Systemic Biologic Treatment for Psoriasis in Elderly Patients
Abstract
1. Introduction
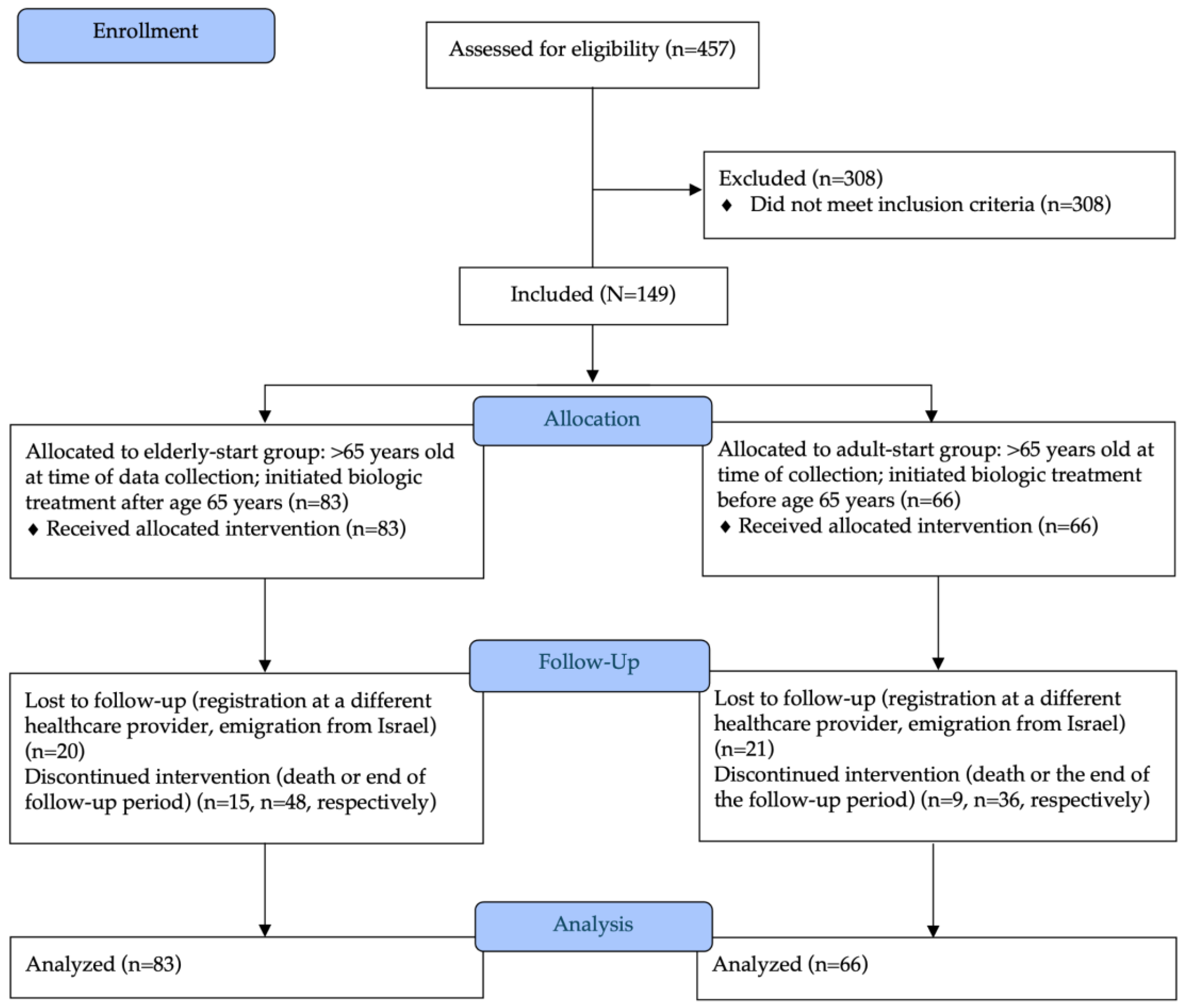
2. Materials and Methods
3. Results
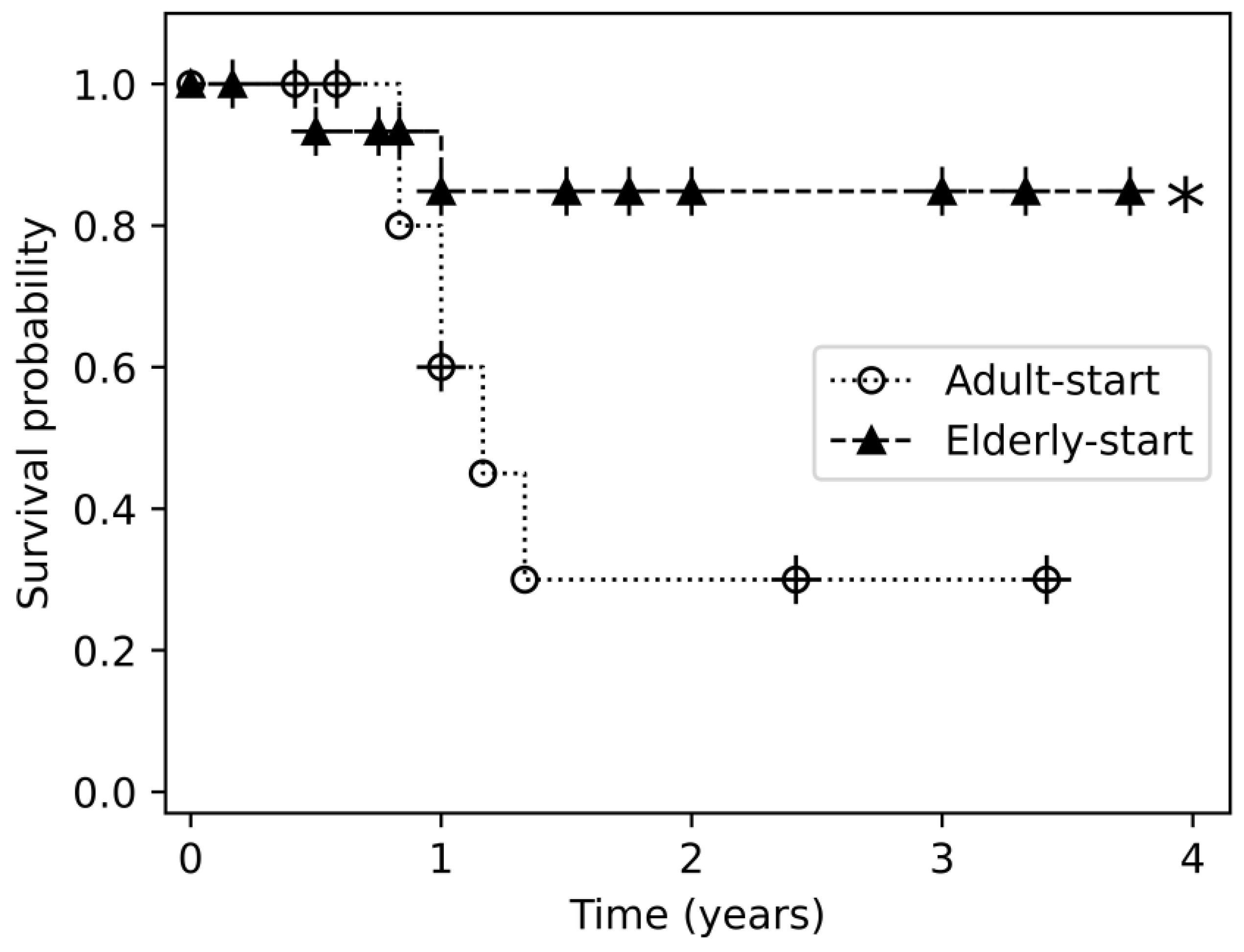
3.1. Efficacy of Biologic Treatment
3.2. Patterns in Biologic Treatment
3.3. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IL | Interleukin |
| PASI | Psoriasis Area and Severity Index |
| TNF | Tumor necrosis factor |
References
- Rustin, M.H.A. Long-Term Safety of Biologics in the Treatment of Moderate-to-Severe Plaque Psoriasis: Review of Current Data. Br. J. Dermatol. 2012, 167 (Suppl. 3), 3–11. [Google Scholar] [CrossRef] [PubMed]
- Michalek, I.M.; Loring, B.; John, S.M. A Systematic Review of Worldwide Epidemiology of Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Parisi, R.; Symmons, D.P.M.; Griffiths, C.E.M.; Ashcroft, D.M. Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team Global Epidemiology of Psoriasis: A Systematic Review of Incidence and Prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef]
- Sandhu, V.K.; Ighani, A.; Fleming, P.; Lynde, C.W. Biologic Treatment in Elderly Patients with Psoriasis: A Systematic Review. J. Cutan. Med. Surg. 2020, 24, 174–186. [Google Scholar] [CrossRef]
- Boehncke, W.-H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Balato, N.; Patruno, C.; Napolitano, M.; Patrì, A.; Ayala, F.; Scarpa, R. Managing Moderate-to-Severe Psoriasis in the Elderly. Drugs Aging 2014, 31, 233–238. [Google Scholar] [CrossRef]
- Ter Haar, E.L.M.; Ten Bruin, E.E.; Bronkhorst, E.E.; Borgonjen, R.J.; Kleinpenning, M.M.; Kop, E.N.; Visch, M.B.; Van de Kerkhof, P.C.M.; De Jong, E.M.G.J.; Lubeek, S.F.K. Safety Assessment of Conventional and Biological Systemic Therapy in Older Adults with Psoriasis, a Real-World Multicentre Cohort Study. Acta Derm. Venereol. 2022, 102, adv00805. [Google Scholar] [CrossRef]
- Garcia-Doval, I.; Carretero, G.; Vanaclocha, F.; Ferrandiz, C.; Daudén, E.; Sánchez-Carazo, J.-L.; Alsina, M.; Herrera-Ceballos, E.; Gómez-García, F.-J.; Ferrán, M.; et al. Risk of Serious Adverse Events Associated with Biologic and Nonbiologic Psoriasis Systemic Therapy: Patients Ineligible vs Eligible for Randomized Controlled Trials. Arch. Dermatol. 2012, 148, 463–470. [Google Scholar] [CrossRef]
