. Reconstr. Surg. 2019, 143, 404e–416e. [Google Scholar] [CrossRef]
- Mallucci, P.; Bistoni, G. Experience and indications for the use of the P4HB scaffold (GalaFLEX) in aesthetic breast surgery: A 100-case experience. Aesthetic Surg. J. 2022, 42, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Karp, N.; Sorenson, T.J.; Boyd, C.J.; Hemal, K.; Lin, A.; Robinson, I.S.; Choi, M. The GalaFLEX “Empanada” for Direct-to-Implant Prepectoral Breast Reconstruction. Plast. Reconstr. Surg. 2025, 155, 488e–491e. [Google Scholar] [CrossRef] [PubMed]
- Sigalove, S.; O’Rorke, E.; Maxwell, G.P.; Gabriel, A. Evaluation of the safety of a GalaFLEX-AlloDerm construct in prepectoral breast reconstruction. Plast. Reconstr. Surg. 2022, 150, 75S–81S. [Google Scholar] [CrossRef] [PubMed]
- Buccheri, E.M.; Villanucci, A.; Mallucci, P.; Bistoni, G.; de Vita, R. Synthetic reabsorbable mesh (GalaFLEX) as soft tissue adjunct in breast augmentation revision surgery. Aesthetic Surg. J. 2023, 43, 559–566. [Google Scholar] [CrossRef]
- Weissler, J.M.; Koltz, P.F.; Carney, M.J.; Serletti, J.M.; Wu, L.C. Sifting through the evidence: A comprehensive review and analysis of neurotization in breast reconstruction. Plast. Reconstr. Surg. 2018, 141, 550–565. [Google Scholar] [CrossRef]
- Shiah, E.; Laikhter, E.; Comer, C.D.; Manstein, S.M.; Bustos, V.P.; Bain, P.A.; Lee, B.T.; Lin, S.J. Neurotization in innervated breast reconstruction: A systematic review of techniques and outcomes. J. Plast. Reconstr. Aesthetic Surg. 2022, 75, 2890–2913. [Google Scholar] [CrossRef]
- Momeni, A.; Meyer, S.; Shefren, K.; Januszyk, M. Flap neurotization in breast reconstruction with nerve allografts: 1-year clinical outcomes. Plast. Reconstr. Surg. –Glob. Open 2021, 9, e3328. [Google Scholar] [CrossRef]
- Djohan, R.; Scomacao, I.; Knackstedt, R.; Cakmakoglu, C.; Grobmyer, S.R. Neurotization of the nipple-areola complex during implant-based reconstruction: Evaluation of early sensation recovery. Plast. Reconstr. Surg. 2020, 146, 250–254. [Google Scholar] [CrossRef]
- Peled, A.W.; von Eyben, R.; Peled, Z.M. Sensory outcomes after neurotization in nipple-sparing mastectomy and implant-based breast reconstruction. Plast. Reconstr. Surg.–Glob. Open 2023, 11, e5437. [Google Scholar] [CrossRef]
- Frey, J.D.; Choi, M.; Salibian, A.A.; Karp, N.S. Comparison of outcomes with tissue expander, immediate implant, and autologous breast reconstruction in greater than 1000 nipple-sparing mastectomies. Plast. Reconstr. Surg. 2017, 139, 1300–1310. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.H.; Ruberg, R.L.; Stevenson, K.B.; Beck, C.E.; Ruppert, A.S.; Harper, J.T.; Boehmler IV, J.H.; Miller, M.J. Independent risk factors for infection in tissue expander breast reconstruction. Plast. Reconstr. Surg. 2009, 124, 1790–1796. [Google Scholar] [CrossRef]
