Current Concepts in the Nonoperative Management of Achilles Tendon Pathologies: A Scoping Review
Abstract
1. Introduction
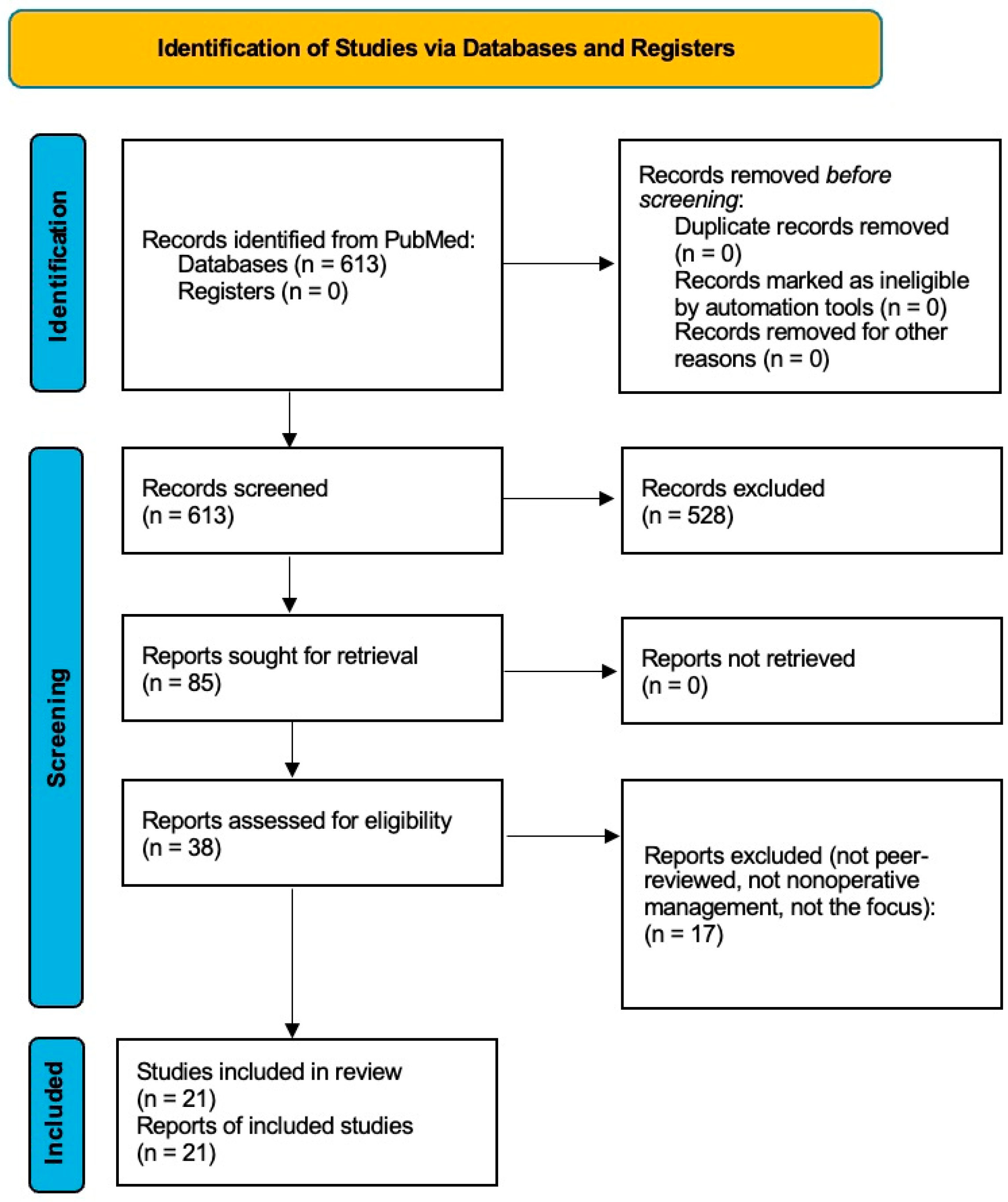
2. Materials and Methods
3. Results
3.1. Achilles Tendon Rupture
3.1.1. Operative Versus Nonoperative Treatment Outcomes
3.1.2. Comparison of Nonoperative Treatment Strategies
3.2. Achilles Tendonitis and Tendinopathy
3.3. Equinus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ESWT | Extracorporeal shockwave therapy |
| NSAID | Nonsteroidal anti-inflammatory drug |
| AFO | Ankle–foot orthoses |
| TBTS | Tilt board with target stretching |
| PRP | Platelet-rich plasma |
References
- Hess, G.W. Achilles tendon rupture: A review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec. 2010, 3, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Olewnik, Ł.; Wysiadecki, G.; Podgórski, M.; Polguj, M.; Topol, M. The Plantaris Muscle Tendon and Its Relationship with the Achilles Tendinopathy. Biomed. Res. Int. 2018, 2018, 9623579. [Google Scholar] [CrossRef] [PubMed]
- Meulenkamp, B.; Stacey, D.; Fergusson, D.; Hutton, B.; Mlis, R.S.; Graham, I.D. Protocol for treatment of Achilles tendon ruptures; a systematic review with network meta-analysis. Syst. Rev. 2018, 7, 247. [Google Scholar] [CrossRef] [PubMed]
- Federer, A.E.; Steele, J.R.; Dekker, T.J.; Liles, J.L.; Adams, S.B. Tendonitis and tendinopathy: What are they and how do they evolve? Foot Ankle Clin. 2017, 22, 665–676. [Google Scholar] [CrossRef]
- Matthews, W.; Ellis, R.; Furness, J.; Hing, W.A. The clinical diagnosis of Achilles tendinopathy: A scoping review. PeerJ 2021, 9, e12166. [Google Scholar] [CrossRef]
- Merry, K.; Napier, C.; Waugh, C.M.; Scott, A. Foundational Principles and Adaptation of the Healthy and Pathological Achilles Tendon in Response to Resistance Exercise: A Narrative Review and Clinical Implications. J. Clin. Med. 2022, 11, 4722. [Google Scholar] [CrossRef]
- DeHeer, P.A. Equinus and Lengthening Techniques. Clin. Podiatr. Med. Surg. 2017, 34, 207–227. [Google Scholar] [CrossRef]
- Myhrvold, S.B.; Brouwer, E.F.; Andresen, T.K.M.; Rydevik, K.; Amundsen, M.; Grün, W.; Butt, F.; Valberg, M.; Ulstein, S.; Hoelsbrekken, S.E. Nonoperative or surgical treatment of acute Achilles tendon rupture. N. Engl. J. Med. 2022, 386, 1409–1420. [Google Scholar] [CrossRef]
- Deng, S.; Sun, Z.; Zhang, C.; Chen, G.; Li, J. Surgical Treatment Versus Conservative Management for Acute Achilles Tendon Rupture: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Foot Ankle Surg. 2017, 56, 1236–1243. [Google Scholar] [CrossRef]
- Lantto, I.; Heikkinen, J.; Flinkkila, T.; Ohtonen, P.; Siira, P.; Laine, V.; Leppilahti, J. A Prospective Randomized Trial Comparing Surgical and Nonsurgical Treatments of Acute Achilles Tendon Ruptures. Am. J. Sports Med. 2016, 44, 2406–2414. [Google Scholar] [CrossRef]
- Seow, D.; Islam, W.; Randall, G.W.; Azam, M.T.; Duenes, M.L.; Hui, J.; Pearce, C.J.; Kennedy, J.G. Lower re-rupture rates but higher complication rates following surgical versus conservative treatment of acute achilles tendon ruptures: A systematic review of overlapping meta-analyses. Knee Surg. Sports Traumatol. Arthrosc. 2023, 31, 3528–3540. [Google Scholar] [CrossRef] [PubMed]