- Van Den Reek, J.M.P.A.; Kievit, W.; Gniadecki, R.; Goeman, J.J.; Zweegers, J.; Van De Kerkhof, P.C.M.; Seyger, M.M.B.; De Jong, E.M.G.J. Drug Survival Studies in Dermatology:Principles, Purposes, and Pitfalls. J. Investig. Dermatol. 2015, 135, 1–5. [Google Scholar] [CrossRef]
- Singh, S.; Bajorek, B. Defining “elderly” in Clinical Practice Guidelines for Pharmacotherapy. Pharm. Pract. 2014, 12, 489. [Google Scholar] [CrossRef]
- Bolognia, J.L.; Schaffer, J.V.; Chrroni, L. Dermatology, 4th ed.; Bolognia, J.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Ter Haar, E.L.M.; Thomas, S.E.; van den Reek, J.M.P.A.; Otero, M.E.; Njoo, M.D.; Ossenkoppele, P.M.; Kop, E.N.; Dodemont, S.R.P.; Körver, J.E.M.; Kuijpers, A.L.A.; et al. Drug Survival, Safety, and Effectiveness of Biologics in Older Patients with Psoriasis: A Comparison with Younger Patients-A BioCAPTURE Registry Study. Drugs Aging 2022, 39, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Osuna, C.G.; García, S.R.; Martín, J.C.; Jiménez, V.G.; López, F.V.; Santos-Juanes, J. Use of Biological Treatments in Elderly Patients with Skin Psoriasis in the Real World. Life 2021, 11, 1348. [Google Scholar] [CrossRef] [PubMed]
- Carrascosa, J.M.; Notario, J. Drug Survival in Biologic Therapy. Do We Know What It Means? Can We Calculate It? Actas Dermo-Sifiliográficas 2014, 105, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Villaverde, R.; Rodriguez-Fernandez-Freire, L.; Armario-Hita, J.C.; Pérez-Gil, A.; Vasquez Chinchay, F.; Galán-Gutiérrez, M. Effectiveness, Survival and Safety of Guselkumab Attending to Basal Characteristics in Moderate-to-Severe Psoriatic Patients: A Cohort Study. F1000Research 2022, 11, 1178. [Google Scholar] [CrossRef]
- Ruggiero, A.; Fabbrocini, G.; Cinelli, E.; Ocampo Garza, S.S.; Camela, E.; Megna, M. Anti-Interleukin-23 for Psoriasis in Elderly Patients: Guselkumab, Risankizumab and Tildrakizumab in Real-World Practice. Clin. Exp. Dermatol. 2022, 47, 561–567. [Google Scholar] [CrossRef]