- Avraham, T.; Weichman, K.E.; Wilson, S.; Weinstein, A.; Haddock, N.T.; Szpalski, C.; Choi, M.; Karp, N.S. Postoperative expansion is not a primary cause of infection in immediate breast reconstruction with tissue expanders. Breast J. 2015, 21, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Perez-Otero, S.; Hemal, K.; Boyd, C.J.; Kabir, R.; Sorenson, T.J.; Jacobson, A.; Thanik, V.D.; Levine, J.P.; Cohen, O.D.; Karp, N.S. Minimizing Nipple-Areolar Complex Complications in Prepectoral Breast Reconstruction After Nipple-Sparing Mastectomy. Ann. Plast. Surg. 2024, 92, S179–S184. [Google Scholar] [CrossRef] [PubMed]
- Kraenzlin, F.; Darrach, H.; Khavanin, N.; Kokosis, G.; Aliu, O.; Broderick, K.; Rosson, G.D.; Manahan, M.A.; Sacks, J.M. Tissue expander–based breast reconstruction in the prepectoral versus subpectoral plane: An analysis of short-term outcomes. Ann. Plast. Surg. 2021, 86, 19–23. [Google Scholar] [CrossRef]
- Soni, S.E.; Le, N.K.; Buller, M.; Modica, A.D.; Kumar, A.; Smith, P.D.; Laronga, C. Complication profile of total submuscular versus prepectoral tissue expander placement: A retrospective cohort study. Ann. Plast. Surg. 2022, 88, S439–S442. [Google Scholar] [CrossRef]
- Nores, G.G.; Carlson, G.W. The Impact of Tissue Expander Nipple Asymmetry on Final Implant Symmetry After Bilateral Nipple Sparing Mastectomy. Ann. Plast. Surg. 2022, 88, S427–S432. [Google Scholar] [CrossRef]
- Brown, C.A.; Carlson, G.W. The Impact of Radiation on Nipple Symmetry After Bilateral Nipple-Sparing Mastectomy and Implant-Based Reconstruction: An Objective Analysis. Ann. Plast. Surg. 2024, 92, 379–382. [Google Scholar] [CrossRef]
- Mercury, O.; Nores, G.G.; Carlson, G.W. Symmetry of nipple position after bilateral nipple-sparing mastectomy and implant-based reconstruction: The impact of reconstructive method. Ann. Plast. Surg. 2022, 88, S422–S426. [Google Scholar] [CrossRef]
- Yoon, A.P.; Qi, J.; Kim, H.M.; Hamill, J.B.; Jagsi, R.; Pusic, A.L.; Wilkins, E.G.; Kozlow, J.H. Patient-reported outcomes after irradiation of tissue expander versus permanent implant in breast reconstruction: A multicenter prospective study. Plast. Reconstr. Surg. 2020, 145, 917e–926e. [Google Scholar] [CrossRef]
- Santosa, K.B.; Chen, X.; Qi, J.; Ballard, T.N.; Kim, H.M.; Hamill, J.B.; Bensenhaver, J.M.; Pusic, A.L.; Wilkins, E.G. Postmastectomy radiation therapy and two-stage implant-based breast reconstruction: Is there a better time to irradiate? Plast. Reconstr. Surg. 2016, 138, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Ogita, M.; Nagura, N.; Kawamori, J.; In, R.; Yoshida, A.; Yamauchi, H.; Takei, J.; Hayashi, N.; Iwahira, Y.; Ohde, S. Risk factors for complications among breast cancer