- Lerch, T.D.; Schwinghammer, A.; Schmaranzer, F.; Anwander, H.; Ecker, T.M.; Schmid, T.; Weber, M.; Krause, F. Return to sport and patient satisfaction at 5-year follow-up after nonoperative treatment for acute Achilles tendon rupture. Foot Ankle Int. 2020, 41, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Ochen, Y.; Beks, R.B.; van Heijl, M.; Hietbrink, F.; Leenen, L.P.H.; van der Velde, D.; Heng, M.; van der Meijden, O.; Groenwold, R.H.H.; Houwert, R.M. Operative treatment versus nonoperative treatment of Achilles tendon ruptures: Systematic review and meta-analysis. BMJ 2019, 364, k5120. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.L.; Achten, J.; Marian, I.R.; Dutton, S.J.; Lamb, S.E.; Ollivere, B.; Maredza, M.; Petrou, S.; Kearney, R.S.; Abdallah, A.; et al. UKSTAR trial collaborators. Plaster cast versus functional brace for non-surgical treatment of Achilles tendon rupture (UKSTAR): A multicentre randomised controlled trial and economic evaluation. Lancet 2020, 395, 441–448. [Google Scholar] [CrossRef]
- Ecker, T.M.; Bremer, A.K.; Krause, F.G.; Müller, T.; Weber, M. Prospective use of a standardized nonoperative early weightbearing protocol for Achilles tendon rupture: 17 years of experience. Am. J. Sports Med. 2016, 44, 1004–1010. [Google Scholar] [CrossRef]
- Prudêncio, D.A.; Maffulli, N.; Migliorini, F.; Serafim, T.T.; Nunes, L.F.; Sanada, L.S.; Okubo, R. Eccentric exercise is more effective than other exercises in the treatment of mid-portion Achilles tendinopathy: Systematic review and meta-analysis. BMC Sports Sci. Med. Rehabil. 2023, 15, 9. [Google Scholar] [CrossRef]
- Rhim, H.C.; Kim, M.S.; Choi, S.; Tenforde, A.S. Comparative Efficacy and Tolerability of Nonsurgical Therapies for the Treatment of Midportion Achilles Tendinopathy: A Systematic Review with Network Meta-analysis. Orthop. J. Sports Med. 2020, 8, 2325967120930567. [Google Scholar] [CrossRef]
- Kearney, R.S.; Ji, C.; Warwick, J.; Parsons, N.; Brown, J.; Harrison, P.; Young, J.; Costa, M.L.; Collaborators, A.T.; Dasari, K.; et al. Effect of Platelet-Rich Plasma Injection vs Sham Injection on Tendon Dysfunction in Patients with Chronic Midportion Achilles Tendinopathy: A Randomized Clinical Trial. JAMA 2021, 326, 137–144. [Google Scholar] [CrossRef]
- Mansur, N.S.B.; Matsunaga, F.T.; Carrazzone, O.L.; dos Santos, B.S.; Nunes, C.G.; Aoyama, B.T.; dos Santos, P.R.D.; Faloppa, F.; Tamaoki, M.J.S. Shockwave Therapy Plus Eccentric Exercises Versus Isolated Eccentric Exercises for Achilles Insertional Tendinopathy: A Double-Blinded Randomized Clinical Trial. J. Bone Joint Surg. Am. 2021, 103, 1295–1302. [Google Scholar] [CrossRef]
- Ko, V.M.; Cao, M.; Qiu, J.; Fong, I.C.-K.; Fu, S.-C.; Yung, P.S.-H.; Ling, S.K.-K. Comparative short-term effectiveness of non-surgical treatments for insertional Achilles tendinopathy: A systematic review and network meta-analysis. BMC Musculoskelet. Disord. 2023, 24, 102. [Google Scholar] [CrossRef]