- Hayashi, M.; Umezawa, Y.; Fukuchi, O.; Ito, T.; Saeki, H.; Nakagawa, H. Efficacy and Safety of Ustekinumab Treatment in Elderly Patients with Psoriasis. J. Dermatol. 2014, 41, 974–980. [Google Scholar] [CrossRef]
- Fiorillo, G.; Ibba, L.; Gargiulo, L.; Vignoli, C.A.; Alfano, A.; Cortese, A.; Toso, F.; Orsini, D.; Iacovelli, P.; Frascione, P.; et al. Effectiveness and Safety of Anti-IL-23 and Anti-IL-17 Biological Therapies for Psoriasis in Elderly Patients: Real-World Experience from Two Italian Hospitals. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1444–e1446. [Google Scholar] [CrossRef]
- Borren, N.Z.; Ananthakrishnan, A.N. Safety of Biologic Therapy in Older Patients with Immune-Mediated Diseases: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2019, 17, 1736–1743.e4. [Google Scholar] [CrossRef]
- Turíbio, D.D.C.D.Q.; Oliveira, F.C.S.; Barreto, S.M.F.; Jabour, T.B.F. Interstitial Lung Disease Due to Anti-TNF Use in the Treatment of Psoriasis. Bras. Dermatol. 2021, 96, 447–450. [Google Scholar] [CrossRef]
- Kawamoto, H.; Hara, H.; Minagawa, S.; Numata, T.; Araya, J.; Kaneko, Y.; Umezawa, Y.; Asahina, A.; Nakagawa, H.; Kuwano, K. Interstitial Pneumonia in Psoriasis. Mayo. Clin. Proc. Innov. Qual. Outcomes 2018, 2, 370–377. [Google Scholar] [CrossRef]
- Ricceri, F.; Bardazzi, F.; Chiricozzi, A.; Dapavo, P.; Ferrara, F.; Mugheddu, C.; Romanelli, M.; Rongioletti, F.; Prignano, F. Elderly Psoriatic Patients under Biological Therapies: An Italian Experience. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Megna, M.; Napolitano, M.; Balato, N.; Monfrecola, G.; Villani, A.; Ayala, F.; Balato, A. Efficacy and Safety of Ustekinumab in a Group of 22 Elderly Patients with Psoriasis over a 2-Year Period. Clin. Exp. Dermatol. 2016, 41, 564–566. [Google Scholar] [CrossRef] [PubMed]
- Drucker, A.M.; Sutradhar, R.; Ling, V.; Gatley, J.M.; Eder, L.; Fahim, C.; Fralick, M.; Gomes, T.; Li, P.; MacDougall, S.; et al. Systemic Therapies for Psoriatic Disease and Serious Infections in Older Adults. JAMA Dermatol. 2025, 161, 490–497. [Google Scholar] [CrossRef]