patients treated with post-mastectomy radiotherapy and immediate tissue-expander/permanent implant reconstruction: A retrospective cohort study. Breast Cancer 2018, 25, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.R.; Freedman, G.; Nicolaou, N.; Sharma, N.; Li, T.; Topham, N.; Morrow, M. Postmastectomy chest wall radiation to a temporary tissue expander or permanent breast implant—Is there a difference in complication rates? Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 81–85. [Google Scholar] [CrossRef]
- Agha, R.; Al Omran, Y.; Wellstead, G.; Sagoo, H.; Barai, I.; Rajmohan, S.; Borrelli, M.; Vella-Baldacchino, M.; Orgill, D.; Rusby, J. Systematic review of therapeutic nipple-sparing versus skin-sparing mastectomy. BJS Open 2019, 3, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Salibian, A.H.; Harness, J.K. Oncologic safety of staged prepectoral implant reconstruction following nipple-sparing mastectomy: A mean 9-year follow-up. Plast. Reconstr. Surg. 2022, 150, 513–522. [Google Scholar] [CrossRef]
- Spillane, S.; Baker, C.; Lippey, J. Therapeutic nipple-sparing mastectomy: A scoping review of oncologic safety and predictive factors for in-breast recurrence. ANZ J. Surg. 2025, 95, 34–40. [Google Scholar] [CrossRef]
- Kokosis, G.; Stern, C.S.; Shamsunder, M.G.; Polanco, T.O.; Patel, V.M.; Slutsky, H.; Morrow, M.; Moo, T.-A.; Sacchini, V.; Coriddi, M.R. Nipple-Sparing Mastectomy and Immediate Reconstruction: A Propensity Score–Matched Analysis of Satisfaction and Quality of Life. Plast. Reconstr. Surg. 2022, 150, 1214e–1223e. [Google Scholar] [CrossRef]
- Galimberti, V.; Vicini, E.; Corso, G.; Morigi, C.; Fontana, S.; Sacchini, V.; Veronesi, P. Nipple-sparing and skin-sparing mastectomy: Review of aims, oncological safety and contraindications. Breast 2017, 34, S82–S84. [Google Scholar] [CrossRef]
- Djohan, R.; Gage, E.; Gatherwright, J.; Pavri, S.; Firouz, J.; Bernard, S.; Yetman, R. Patient satisfaction following nipple-sparing mastectomy and immediate breast reconstruction: An 8-year outcome study [outcomes article]. Plast. Reconstr. Surg. 2010, 125, 818–829. [Google Scholar] [CrossRef]
- Bailey, C.R.; Ogbuagu, O.; Baltodano, P.A.; Simjee, U.F.; Manahan, M.A.; Cooney, D.S.; Jacobs, L.K.; Tsangaris, T.N.; Cooney, C.M.; Rosson, G.D. Quality-of-life outcomes improve with nipple-sparing mastectomy and breast reconstruction. Plast. Reconstr. Surg. 2017, 140, 219–226. [Google Scholar] [CrossRef]
- Clarijs, M.E.; Peeters, N.J.V.; van Dongen, S.A.; Koppert, L.B.; Pusic, A.L.; Mureau, M.A.; Rijken, B.F. Quality of life and complications after nipple-versus skin-sparing mastectomy followed by immediate breast reconstruction: A systematic review and meta-analysis. Plast. Reconstr. Surg. 2023, 152, 12e–24e. [Google Scholar] [CrossRef] [PubMed]