- Zhi, X.; Liu, X.; Han, J.; Xiang, Y.; Wu, H.; Wei, S.; Xu, F. Nonoperative treatment of insertional Achilles tendinopathy: A systematic review. J. Orthop. Surg. Res. 2021, 16, 233. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumar, R.; Mudgal, S.K.; Ranjan, P.; Kumar, S. The Effects of Botulinum Toxin and Casting in Spastic Children with Cerebral Palsy: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e36851. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, X.; Pu, F.; Yang, Y.; Wang, L.; Liu, H.; Fan, Y. Conservative treatment for equinus deformity in children with cerebral palsy using an adjustable splint-assisted ankle-foot orthosis. Medicine 2017, 96, e8186. [Google Scholar] [CrossRef]
- Klaewkasikum, K.; Patathong, T.; Woratanarat, P.; Woratanarat, T.; Thadanipon, K.; Rattanasiri, S.; Thakkinstian, A. Efficacy of conservative treatment for spastic cerebral palsy children with equinus gait: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2022, 17, 411. [Google Scholar] [CrossRef]
- Tustin, K.; Patel, A. A Critical Evaluation of the Updated Evidence for Casting for Equinus Deformity in Children with Cerebral Palsy. Physiother. Res. Int. 2017, 22, e1646. [Google Scholar] [CrossRef]
- Yang, S.M.; Chen, Y.H.; Lu, Y.L.; Wu, C.H.; Chen, W.S.; Lin, M.T. The dose effectiveness of extracorporeal shockwave on plantar flexor spasticity of ankle in stroke patients: A randomized controlled trial. J. Neuroeng. Rehabil. 2024, 21, 176. [Google Scholar] [CrossRef]
- Campanini, I.; Bò, M.C.; Bassi, M.C.; Damiano, B.; Scaltriti, S.; Lusuardi, M.; Merlo, A.; Hoover, D.L. Outcome measures for assessing the effectiveness of physiotherapy interventions on equinus foot deformity in post-stroke patients with triceps surae spasticity: A scoping review. PLoS ONE 2023, 18, e0287220. [Google Scholar] [CrossRef]
- Muzaffar, T.; Rather, A.H.; Haque, K.U.; Ahmad, S.J. To Evaluate the Effectiveness of TBTS—A Novel Device to do Self-Stretching of Gastroc-Soleus Muscle in Patients with Equinus Deformity. J. Clin. Diagn. Res. 2017, 11, YC01–YC04. [Google Scholar] [CrossRef]
- Glazebrook, M.; Rubinger, D. Functional rehabilitation for nonsurgical treatment of acute Achilles tendon rupture. Foot Ankle Clin. 2019, 24, 387–398. [Google Scholar] [CrossRef]
- Mubark, I.; Abouelela, A.; Arya, S.; Buchanan, D.; Elgalli, M.; Parker, J.; Ashwood, N.; Karagkevrekis, C. Achilles Tendon Rupture: Can the Tendon Gap on Ultrasound Scan Predict the Outcome of Functional Rehabilitation Program? Cureus 2020, 12, e10298. [Google Scholar] [CrossRef]
- Yassin, M.; Myatt, R.; Thomas, W.; Gupta, V.; Hoque, T.; Mahadevan, D. Does size of tendon gap affect patient-reported outcome following Achilles tendon rupture treated with functional rehabilitation? Bone Jt. J. 2020, 102-B, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.M.; Tan, C.O.; Tenforde, A.S. Functional Gains Using Radial and Combined Shockwave Therapy in the Management of Achilles Tendinopathy. J. Foot Ankle Surg. 2022, 61, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Nauwelaers, A.K.; Van Oost, L.; Peers, K. Evidence for the use of PRP in chronic midsubstance Achilles tendinopathy: A systematic review with meta-analysis. Foot Ankle Surg. 2021, 27, 486–495. [Google Scholar] [CrossRef]