- Ibba, L.; Di Giulio, S.; Gargiulo, L.; Facheris, P.; Perugini, C.; Costanzo, A.; Narcisi, A.; Valenti, M. Long-Term Effectiveness and Safety of Risankizumab in Patients with Moderate-to-Severe Psoriasis with and without Cardiometabolic Comorbidities: A Single-Center Retrospective Study. J. Dermatol. Treat. 2024, 35, 2425029. [Google Scholar] [CrossRef]
- Bellinato, F.; Maurelli, M.; Geat, D.; Girolomoni, G.; Gisondi, P. Managing the Patient with Psoriasis and Metabolic Comorbidities. Am. J. Clin. Dermatol. 2024, 25, 527–540. [Google Scholar] [CrossRef]
- Garber, C.; Plotnikova, N.; Au, S.; Sorensen, E.P.; Gottlieb, A. Biologic and Conventional Systemic Therapies Show Similar Safety and Efficacy in Elderly and Adult Patients with Moderate to Severe Psoriasis. J. Drugs Dermatol. 2015, 14, 846–852. [Google Scholar]
- Alabas, O.A.; Mason, K.J.; Yiu, Z.Z.N.; Smith, C.H.; Warren, R.B.; Griffiths, C.E.M.; BADBIR Study Group. Age and Biologic Survival in Patients with Moderate-to-Severe Psoriasis: A Cohort Study from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR). Br. J. Dermatol. 2025, 192, 907–916. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Coscarella, G.; Puig, L.; Vender, R.; Yeung, J.; Carrascosa, J.; Piaserico, S.; Gisondi, P.; Lynde, C.; Ferreira, P.; et al. Age Affects Drug Survival Rates of Interleukin (IL)-17 and IL-23 Inhibitors in Patients with Plaque Psoriasis: Results from a Retrospective, Multicentric, Multi-country, Cohort Study. Acad. Dermatol. Venereol. 2024, 38, 2175–2185. [Google Scholar] [CrossRef]
- Penso, L.; Dray-Spira, R.; Weill, A.; Pina Vegas, L.; Zureik, M.; Sbidian, E. Association Between Biologics Use and Risk of Serious Infection in Patients with Psoriasis. JAMA Dermatol. 2021, 157, 1056–1065. [Google Scholar] [CrossRef]
- Bagit, A.; Sood, S.; Sriranganathan, A.; Maliyar, K.; Georgakopoulos, J.R.; Abduelmula, A.; Sachdeva, M.; Mufti, A.; Yeung, J. 54665 The Efficacy and Safety of Switching From Different Biologics To Interleukin-23 Inhibitors: A Systematic Review. J. Am. Acad. Dermatol. 2024, 91, AB327. [Google Scholar] [CrossRef]