- Peled, A.W.; Amara, D.; Piper, M.L.; Klassen, A.F.; Tsangaris, E.; Pusic, A.L. Development and validation of a nipple-specific scale for the BREAST-Q to assess patient-reported outcomes following nipple-sparing mastectomy. Plast. Reconstr. Surg. 2019, 143, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
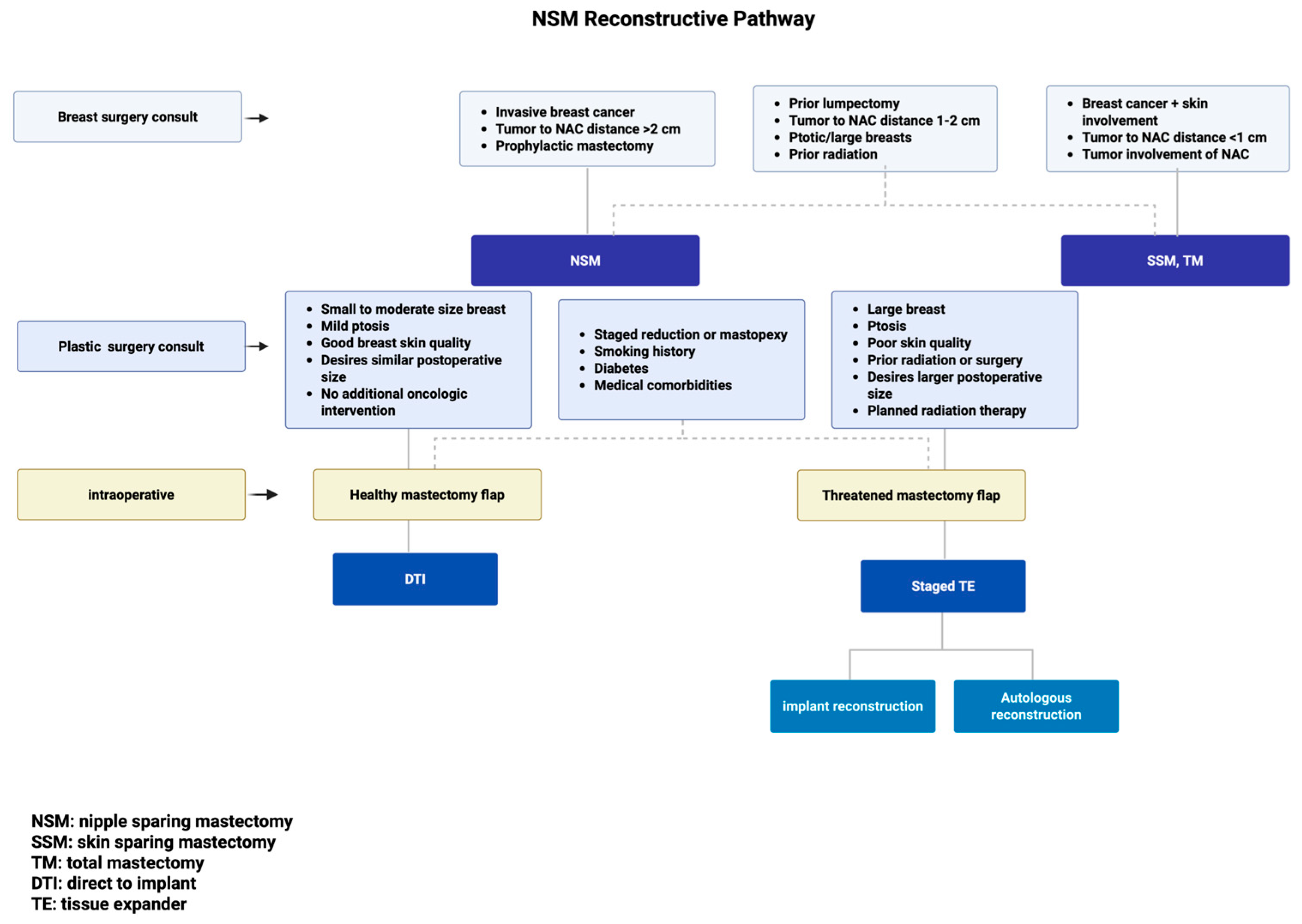
| Indications | Contraindications | Special Consideration |
|---|---|---|
| Invasive breast cancer (including large size > 3 cm) | Breast cancer with skin involvement | Prior lumpectomy |
| Tumor > 2 cm away from NAC | Tumor < 1 cm away from NAC or with direct NAC involvement | Tumor 1–2 cm from NAC |
| Prophylactic mastectomy | Ptotic and/or large breasts | |
| Prior radiation therapy |
| DTI | TE Staged Reconstruction | Special Consideration |
|---|---|---|
| Small to moderate-size breast (A, B, C cup) | Large breast (large C cup, D cup, or greater) | Staged reduction or mastopexy procedure |
| Mild ptosis (no ptosis, grade 1 ptosis, pseudoptosis, SN-N < 27 cm) | Ptosis (grade 2, 3 ptosis) | |
| Good breast skin quality, minimal laxity (skin pinch test > 2 cm, skin stretch in each breast quadrant) | Poor skin quality, history of prior radiation or surgery | Smoking history, diabetes, medical comorbidities |
| Desires similar postoperative size | Desires larger postoperative size | |
| Healthy mastectomy flaps | Intraoperative assessment with threatened mastectomy flaps | |
| No further oncologic intervention | Planned radiation therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.J.; Boyd, C.J.; Hemal, K.; Sorenson, T.J.; Amro, C.; Vernice, N.A.; Lakatta, A.C.; Cohen, O.; Choi, M.; Karp, N.S. Techniques for Success in Nipple-Sparing Mastectomy and Immediate Reconstruction. J. Clin. Med. 2025, 14, 4363. https://doi.org/10.3390/jcm14124363
Park JJ, Boyd CJ, Hemal K, Sorenson TJ, Amro C, Vernice NA, Lakatta AC, Cohen O, Choi M, Karp NS. Techniques for Success in Nipple-Sparing Mastectomy and Immediate Reconstruction. Journal of Clinical Medicine. 2025; 14(12):4363. https://doi.org/10.3390/jcm14124363
Chicago/Turabian StylePark, Jenn J., Carter J. Boyd, Kshipra Hemal, Thomas J. Sorenson, Chris Amro, Nicholas A. Vernice, Alexis C. Lakatta, Oriana Cohen, Mihye Choi, and Nolan S. Karp. 2025. "Techniques for Success in Nipple-Sparing Mastectomy and Immediate Reconstruction" Journal of Clinical Medicine 14, no. 12: 4363. https://doi.org/10.3390/jcm14124363
APA StylePark, J. J., Boyd, C. J., Hemal, K., Sorenson, T. J., Amro, C., Vernice, N. A., Lakatta, A. C., Cohen, O., Choi, M., & Karp, N. S. (2025). Techniques for Success in Nipple-Sparing Mastectomy and Immediate Reconstruction. Journal of Clinical Medicine, 14(12), 4363. https://doi.org/10.3390/jcm14124363