- Madhi, M.I.; Yausep, O.E.; Khamdan, K.; Trigkilidas, D. The use of PRP in treatment of Achilles Tendinopathy: A systematic review of literature. Study design: Systematic review of literature. Ann. Med. Surg. 2020, 55, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Xu, S.-Z.; Gu, P.-C.; Du, J.-Y.; Cai, Y.-Z.; Zhang, C.; Lin, X.-J. Is Platelet-rich Plasma Injection Effective for Chronic Achilles Tendinopathy? A Meta-analysis. Clin. Orthop. Relat. Res. 2018, 476, 1633–1641. [Google Scholar] [CrossRef]
- Arthur Vithran, D.T.; Xie, W.; Opoku, M.; Essien, A.E.; He, M.; Li, Y. The Efficacy of Platelet-Rich Plasma Injection Therapy in the Treatment of Patients with Achilles Tendinopathy: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 995. [Google Scholar] [CrossRef]
- Chimenti, R.L.; Neville, C.; Houck, J.; Cuddeford, T.; Carreira, D.; Martin, R.L. Achilles Pain, Stiffness, and Muscle Power Deficits: Midportion Achilles Tendinopathy Revision-2024. J. Orthop. Sports Phys. Ther. 2024, 54, CPG1–CPG32. [Google Scholar] [CrossRef]
- Grady, J.F.; Saxena, A. Effects of stretching the gastrocnemius muscle. J. Foot Surg. 1991, 30, 465–469. [Google Scholar]
- Macklin, K.; Healy, A.; Chockalingam, N. The effect of calf muscle stretching exercises on ankle joint dorsiflexion and dynamic foot pressures, force and related temporal parameters. Foot 2012, 22, 10–17. [Google Scholar] [CrossRef]
- Radford, J.A.; Burns, J.; Buchbinder, R.; Landorf, K.B.; Cook, C. Does stretching increase ankle dorsiflexion range of motion? A systematic review. Br. J. Sports Med. 2006, 40, 870–875. [Google Scholar] [CrossRef]
- Kunkle, B.F.; Baxter, N.A.; Hoch, C.P.; Caughman, A.; Barcel, J.; Scott, D.J.; Gross, C.E. Success Rate of Non-Operative Treatment of Insertional Achilles Tendinopathy. Foot Ankle Orthop. 2022, 7, 2473011421S00735. [Google Scholar] [CrossRef]
- Dombrowski, M.; Murawski, C.D.; Yasui, Y.; Chen, A.F.; Ewalefo, S.O.; Fourman, M.S.; Kennedy, J.G.; Hogan, M.V. Medical comorbidities increase the rate of surgical site infection in primary Achilles tendon repair. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2840–2851. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Schiavone, C.; Salini, V.; Andia, I. Occurrence of tendon pathologies in metabolic disorders. Rheumatology 2013, 52, 599–608. [Google Scholar] [CrossRef] [PubMed]
| Citation | Study Design | Sample Size | Treatment | Primary Outcomes | Key Findings |
|---|---|---|---|---|---|
| Myhrvold et al., [8] | Multicenter RCT | 554 | Nonoperative vs. surgical treatment | ATRS, re-rupture rate, complications | No difference in functional outcomes; higher re-rupture rate in non-op group (6.2%) vs. surgical group (0.6%); fewer complications in non-op group. |
| Deng et al. [9] | Systematic review and meta-analysis | Pooled data | Surgical vs. conservative | Re-rupture rate, complications, return to activity | Surgery had lower re-rupture rate, but more complications; no difference in long-term function. |