- Tsai, S.H.L.; Yang, C.-Y.; Huo, A.-P.; Wei, J.C.-C. Interleukin 23 versus Interleukin 12/23 Inhibitors on Preventing Incidental Psoriatic Arthritis in Patients with Psoriasis? A Real-World Comparison from the TriNetX Global Collaborative Network. J. Am. Acad. Dermatol. 2024, 91, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Stangelberger, A.; Waldert, M.; Djavan, B. Prostate Cancer in Elderly Men. Rev. Urol. 2008, 10, 111–119. [Google Scholar] [PubMed]
- Patel, R.V.; Clark, L.N.; Lebwohl, M.; Weinberg, J.M. Treatments for Psoriasis and the Risk of Malignancy. J. Am. Acad. Dermatol. 2009, 60, 1001–1017. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.M.; Kim, Y.-J.; Chang, S.E.; Lee, M.W.; Won, C.H.; Lee, W.J. Cancer Risks in Patients with Psoriasis Administered Biologics Therapy: A Nationwide Population-Based Study. J. Cancer Res. Clin. Oncol. 2023, 149, 17093–17102. [Google Scholar] [CrossRef]
- Battista, T.; Gallo, L.; Martora, F.; Fattore, D.; Potestio, L.; Cacciapuoti, S.; Scalvenzi, M.; Megna, M. Biological Therapy for Psoriasis in Cancer Patients: An 8-Year Retrospective Real-Life Study. J. Clin. Med. 2024, 13, 1940. [Google Scholar] [CrossRef]
- Riaz, S.; Emam, S.; Wang, T.; Gniadecki, R. Negative Impact of Comorbidities on All-Cause Mortality of Patients with Psoriasis Is Partially Alleviated by Biologic Treatment: A Real-World Case-Control Study. J. Am. Acad. Dermatol. 2024, 91, 43–50. [Google Scholar] [CrossRef]

| Variable | Elderly-Start Group (≥65 y) n = 83 | Adult-Start Group (<65 y) n = 66 | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Patient years | 695.6 | 784.3 | 0.86 | |
| Age at the end of follow-up period | 79.4 ± 6.3 | 72.2 ± 3 | ||
| Age at diagnosis | 56.8 ± 13.3 | 47.4 ± 10.5 | ||
| Sex | Male | 49 (59%) | 45 (68.1%) | 0.25 |
| Female | 34 (40.9%) | 21 (31.8%) | ||
| Age at onset of biologic treatment | 70.3 ± 4.9 | Age 65–70: 50 (60.2%) | 59.7 ± 3.6 | |
| Age 70–75: 17 (20.2%) | ||||
| Age > 75: 16 (19.2%) | ||||
| Disease duration (years) | 21.9 ± 11.7 | 24 ± 10.5 | 0.06 | |
| Follow-up time (years) | 8.3 ± 3.6 | 11.8 ± 4.1 | 0.00 | |
| Comorbidities | ||||
| Weight (kg) | 81.8 ± 15.1 | 82.3 ± 17.8 | 0.97 | |
| BMI (kg/m2) | 28.9 ± 4.9 | 28.4 ± 5.1 | 0.59 | |
| Obese | ||||
| (BMI * > 30) | 33 (39.7%) | 24 (36.3%) | 0.58 | |
| Diabetes mellitus | 36 (43.3%) | 29 (43.9%) | 0.99 | |
| Hyperlipidemia | 69 (83.1%) | 50 (75.7%) | 0.2 | |
| Hypertension | 55 (66.2%) | 41 (62.1%) | 0.53 | |
| History of malignancy | 15 (18%) | 9 (13.6%) | 0.44 | |
| Psoriatic arthritis | 24 (28.9%) | 29 (43.9%) | 0.05 | |
| History of psoriasis treatment failure | ||||
| Methotrexate | 51 (61.44) | 47 (71.21) | 0.21 | |
| Acitretin | 58 (69.87) | 50 (75.75) | 0.42 | |
| Cyclosporine | 1 (1.20) | 9 (13.63) | 0.005 | |
| Phototherapy | 82 (98.79) | 63 (95.45) | 0.79 | |
| Variable | Elderly-Start Group (≥65 y) n = 83 | Adult-Start Group (<65 y) n = 66 | p Value | |
|---|---|---|---|---|
| Initial disease severity by mean PASI * | 21.3 | 22.7 | 0.17 | |
| Disease severity after first biologic treatment (delta PASI) | <50 | 47 (62.6%) | 39 (68.4%) | 0.84 |
| ≥50 | 17 (22.7%) | 16 (28.1%) | ||
| 100 | 11 (14.7%) | 2 (3.5%) | ||
| Disease severity after final biologic treatment (delta PASI) | <50 | 4 (5.1%) | 18 (29%) | 0.003 |
| ≥50 | 27 (34.6%) | 18 (29%) | ||
| 100 | 47 (60.3%) | 26 (41.9%) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glazer Levavi, S.; Maman, R.; Sherman, S.; Mimouni, D.; Pavlovsky, L. Systemic Biologic Treatment for Psoriasis in Elderly Patients. J. Clin. Med. 2025, 14, 4779. https://doi.org/10.3390/jcm14134779
Glazer Levavi S, Maman R, Sherman S, Mimouni D, Pavlovsky L. Systemic Biologic Treatment for Psoriasis in Elderly Patients. Journal of Clinical Medicine. 2025; 14(13):4779. https://doi.org/10.3390/jcm14134779
Chicago/Turabian StyleGlazer Levavi, Sapir, Ronny Maman, Shany Sherman, Daniel Mimouni, and Lev Pavlovsky. 2025. "Systemic Biologic Treatment for Psoriasis in Elderly Patients" Journal of Clinical Medicine 14, no. 13: 4779. https://doi.org/10.3390/jcm14134779
APA StyleGlazer Levavi, S., Maman, R., Sherman, S., Mimouni, D., & Pavlovsky, L. (2025). Systemic Biologic Treatment for Psoriasis in Elderly Patients. Journal of Clinical Medicine, 14(13), 4779. https://doi.org/10.3390/jcm14134779