| Lantto et al. [10] | Prospective RCT | 60 | Surgical vs. nonoperative with rehab | ATRS, re-rupture, strength | Similar function; slightly better strength in surgery group; fewer complications in non-op group. |
| Seow et al. [11] | Systematic review and meta-analyses | Meta-analysis of meta-analyses | Surgical vs. conservative | Re-rupture, complications, function | Surgery had lower re-rupture rate, but higher complications. Functional rehab was important in non-op group for successful outcomes. |
| Lerch et al. [12] | Retrospective cohort | 89 | Nonoperative with early rehab | Return to sport, satisfaction | 70% returned to sport; 67% for high-activity patients. High patient satisfaction overall. |
| Ochen et al. [13] | Systematic review and meta-analysis | 15,862 | Surgical vs. nonoperative (with and without rehab) | Re-rupture, complications | Surgery had significantly lower re-rupture rate (2.3% vs. 3.9%), but more complications. In patients with early rehab, re-rupture rates were similar. |
| Citation | Study Design | Sample Size | Intervention | Weightbearing | Primary Outcomes | Key Findings |
|---|---|---|---|---|---|---|
| Costa et al. (UK STAR Trial) [14] | Multicenter randomized control trial | 540 | Functional bracing vs. plaster casting | Full weightbearing allowed within 48 h with brace | ATRS, return to work, complications | Higher ATRS, earlier return to work, and fewer complications in bracing group |
| Ecker et al. [15] | Prospective case series | 114 | Early weightbearing protocol using walking boot | Immediate weightbearing with progressive dorsiflexion | Functional outcomes, re-rupture rates | Low re-rupture rate and favorable functional recovery with early mobilization |
| Citation | Study Design | Condition Focus | Intervention | Key Findings |
|---|---|---|---|---|
| Prudêncio et al. [16] | Systematic review and meta-analysis | Midportion tendinopathy | Various conservative treatments | Eccentric exercise was most effective at improving pain and function. |
| Rhim et al. [17] | Network meta-analysis | Midportion tendinopathy | HVI, ESWT, eccentric loading | Adding HVI with corticosteroids or ESWT to eccentric exercise may improve long-term outcomes. |
| Kearney et al. [18] | Randomized clinical trial | Midportion tendinopathy | PRP injection vs. placebo injection | No difference in outcomes between both groups. |
| Mansur et al. [19] | Double-blinded randomized clinical trial | Insertional tendinopathy | ESWT and eccentric exercise vs. eccentric exercise alone | No difference in outcomes between both groups. |
| Ko et al. [20] | Systematic review and network meta-analysis | Insertional tendinopathy | Eccentric exercise, ESWT, cryotherapy, orthotics | ESWT and eccentric loading were among the most effective short-term treatments. |
| Zhi et al. [21] | Systematic review | Insertional tendinopathy | NSAIDs, physical therapy, ESWT, heel lifts, orthoses | ESWT was most effective; combining with physical therapy was also beneficial. |
| Citation | Study Design | Population | Intervention | Key Findings |
|---|---|---|---|---|
| Kumar et al. [22] | Systematic review and meta-analysis | Children with spastic cerebral palsy | Botulinum toxin + casting | Improved ankle dorsiflexion and gait short-term; combination therapy was more effective than either alone. |
| Chen et al. [23] | Prospective cohort | Children with cerebral palsy | Adjustable splint-assisted AFO | Orthotic use improved passive ROM and gait parameters. |
| Klaewkasikum et al. [24] | Systematic review and meta-analysis | Children with spastic CP and equinus gait | Various conservative treatments | Conservative approaches (casting, AFO, stretching) significantly improved dorsiflexion and gait. |
| Tustin & Patel [25] | Narrative review | Children with cerebral palsy | Serial casting | Temporary gains in dorsiflexion; adjunct therapies needed for sustained results. |
| Yang et al. [26] | Randomized controlled trial | Stroke patients with plantar flexor spasticity | ESWT | ESWT reduced spasticity and increased ankle dorsiflexion. |
| Campanini et al. [27] | Scoping review | Stroke patients with equinus | Physical therapy modalities (e.g., stretching, AFO) | PT strategies were generally effective at reducing triceps spasticity and improving gait. |
| Muzzafar et al. [28] | Randomized controlled trial | Patients with equinus, both spastic and non-spastic | TBTS control | Significant decrease in equinus after 1 month of TBTS use. |
| Achilles Tendon Pathology | Treatment | Key Considerations |
|---|---|---|
| Tendon Rupture: Operative Fixation | Follow surgeon’s postoperative rehabilitation protocol. | Lower re-rupture rate. |
| Tendon Rupture: Conservative Treatment | Early functional rehabilitation. If concerned about compliance with rehab protocol, consider operative fixation. | Higher re-rupture rate; fewer complications. |
| Achilles Tendinosis | Eccentric exercise as primary treatment; ESWT or PRP injections may be considered as secondary modalities. | Eccentric exercise is most consistently supported as the superior treatment option. |
| Equinus | Serial casting, static stretching, and dynamic bracing. | Treatment is patient-specific and guided by the underlying cause of contracture. Long-term effectiveness may vary. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kipp, J.A.; Blazek, C.D. Current Concepts in the Nonoperative Management of Achilles Tendon Pathologies: A Scoping Review. J. Clin. Med. 2025, 14, 4736. https://doi.org/10.3390/jcm14134736
Kipp JA, Blazek CD. Current Concepts in the Nonoperative Management of Achilles Tendon Pathologies: A Scoping Review. Journal of Clinical Medicine. 2025; 14(13):4736. https://doi.org/10.3390/jcm14134736
Chicago/Turabian StyleKipp, Jennifer A., and Cody D. Blazek. 2025. "Current Concepts in the Nonoperative Management of Achilles Tendon Pathologies: A Scoping Review" Journal of Clinical Medicine 14, no. 13: 4736. https://doi.org/10.3390/jcm14134736
APA StyleKipp, J. A., & Blazek, C. D. (2025). Current Concepts in the Nonoperative Management of Achilles Tendon Pathologies: A Scoping Review. Journal of Clinical Medicine, 14(13), 4736. https://doi.org/10.3390/jcm14134736





